Gold-Catalyzed Addition of Carboxylic Acids to Alkynes and Allenes: Valuable Tools for Organic Synthesis
Abstract
1. Introduction
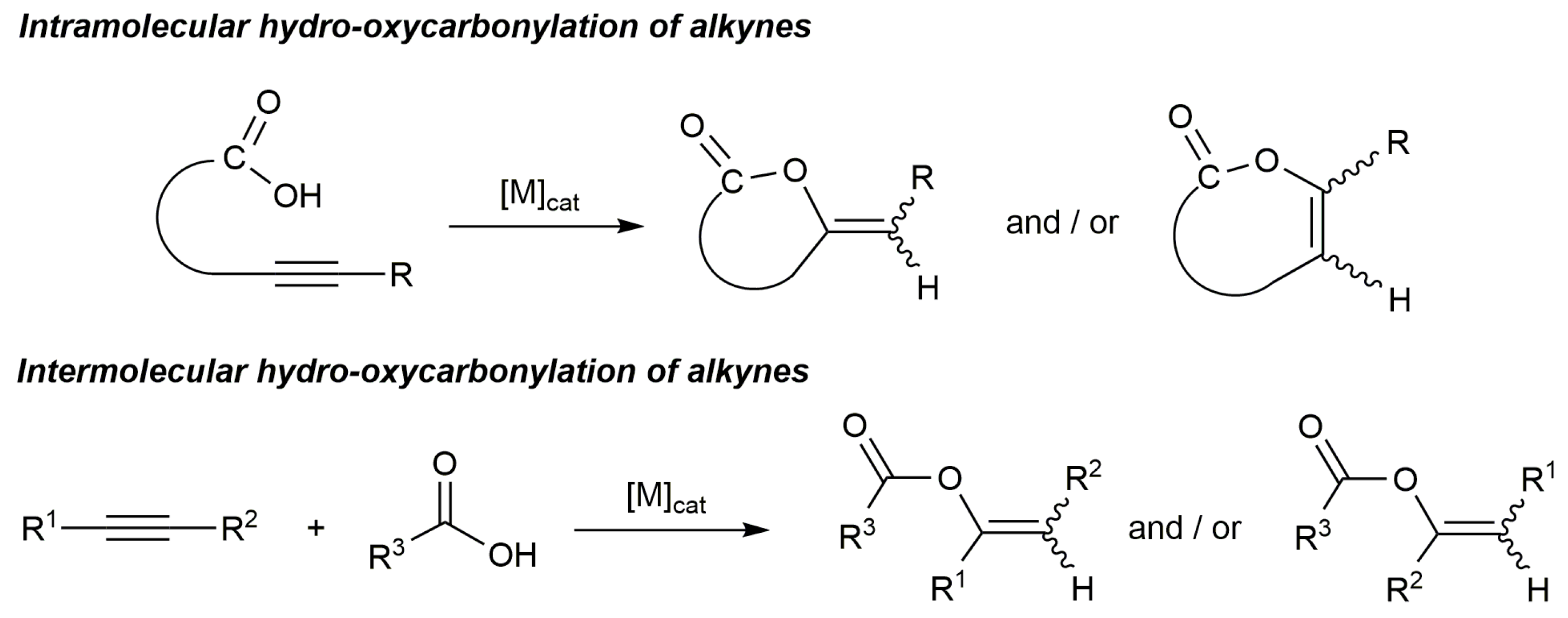
2. Additions of Carboxylic Acids to Alkynes
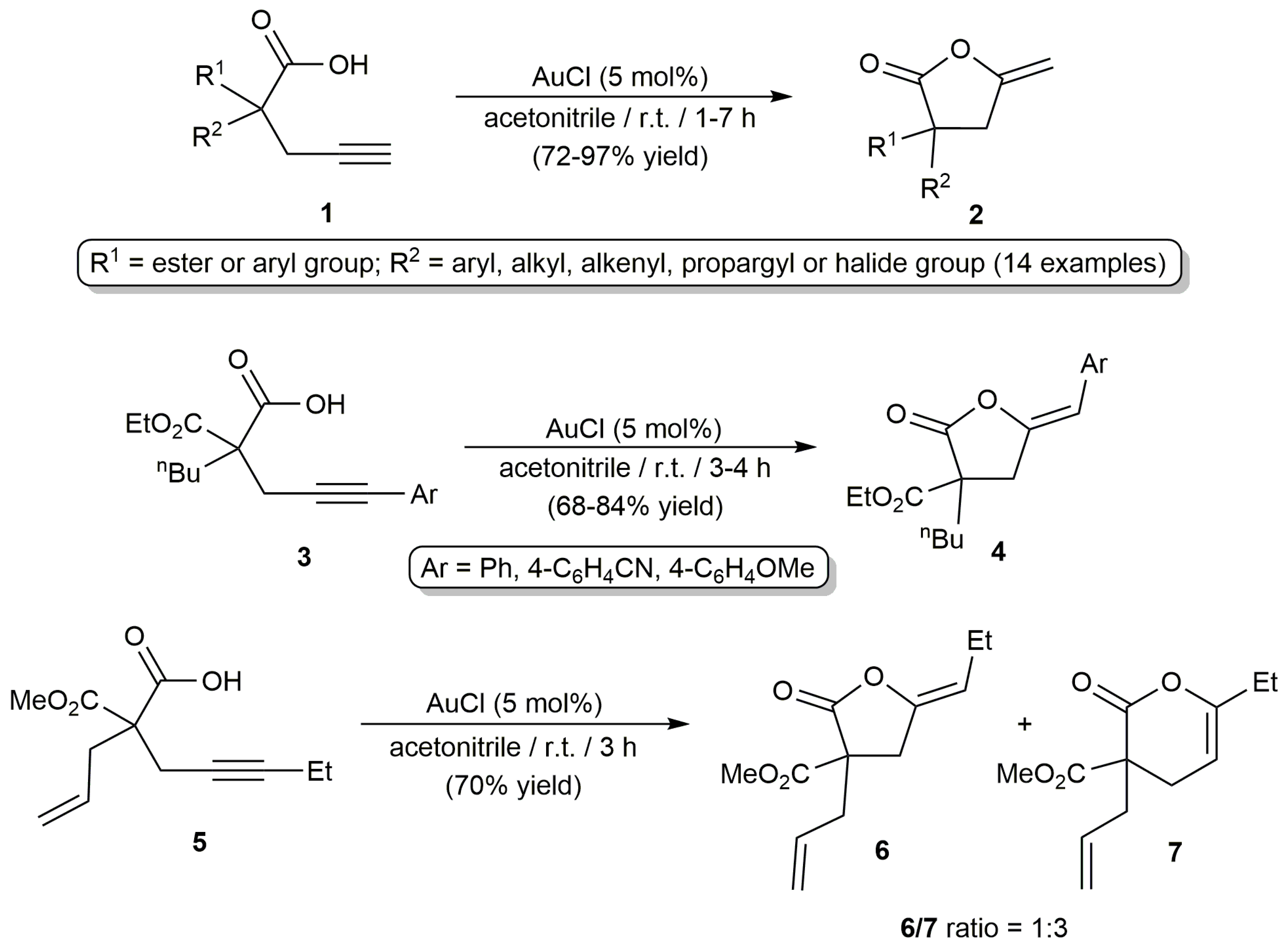
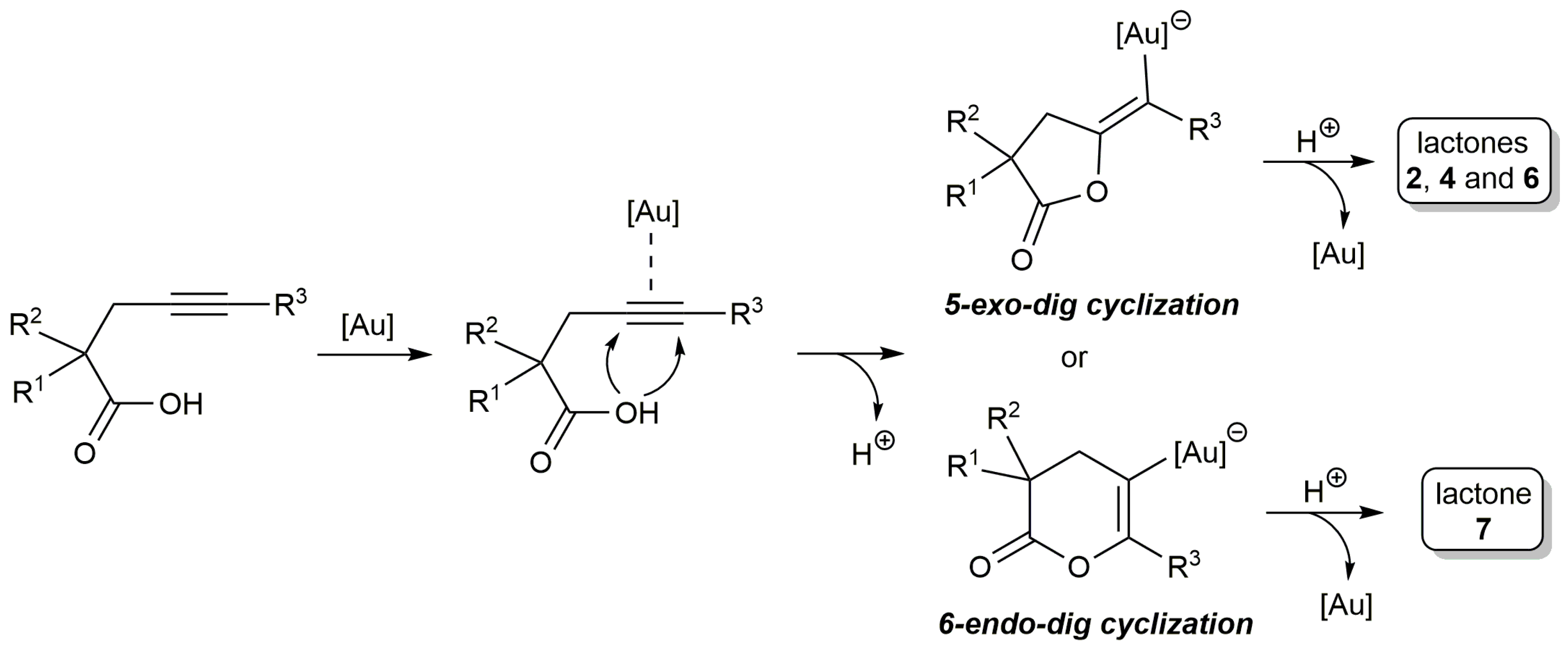
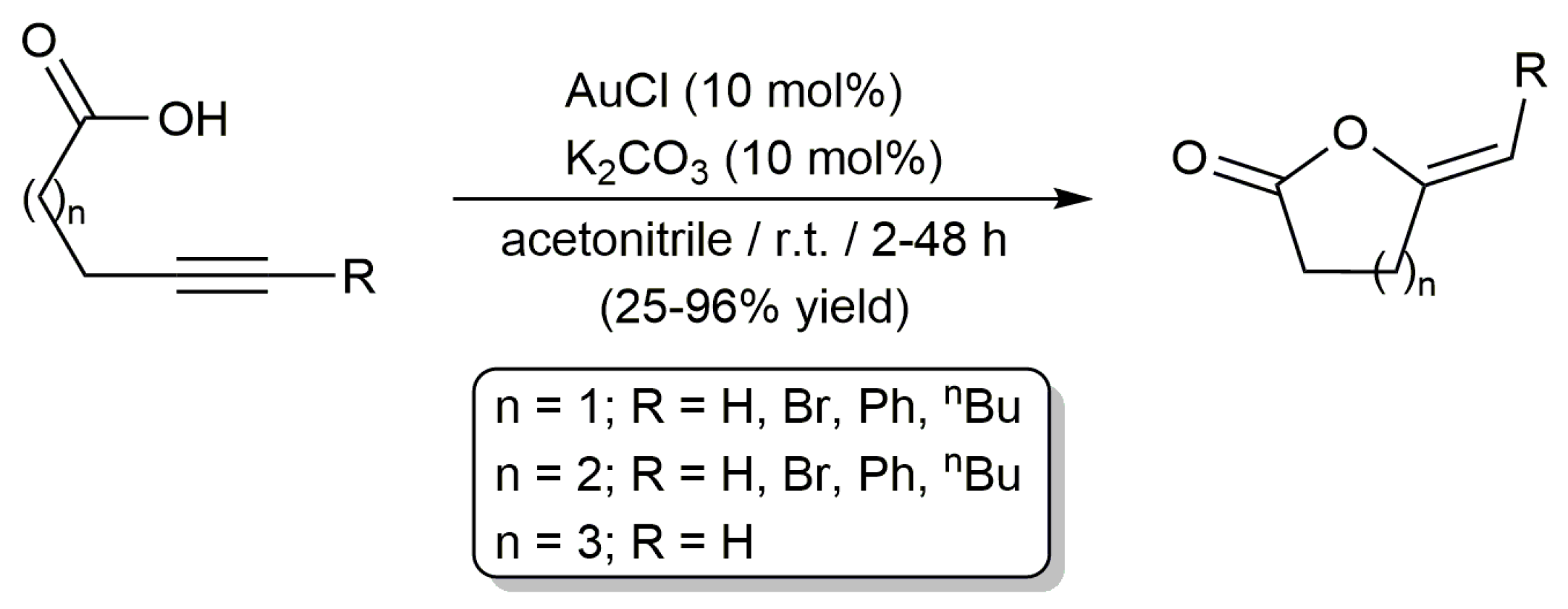
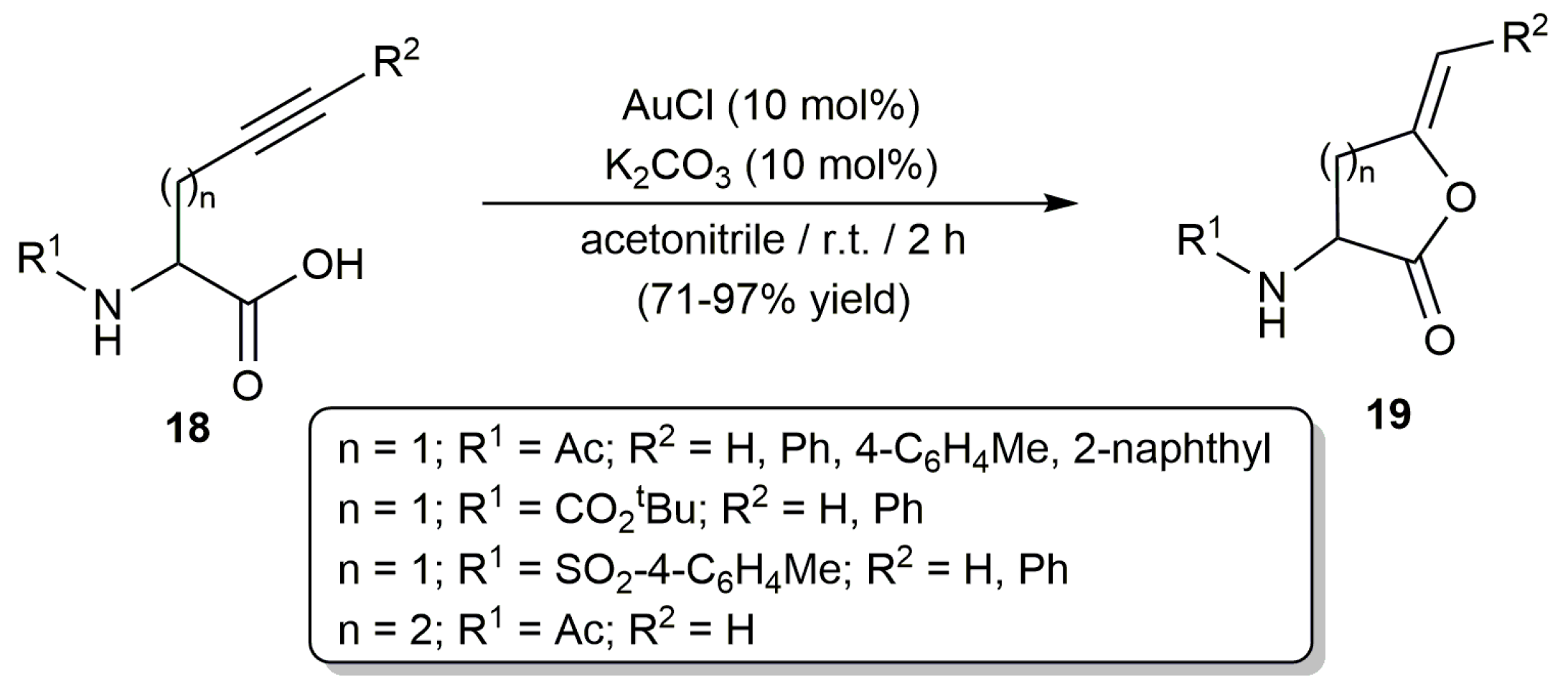
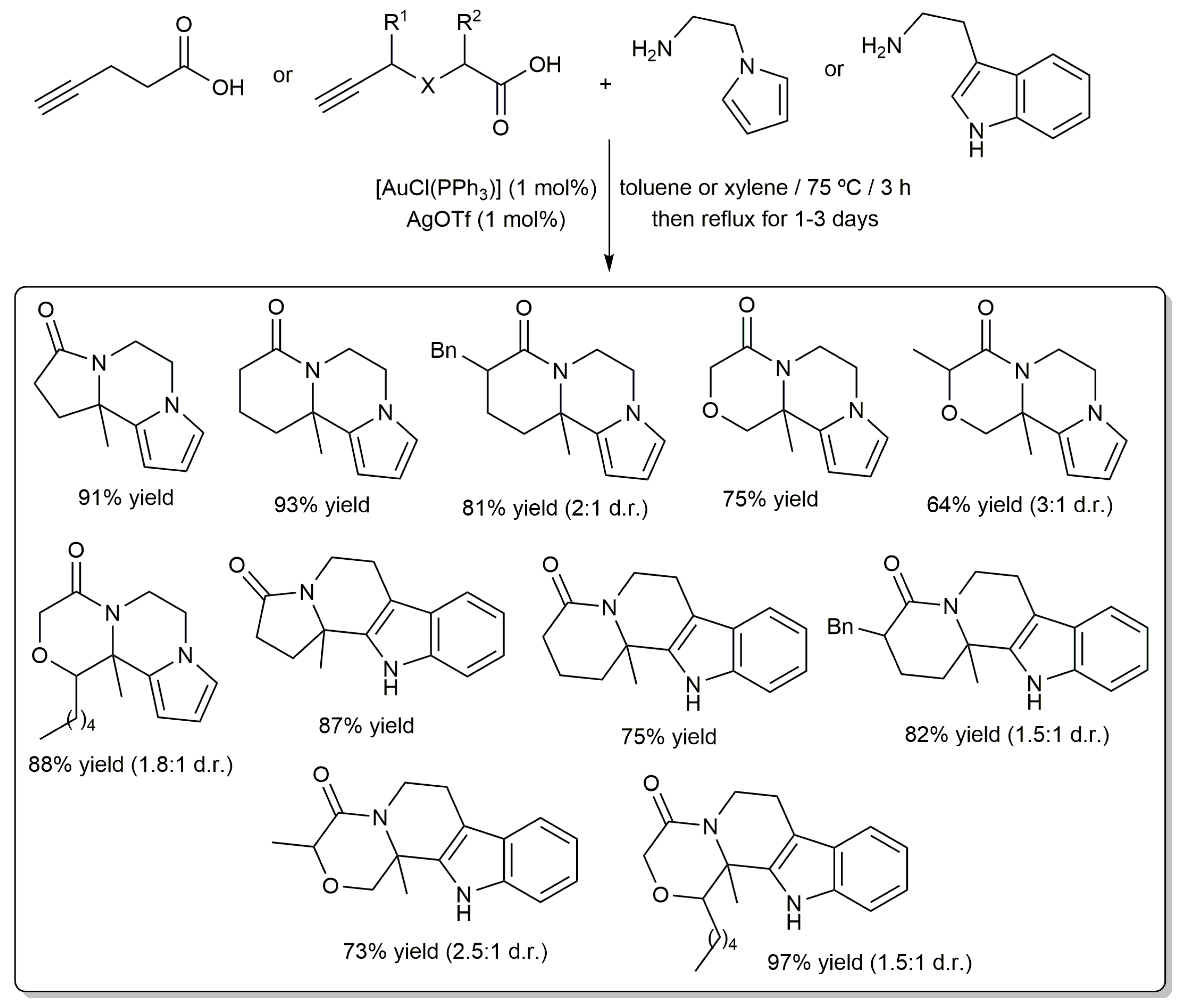
2.1. Cycloisomerization of Alkynoic Acids
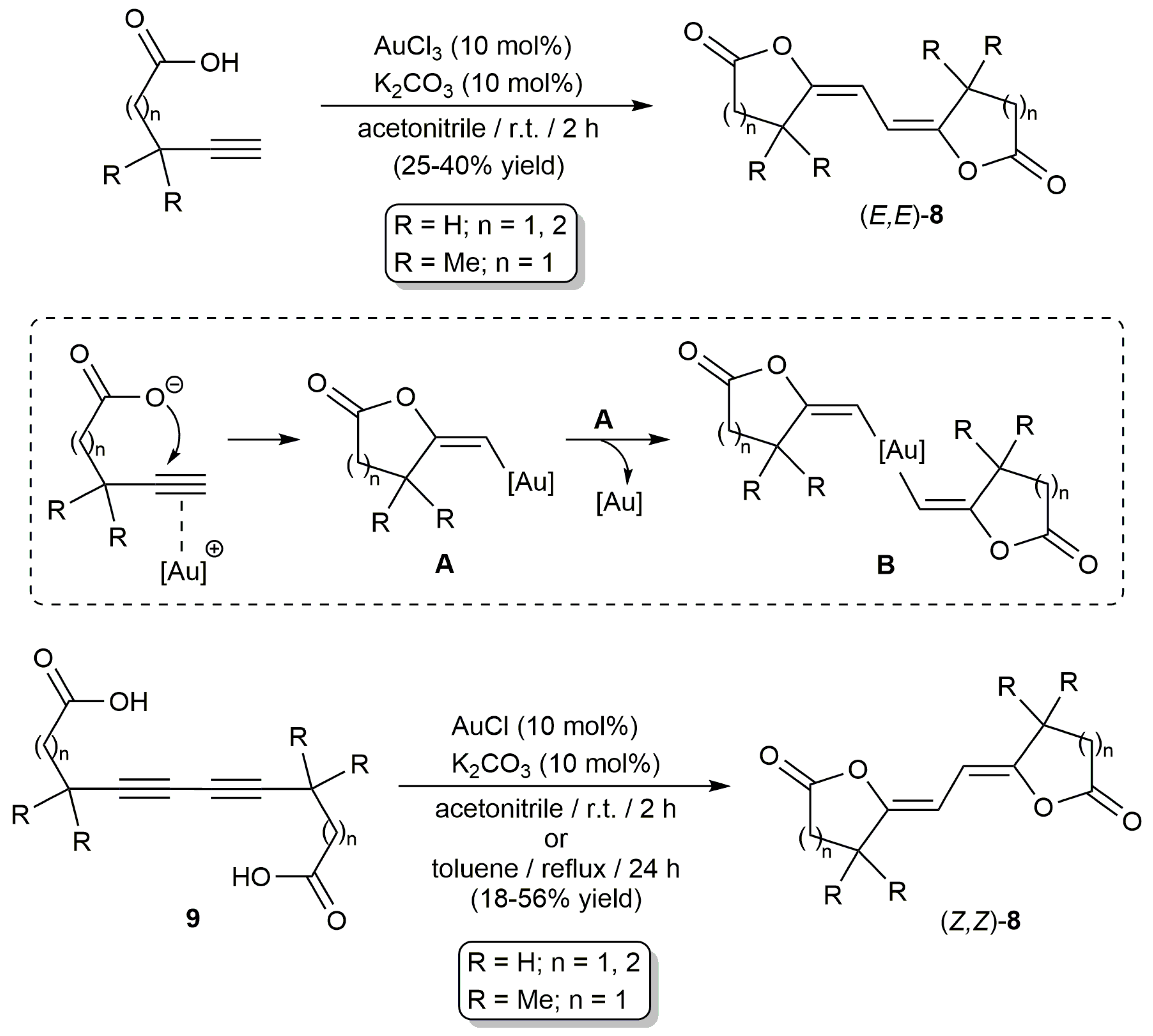
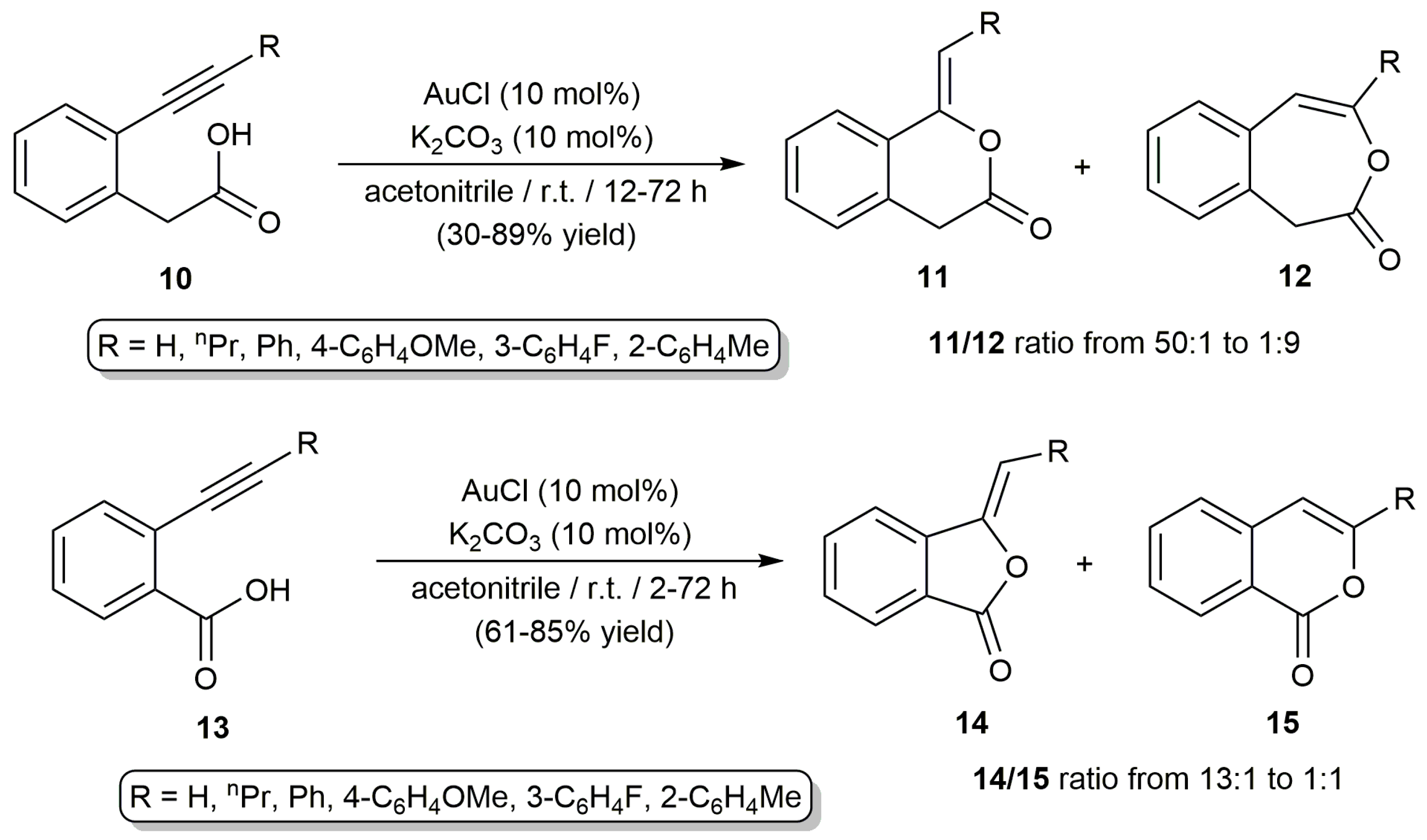
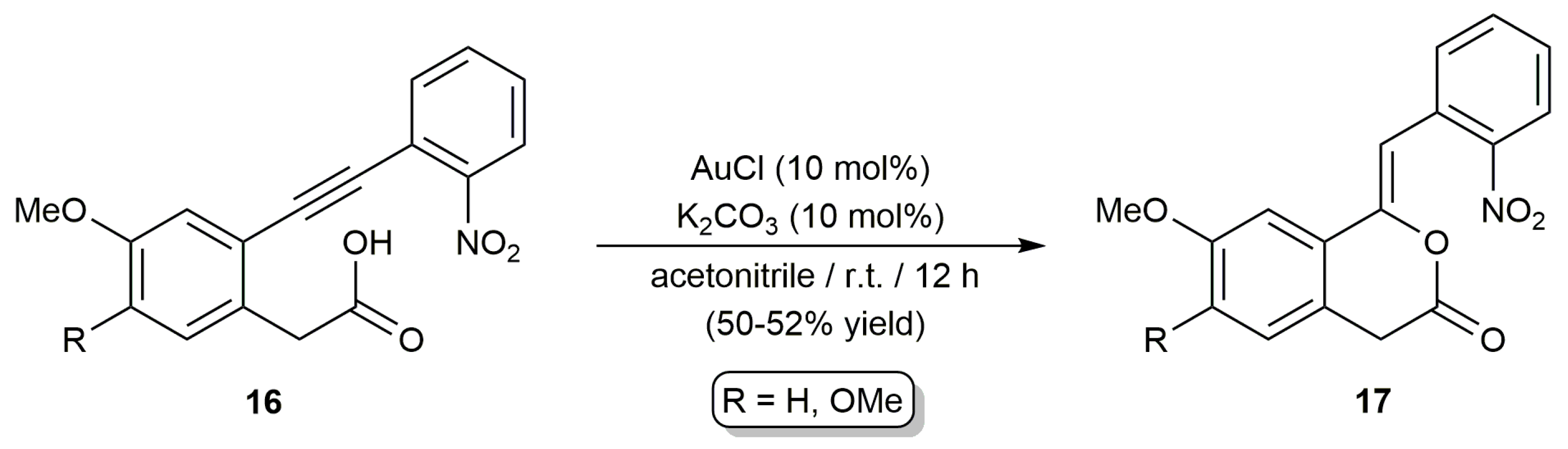
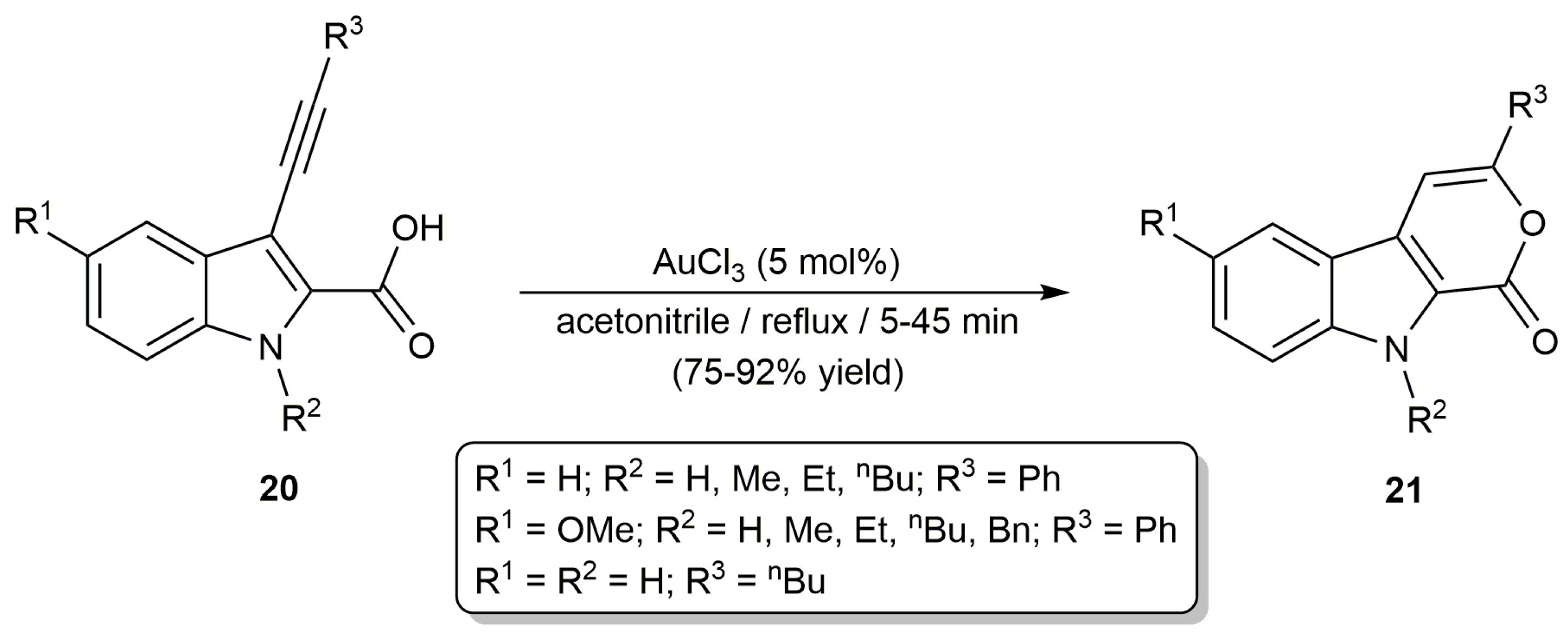
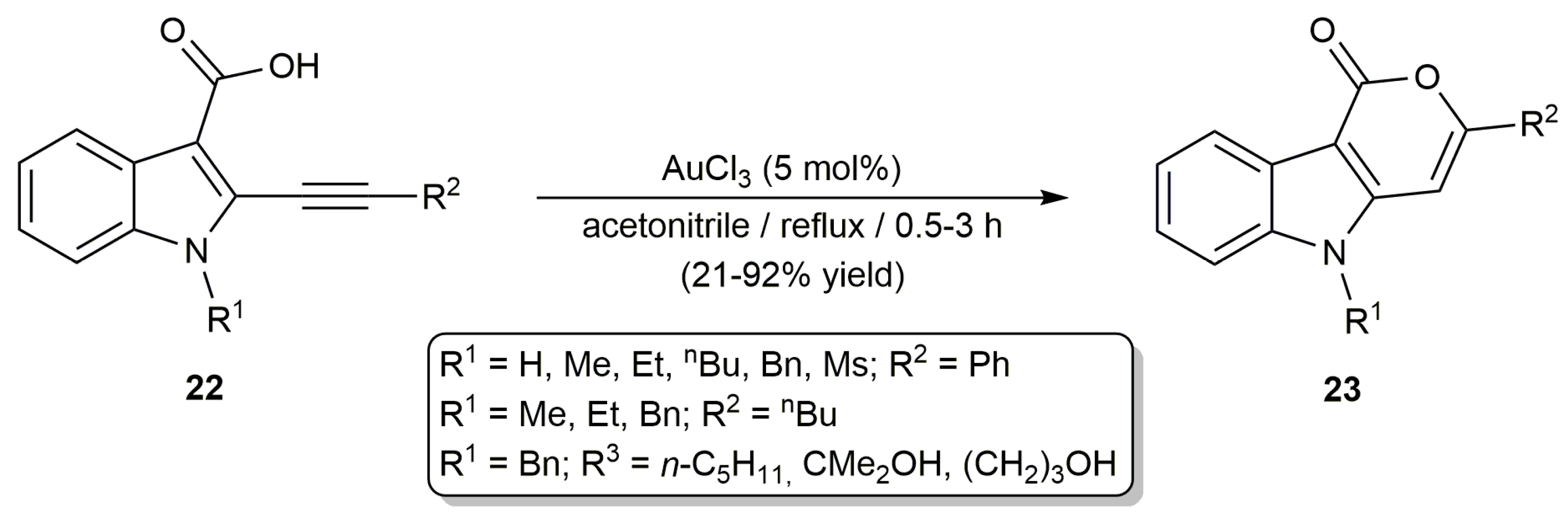
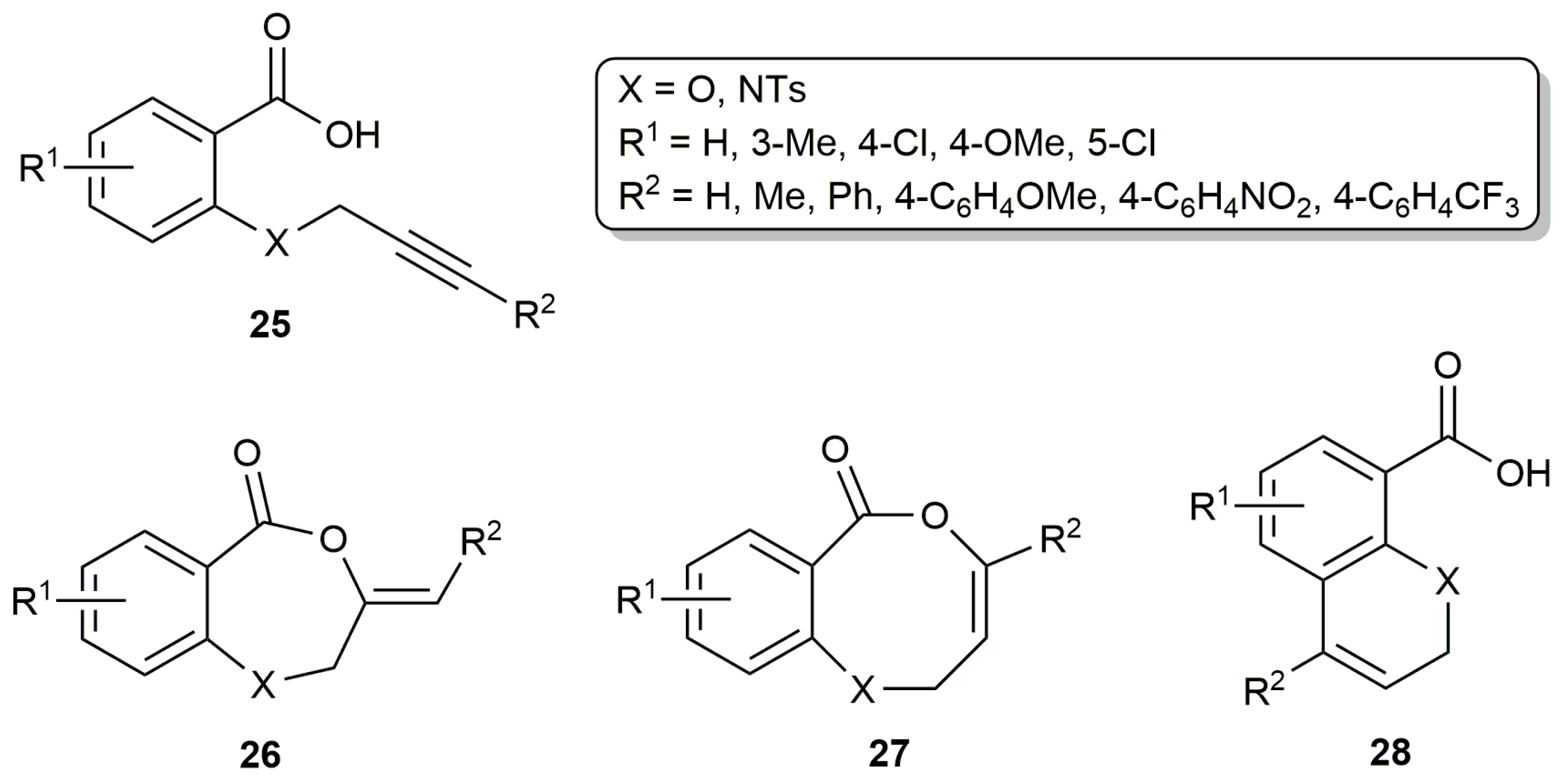
2.2. Cascade Processes Involving the Cycloisomerization of Alkynoic Acids
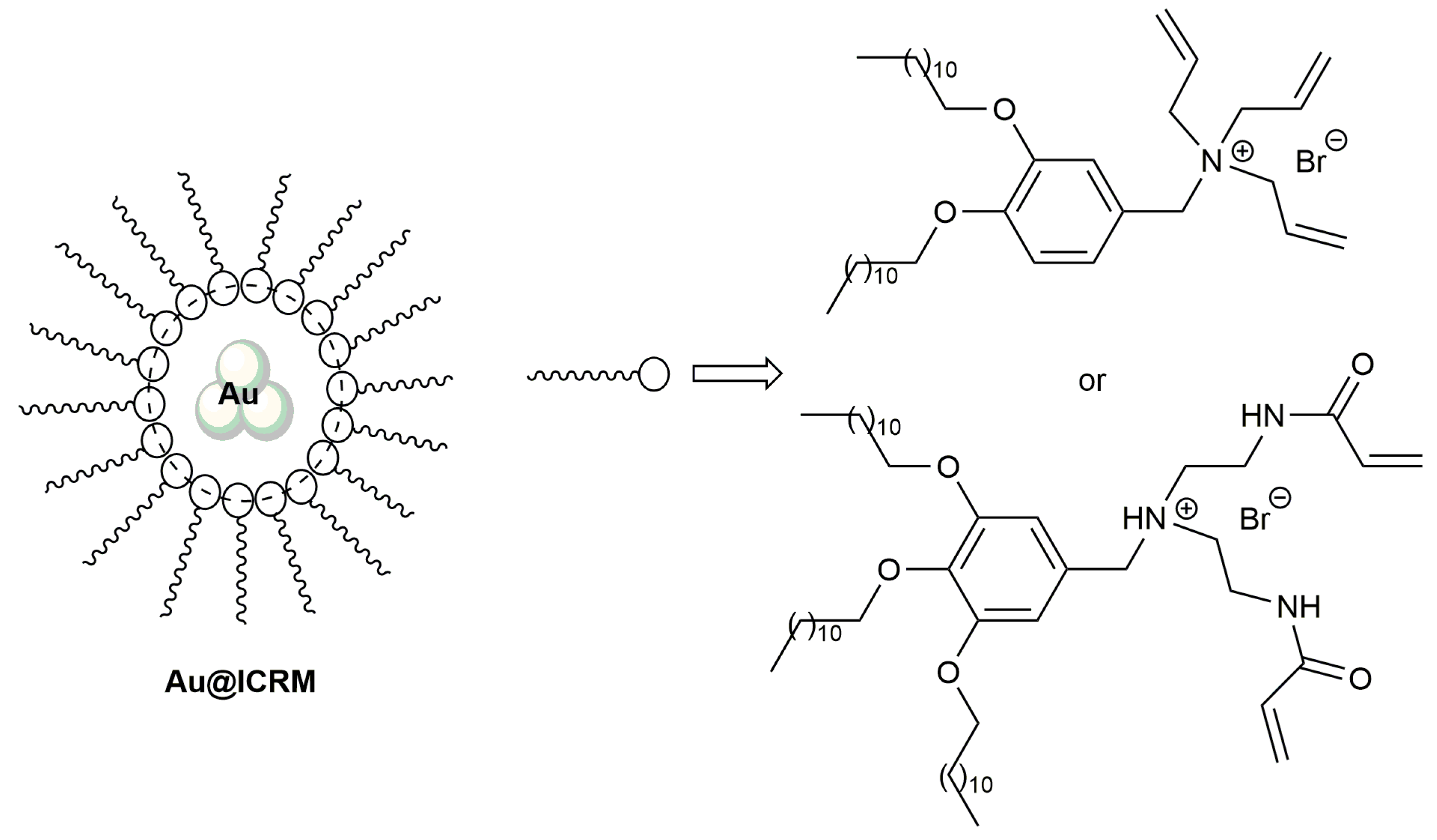
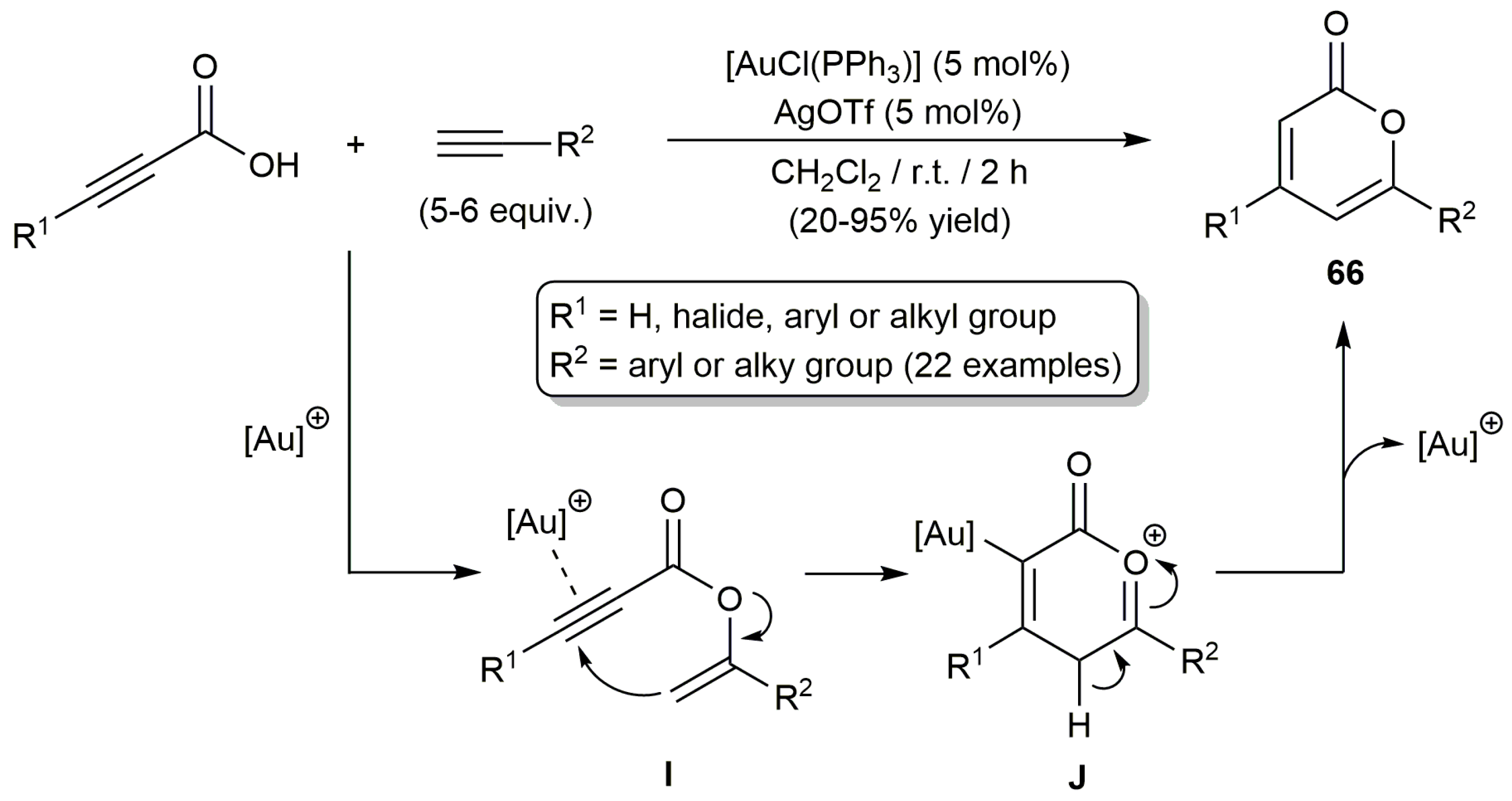
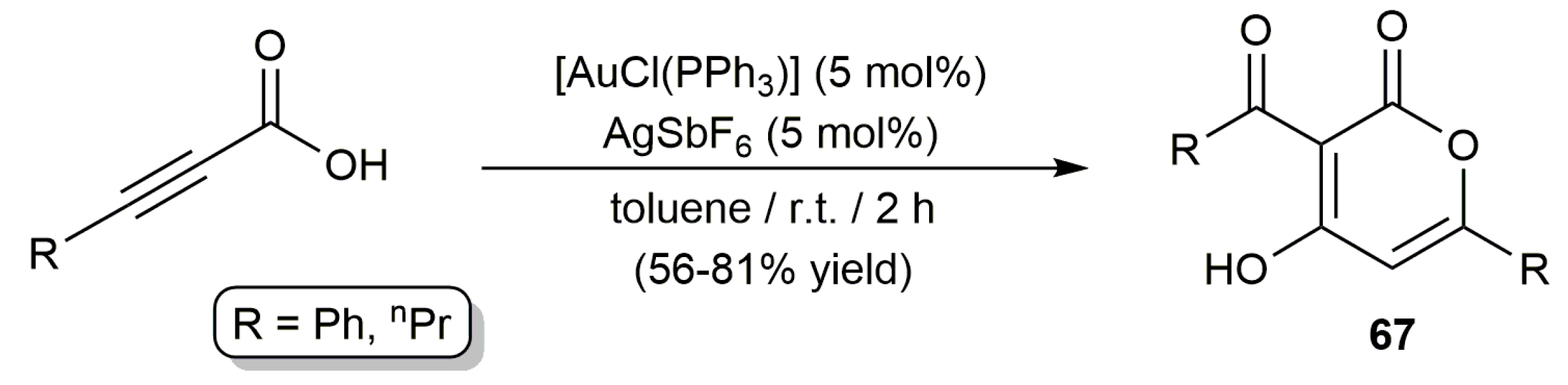
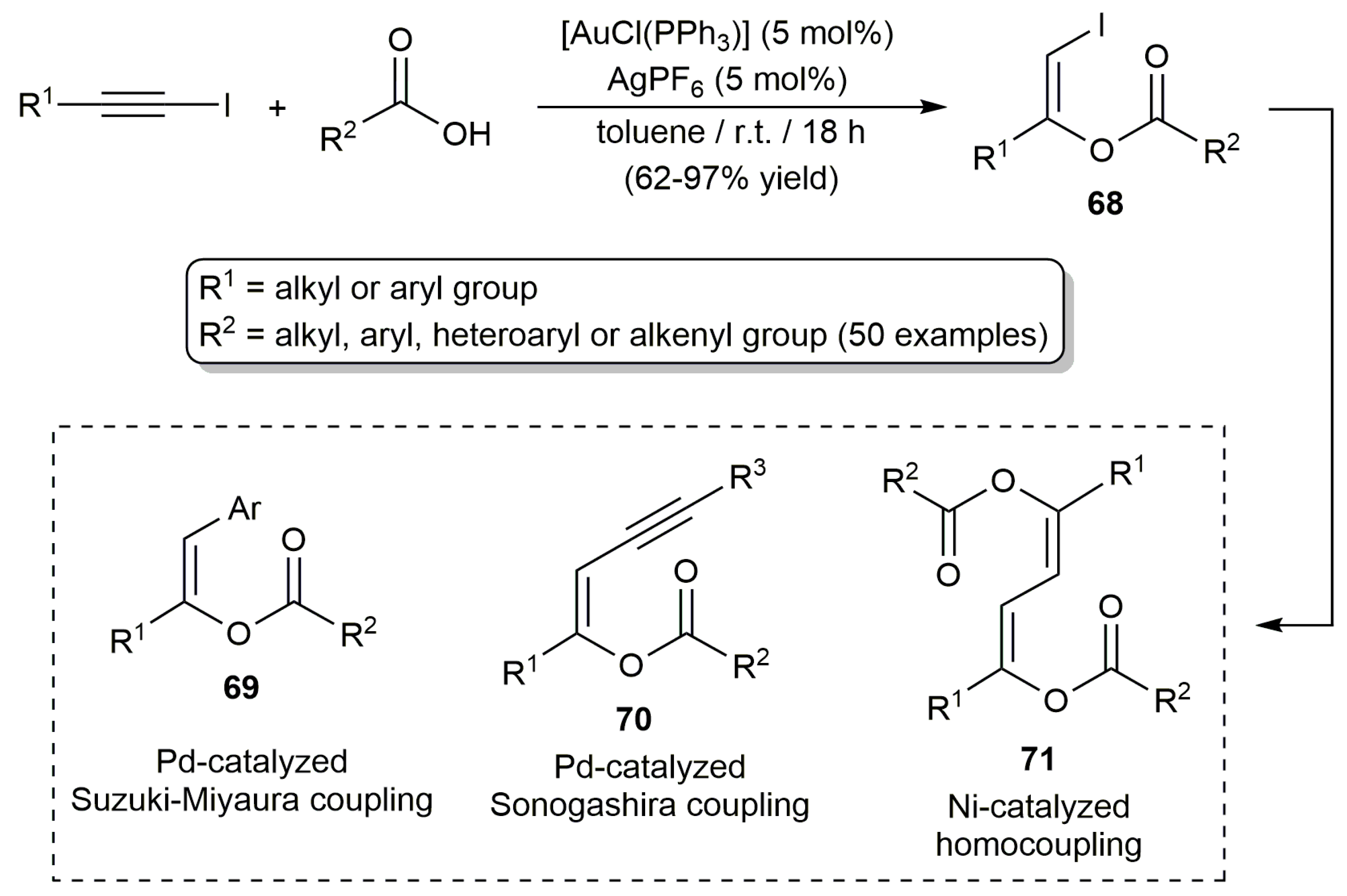
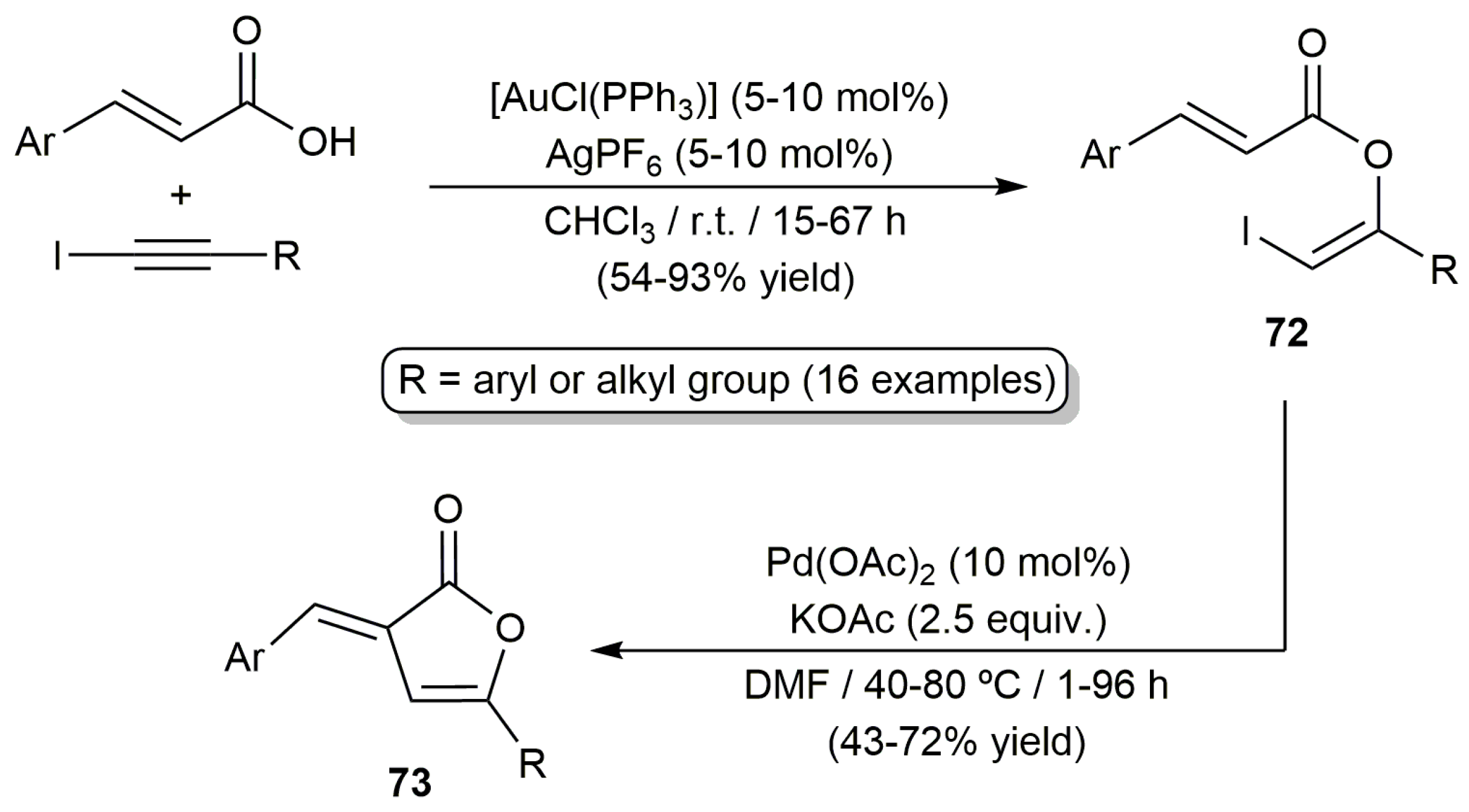
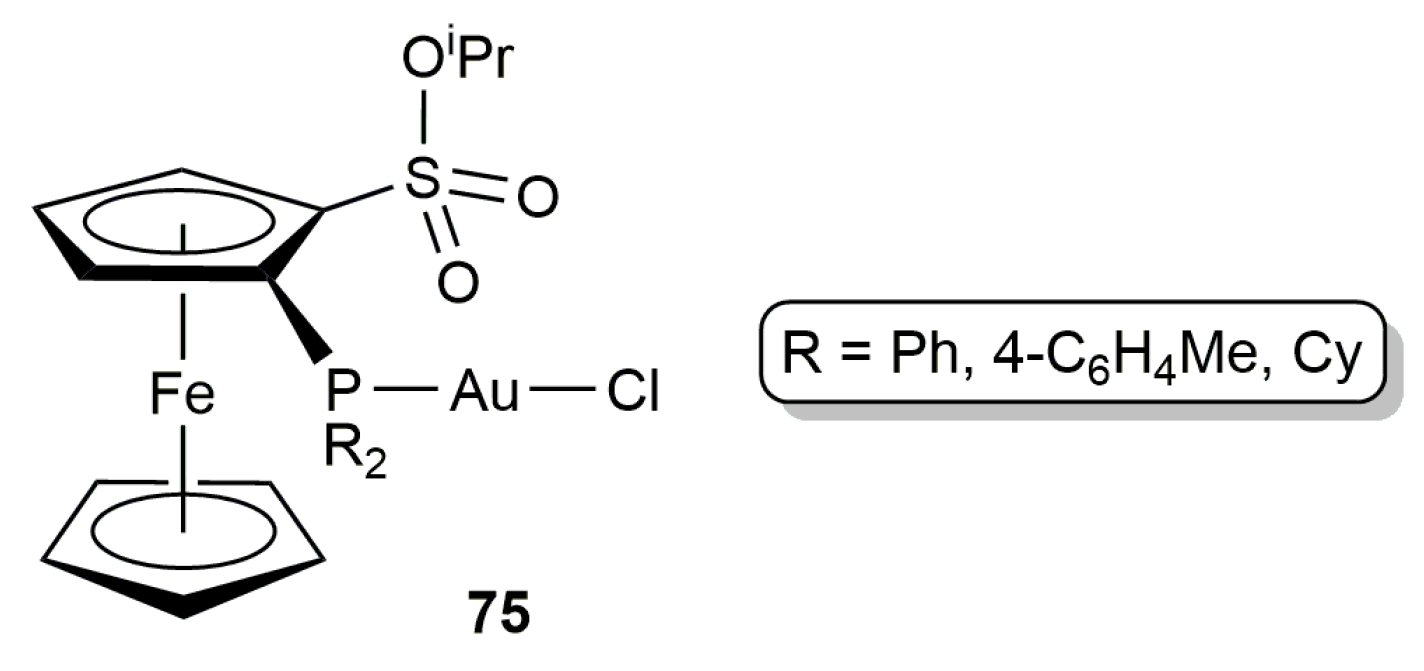
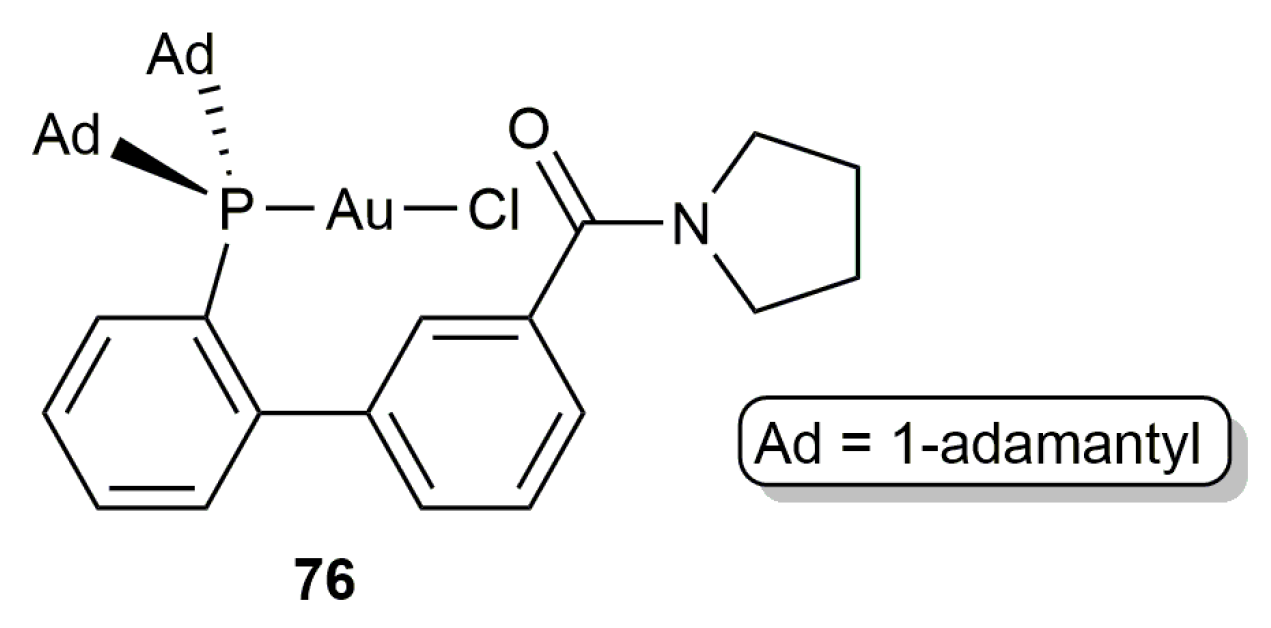
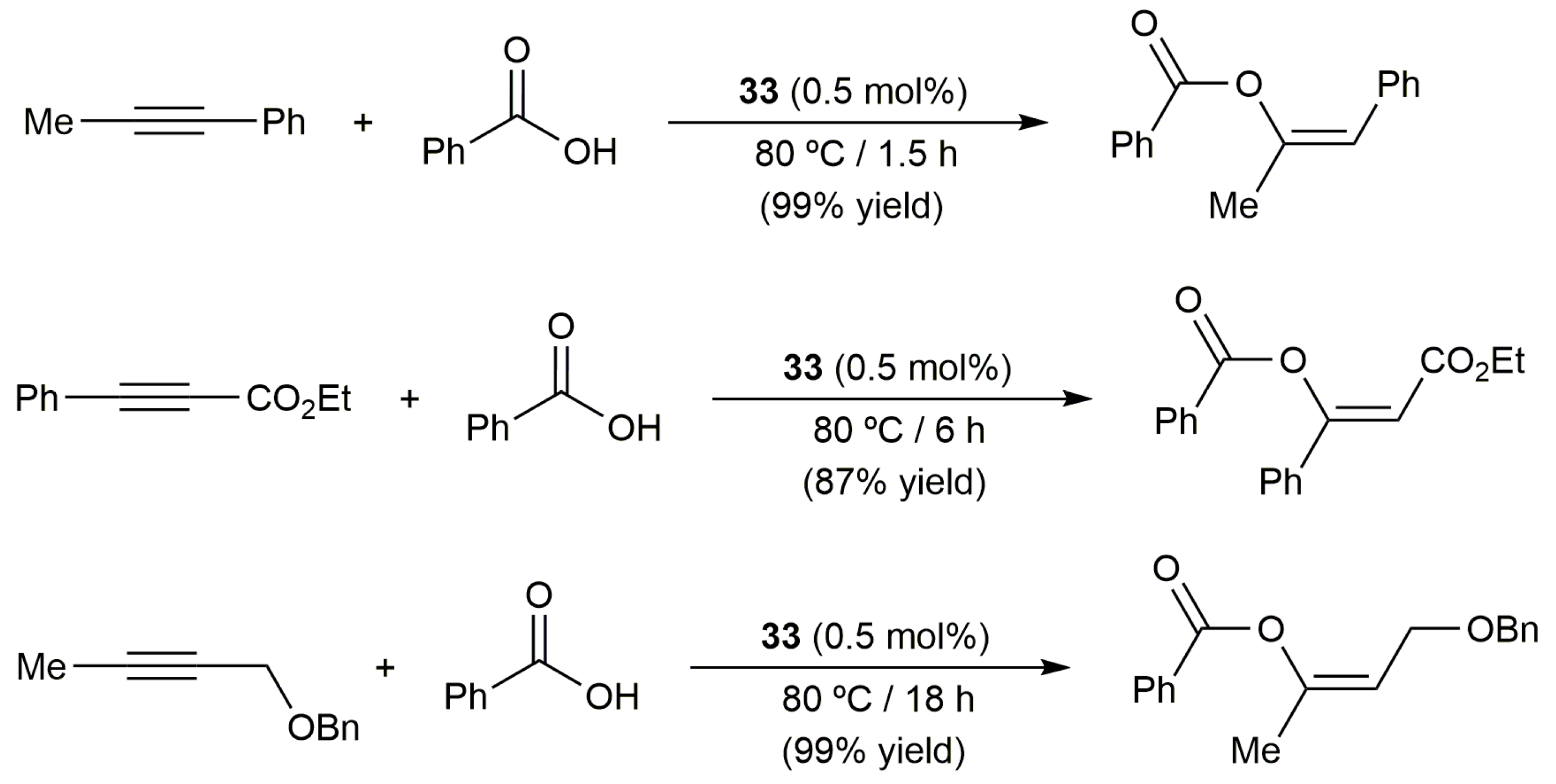
2.3. Intermolecular Addition of Carboxylic Acids to Alkynes
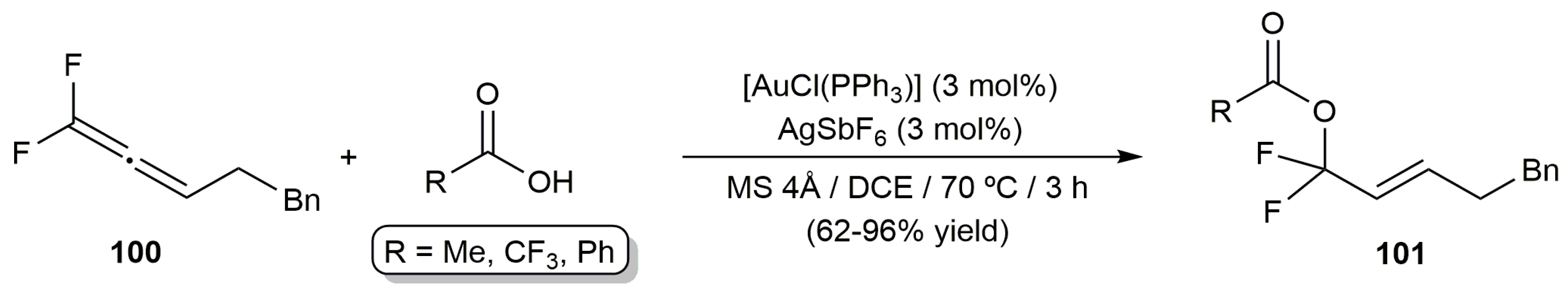
3. Addition of Carboxylic Acids to Allenes
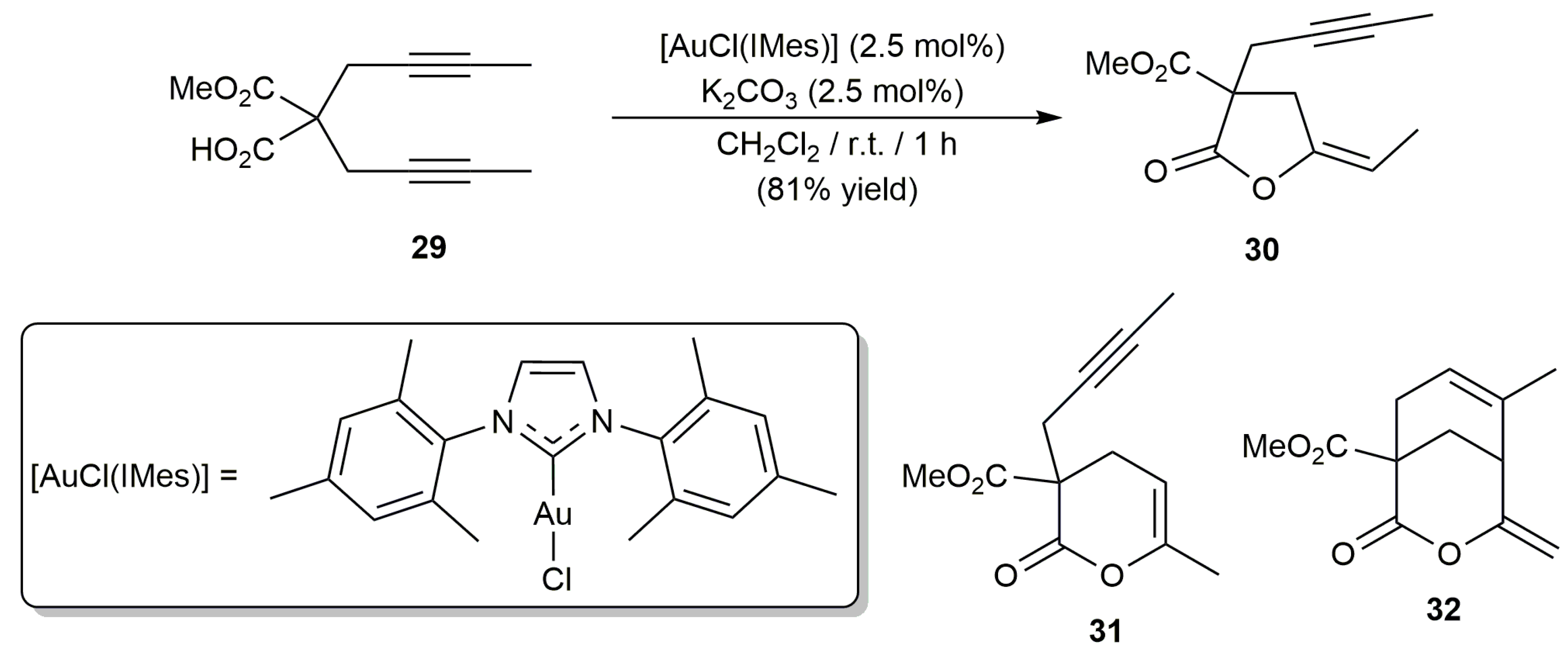
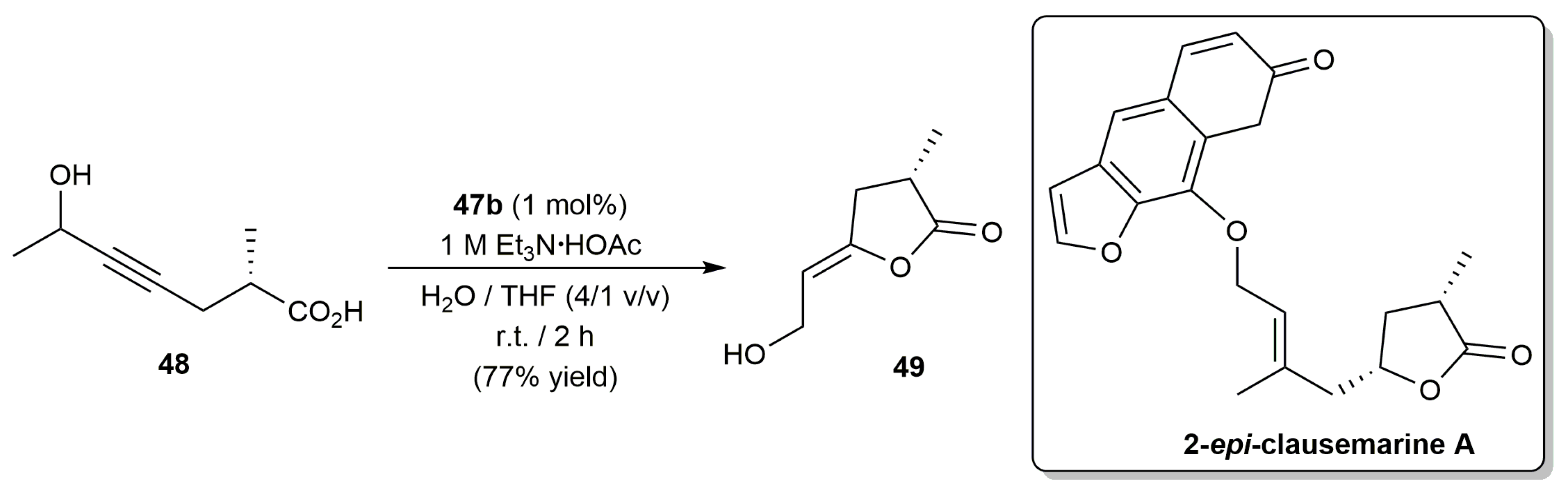
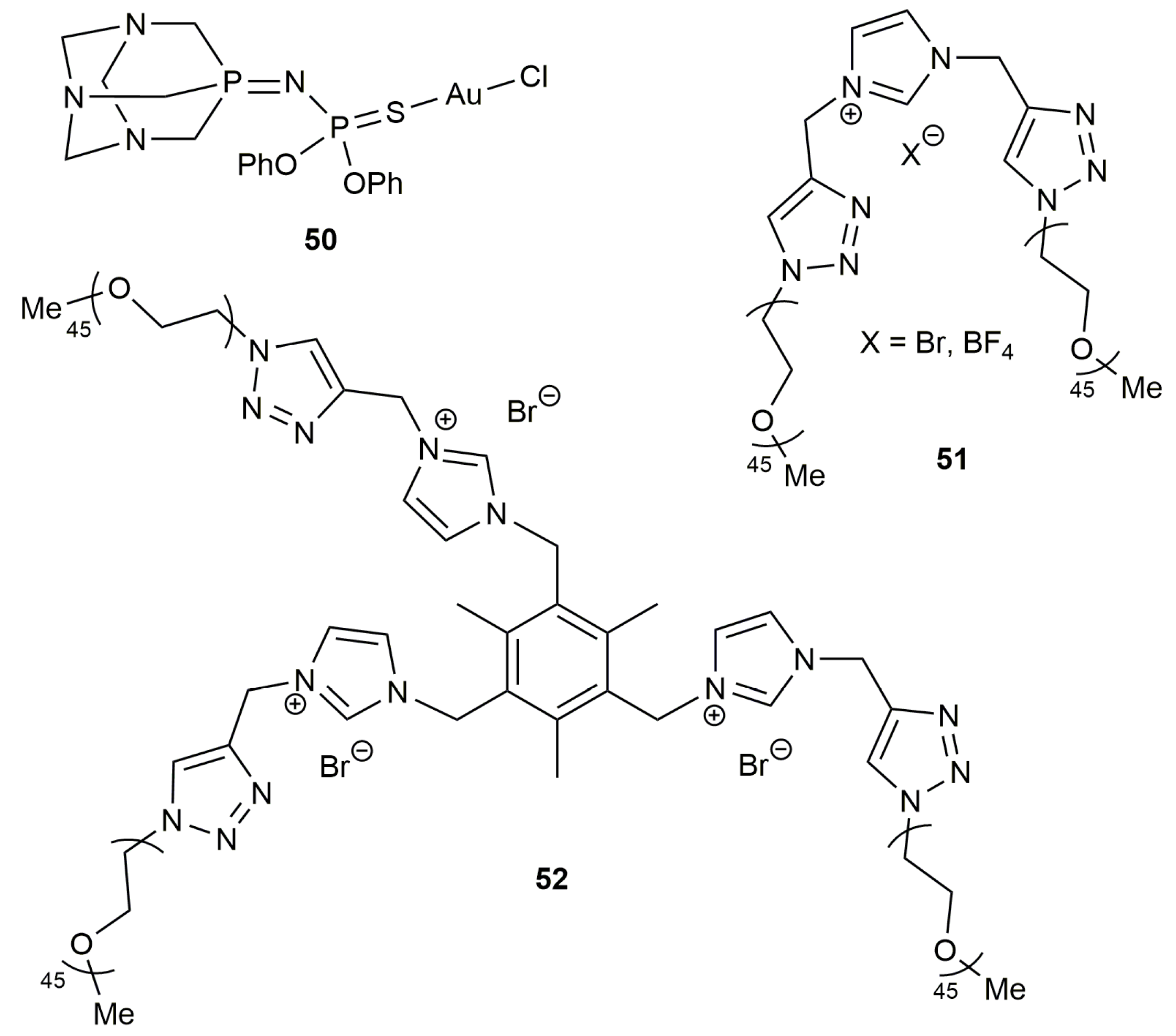
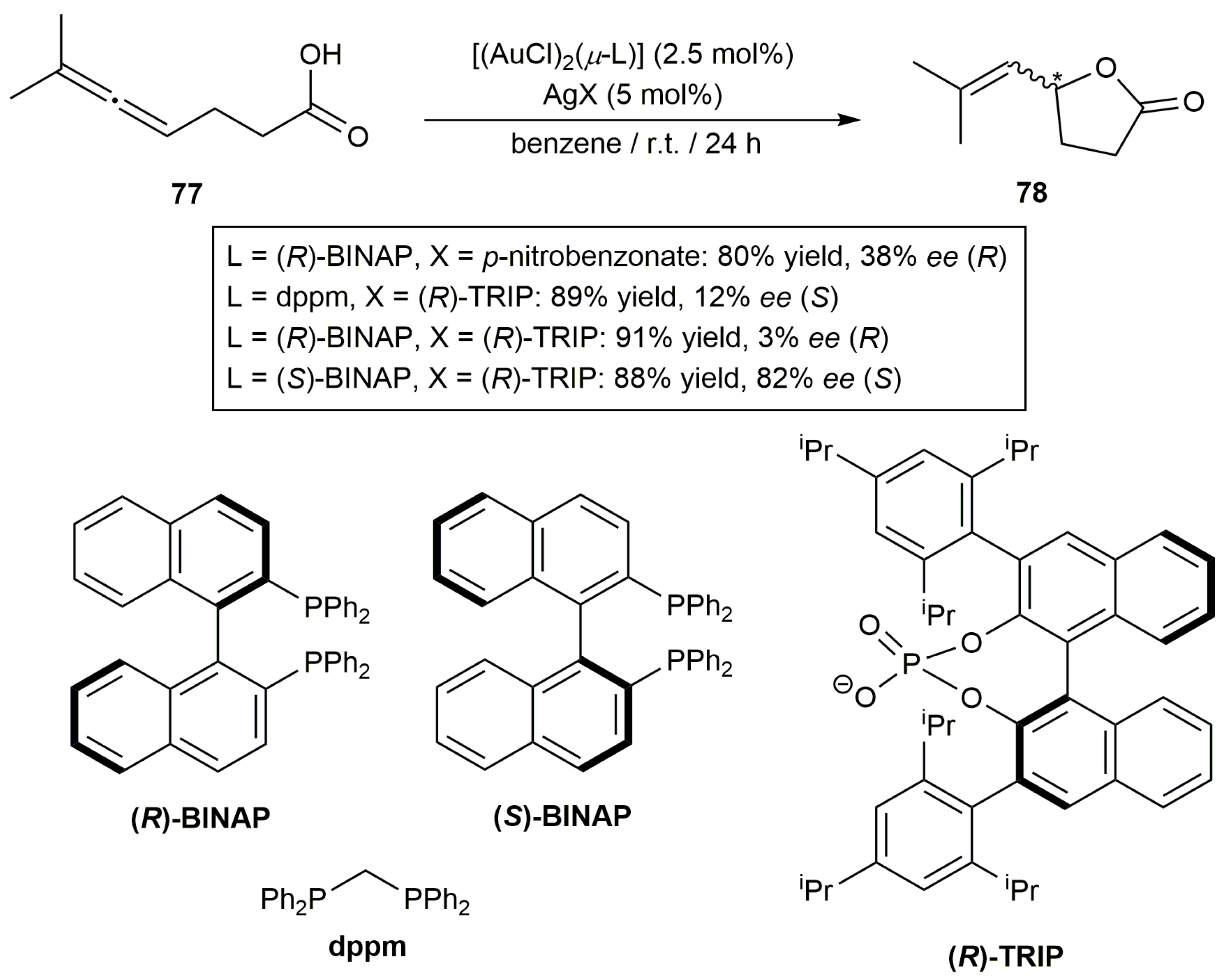
3.1. Cycloisomerization of Allenoic Acids
3.2. Intermolecular Addition of Carboxylic Acids to Allenes
4. Summary
Funding
Conflicts of Interest
References
- Ananikov, V.P.; Tanaka, M. (Eds.) Hydrofunctionalization; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom-hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef]
- Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes: Recent developments and trends. Angew. Chem. Int. Ed. 2004, 43, 3368–3398. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Kavthe, R.D.; Shinde, V.S. Transition metal-catalyzed addition of C-, N- and O-nucleophiles to unactivated C-C multiple bonds. Tetrahedron 2012, 68, 8079–8146. [Google Scholar] [CrossRef]
- Zeng, X. Recent advances in catalytic sequential reactions involving heteroelement addition to carbon-carbon multiple bonds. Chem. Rev. 2013, 113, 6864–6900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zi, W. Transition-metal catalyzed enantioselective functionalization of alkynes. Tetrahedron Lett. 2018, 59, 2205–2213. [Google Scholar] [CrossRef]
- Cadierno, V. Metal-catalyzed hydrofunctionalization reactions of haloalkynes. Eur. J. Inorg. Chem. 2020, 886–898. [Google Scholar] [CrossRef]
- Bruneau, C.; Neveux, M.; Kabouche, Z.; Ruppin, C.; Dixneuf, P.H. Ruthenium-catalyzed additions to alkynes: Synthesis of activated esters and their use in acylation reactions. Synlett 1991, 1991, 755–763. [Google Scholar] [CrossRef]
- Gooβen, L.J.; Rodríguez, N.; Gooβen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 2008, 47, 3100–3120. [Google Scholar]
- Hintermann, L. Recent developments in metal-catalyzed additions of oxygen nucleophiles to alkenes and alkynes. Top. Organomet. Chem. 2010, 31, 123–155. [Google Scholar]
- Bruneau, C. Group 8 metals-catalyzed O-H bond addition to unsaturated molecules. Top. Organomet. Chem. 2013, 43, 203–230. [Google Scholar]
- Francos, J.; Cadierno, V. Metal-catalyzed intra- and intermolecular addition of carboxylic acids to alkynes in aqueous media: A review. Catalysts 2017, 7, 328. [Google Scholar] [CrossRef]
- González-Liste, P.J.; Francos, J.; García-Garrido, S.E.; Cadierno, V. The intermolecular hydro-oxycarbonylation of internal alkynes: Current state of the art. Arkivoc 2018, 2, 17–39. [Google Scholar]
- Rao, Y.S. Recent advances in the chemistry of unsaturated lactones. Chem. Rev. 1976, 76, 625–694. [Google Scholar] [CrossRef]
- Libiszewska, K. Lactones as biologically active compounds. Biotechnol. Food Sci. 2011, 75, 45–53. [Google Scholar]
- Janecki, T. (Ed.) Natural Lactones and Lactams: Synthesis, Ocurrence and Biological Activity; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Cadierno, V. Metal-catalyzed synthesis and transformations of β-haloenol esters. Catalysts 2020, 10, 399. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. Gold-catalyzed organic reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Leyva-Pérez, A.; Sabater, M.J. Gold-catalyzed carbon-heteroatom bond-forming reactions. Chem. Rev. 2011, 111, 1657–1712. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Y.; Liu, H. Recent advances in the gold-catalyzed additions to multiple C-C bonds. Beilstein J. Org. Chem. 2011, 7, 897–936. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Toste, F.D. (Eds.) Modern Gold Catalyzed Synthesis; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Toste, F.D.; Michelet, V. (Eds.) Gold Catalysis: An Homogeneous Approach; Imperial College Press: London, UK, 2014. [Google Scholar]
- Praveen, C. Carbophilic activation of π-systems via gold coordination: Towards regioselective access of intermolecular addition products. Coord. Chem. Rev. 2019, 392, 1–34. [Google Scholar] [CrossRef]
- Alyebyev, S.B.; Beletskaya, I.P. Gold as a catalyst. Part I. Nucleophilic addition to the triple bond. Russ. Chem. Rev. 2017, 86, 689–749. [Google Scholar] [CrossRef]
- Alyebyev, S.B.; Beletskaya, I.P. Gold as a catalyst. Part II. Alkynes in the reactions of carbon-carbon bond formation. Russ. Chem. Rev. 2018, 87, 984–1047. [Google Scholar] [CrossRef]
- Gorin, D.J.; Sherry, B.D.; Toste, F.D. Ligand effects in homogeneous Au catalysis. Chem. Rev. 2008, 108, 3351–3378. [Google Scholar] [CrossRef] [PubMed]
- Krauser, N.; Winter, C. Gold-catalyzed nucleophilic cyclization of functionalized allenes: A powerful access to carbo and heterocycles. Chem. Rev. 2011, 111, 1994–2009. [Google Scholar] [CrossRef]
- Yang, W.; Hashmi, A.S.K. Mechanistic insights into gold chemistry of allenes. Chem. Soc. Rev. 2014, 43, 2941–2955. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. New and selective transition metal catalyzed reactions of allenes. Angew. Chem. Int. Ed. 2000, 39, 3590–3593. [Google Scholar] [CrossRef]
- Genin, E.; Toullec, P.Y.; Antoniotti, S.; Brancour, C.; Genêt, J.-P.; Michelet, V. Room temperature Au(I)-catalyzed exo-selective cyclosimerization of acetylenic acids: An entry to functionalized γ-lactones. J. Am. Chem. Soc. 2006, 128, 3112–3113. [Google Scholar] [CrossRef]
- Genin, E.; Toullec, P.Y.; Marie, P.; Antoniotti, S.; Brancour, C.; Genêt, J.-P.; Michelet, V. Gold catalysis in organic synthesis: Efficient intramolecular cyclization of γ-acetylenic carboxylic acids to 5-exo-alkylidene-butyrolactones. Arkivoc 2007, 67–78. [Google Scholar] [CrossRef]
- Harkat, H.; Weibel, J.-M.; Pale, P. A mild access to γ- or δ-alkylidene lactones through gold catalysis. Tetrahedron Lett. 2006, 47, 6273–6276. [Google Scholar] [CrossRef]
- Harkat, H.; Dembelé, A.Y.; Weibel, J.-M.; Blanc, A.; Pale, P. Cyclization of alkynoic acids with gold catalysts: A surprising dichotomy between AuI and AuIII. Tetrahedron 2009, 65, 1871–1879. [Google Scholar] [CrossRef]
- Marchal, E.; Uriac, P.; Legouin, B.; Toupet, L.; van de Weghe, P. Cycloisomerization of γ- and δ-acetylenic acids catalyzed by gold(I) chloride. Tetrahedron 2007, 63, 9979–9990. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, X.; De Brabender, J.K. Studies toward the unique pederin family member psymberin: Full structure elucidation, two alternative total syntheses, and analogs. J. Am. Chem. Soc. 2012, 134, 17083–17093. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.O.; Reboredo, F.J.; Estévez, J.C.; Tapia, R.A.; Estévez, R.J. Gold-facilitated “6-exo-dig” intramolecular cyclization of 2-[(2-nitrophenyl)ethynyl]phenylacetic acids: General access to 5H-benzo[b]carbazole-6,11-diones. Synlett 2009, 2009, 3107–3110. [Google Scholar] [CrossRef]
- Medran, N.S.; Villalba, M.; Mata, E.G.; Testero, S.A. Gold-catalyzed cycloisomerization of alkyne-containing amino acids: Controlled tuning of C-N vs. C-O reactivity. Eur. J. Org. Chem. 2016, 2016, 3757–3764. [Google Scholar] [CrossRef]
- Praveen, C.; Ayyanar, A.; Perumal, P.T. Gold(III) chloride catalyzed regioselective synthesis of pyrano[3,4-b]indol-1(9H)-ones and evaluation of anticancer potential towards human cervix adenocarcinoma. Bioorg. Med. Chem. Lett. 2011, 21, 4170–4173. [Google Scholar] [CrossRef] [PubMed]
- Praveen, C.; Ananth, D.B. Design, synthesis and cytotoxicity of pyrano [4,3-b]indol-1(5H)-ones: A hybrid pharmacophore approach via gold catalyzed cyclization. Bioorg. Med. Chem. Lett. 2016, 26, 2507–2512. [Google Scholar] [CrossRef]
- Taskaya, S.; Menges, N.; Balci, M. Gold-catalyzed formation of pyrrolo- and indolo-oxacin-1-one derivatives: The key structure of some marine natural products. Beilstein J. Org. Chem. 2015, 11, 897–905. [Google Scholar] [CrossRef]
- Wang, W.; Kumar, M.; Hammond, G.B.; Xu, B. Enhanced reactivity in homogeneous gold catalysis through hydrogen bonding. Org. Lett. 2014, 16, 636–639. [Google Scholar] [CrossRef]
- Kumar, M.; Hammond, G.B.; Xu, B. Cationic gold catalyst poisoning and reactivation. Org. Lett. 2014, 16, 3452–3455. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Mashuta, M.S.; Hammond, G.B.; Xu, B. A highly efficient and broadly applicable cationic gold catalyst. Angew. Chem. Int. Ed. 2014, 53, 4456–4459. [Google Scholar] [CrossRef] [PubMed]
- Leenders, S.H.A.M.; Dürr, M.; Ivanović-Burmazović, I.; Reek, J.N.H. Gold functionalized platinum M12L24-nanospheres and their application in cyclization reactions. Adv. Synth. Catal. 2016, 345, 1509–1518. [Google Scholar] [CrossRef]
- Ho, T.D.; Schramm, M.P. Au-cavitand catalyzed alkyne-acid cyclizations. Eur. J. Org. Chem. 2019, 2019, 5678–5684. [Google Scholar] [CrossRef]
- Nolla-Saltiel, R.; Robles-Marín, E.; Porcel, S. Silver(I) and gold(I)-promoted synthesis of alkylidene lactones and 2H-chromenes from salicylic and anthranilic acid derivatives. Tetrahedron Lett. 2014, 55, 4484–4488. [Google Scholar] [CrossRef]
- Carrillo-Arcos, U.A.; Porcel, S. Gold promoted arylative cyclization of alkynoic acids with arenediazonium salts. Org. Biomol. Chem. 2018, 16, 1837–1842. [Google Scholar] [CrossRef]
- Sperger, C.A.; Friksdahl, A. Gold-catalyzed tandem cyclizations of 1,6-diynes triggered by internal N- and O-nucleophiles. J. Org. Chem. 2010, 75, 4542–4553. [Google Scholar] [CrossRef]
- Gold(I)-catalysed cyclisation of alkynoic acids: Towards an efficient and eco-friendly synthesis of γ-, δ- and ε-lactones. Adv. Synth. Catal. 2016, 358, 3857–3862. [CrossRef]
- Fernández-García, J.M.; García-García, P.; Fernández-Rodríguez, M.A.; Pérez-Anes, A.; Aguilar, E. Regioselective synthesis of oxepinones and azepinones by gold-catalyzed cycloisomerization of functionalized cyclopropyl alkynes. Chem. Commun. 2013, 49, 11185–11187. [Google Scholar] [CrossRef] [PubMed]
- Garre, M.S.; Sucunza, D.; Aguilar, E.; García-García, P.; Vaquero, J.J. Regiodivergent electrophilic cyclizations of alkynylcyclobutanes for the synthesis of cyclobutane-fused O-heterocycles. J. Org. Chem. 2019, 84, 5712–5725. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Toullec, P.Y.; Díez, J.; Conejero, S.; Michelet, V.; Cadierno, V. Cycloisomerization versus hydration reactions in aqueous media: A Au(III)-NHC catalyst that makes the difference. Org. Lett. 2012, 14, 2520–2523. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Toullec, P.Y.; Borge, J.; Conejero, S.; Michelet, V.; Cadierno, V. Water-soluble gold(I) and gold(III) complexes with sulfonated N-heterocyclic carbene ligands: Synthesis, characterization, and application in the catalytic cycloisomerization of γ-alkynoic acids into enol-lactones. ACS Catal. 2013, 3, 3086–3098. [Google Scholar] [CrossRef]
- Ferré, M.; Cattoën, X.; Man, M.W.C.; Pleixats, R. Sol-gel immobilized N-heterocyclic carbene gold complex as a recyclable catalyst for the rearrangement of allylic esters and the cycloisomerization of γ-alkynoic acids. ChemCatChem 2016, 8, 2824–2831. [Google Scholar] [CrossRef]
- Belger, K.; Krause, N. Smaller, faster, better: Modular synthesis of unsymmetrical ammonium salt-tagged NHC-gold(I) complexes and their application as recyclable catalysts in water. Org. Biomol. Chem. 2015, 13, 8556–8560. [Google Scholar] [CrossRef] [PubMed]
- Sak, H.; Mawick, M.; Krause, N. Sustainable gold catalysis in water using cyclodextrin-tagged NHC-gold complexes. ChemCatChem 2019, 11, 5821–5829. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, M.J.; Vidal, C.; Díez, J.; García-Álvarez, J. Introducing deep eutectic solvents as biorenewable media for Au(I)-catalyzed cycloisomerisation of γ-alkynoic acids: An unprecedented catalytic system. Chem. Commun. 2014, 50, 12927–12929. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, W.J.; Bell, N.A.W.; Qing, Y.; Bayley, H. Single-molecule observation of the intermediates in a catalytic cycle. J. Am. Chem. Soc. 2018, 140, 17538–17546. [Google Scholar] [CrossRef]
- Fernández, G.; Bernardo, L.; Villanueva, A.; Pleixats, R. Gold nanoparticles stabilized by PEG-tagged imidazolium salts as recyclable catalysts for the synthesis of propargylamines and the cycloisomerization of γ-alkynoic acids. New J. Chem. 2020, 44, 6130–6141. [Google Scholar] [CrossRef]
- Neaţu, F.; Li, Z.; Richards, R.; Toullec, P.Y.; Genêt, J.-P.; Dumbuya, K.; Gottfried, J.M.; Steinrück, H.-P.; Pârvulescu, V.I.; Michelet, V. Heterogeneous gold catalysts for efficient access to functionalized lactones. Chem. Eur. J. 2008, 14, 9412–9418. [Google Scholar] [CrossRef] [PubMed]
- Neaţu, F.; Toullec, P.Y.; Michelet, V.; Pârvulescu, V.I. Heterogeneous Au and Rh catalysts for cycloisomerization reactions of γ-acetylenic acids. Pure Appl. Chem. 2009, 81, 2387–2396. [Google Scholar] [CrossRef]
- Neaţu, F.; Pârvulescu, V.I.; Michelet, V.; Gênet, J.-P.; Goguet, A.; Hardacre, C. Gold imidazolium-based ionic liquids, efficient catalysts for cycloisomerization of γ-acetylenic carboxylic acids. New J. Chem. 2009, 33, 102–106. [Google Scholar] [CrossRef]
- Verho, O.; Gao, F.; Johnston, E.V.; Wan, W.; Nagendiran, A.; Zheng, H.; Bäckvall, J.-E.; Zou, X. Mesoporous silica nanoparticles applied as a support for Pd and Au nanocatalysts in cycloisomerization reactions. APL Mater. 2014, 2, 113316. [Google Scholar] [CrossRef]
- Eriksson, K.; Verho, O.; Nyholm, L.; Oscarsson, S.; Bäckvall, J.-E. Disperse gold nanoparticles supported in the pores of siliceous mesocellular foam: A catalyst for cyclosiomerization of alkynoic acids to γ-alkylidene lactones. Eur. J. Org. Chem. 2015, 2015, 2250–2255. [Google Scholar] [CrossRef]
- Lee, L.-C.; Zhao, Y. Metalloenzyme-mimicking supramolecular catalyst for highly active and selective intramolecular alkyne carboxylation. J. Am. Chem. Soc. 2014, 136, 5579–5582. [Google Scholar] [CrossRef] [PubMed]
- Toullec, P.Y.; Genin, E.; Antoniotti, S.; Genêt, J.-P.; Michelet, V. Au2O3 as a stable and efficient catalyst for the selective cycloisomerization of γ-acetylenic carboxylic acids to γ-alkylidene-γ-butyrolactones. Synlett 2008, 2008, 707–711. [Google Scholar]
- Shu, X.-Z.; Nguyen, S.C.; He, Y.; Oba, F.; Zhang, Q.; Canlas, C.; Somorjai, G.A.; Alivisatos, A.P.; Toste, F.D. Silica-supported cationic gold(I) complexes as heterogeneous catalysts for regio- and enantioselective lactonization reactions. J. Am. Chem. Soc. 2015, 137, 7083–7086. [Google Scholar] [CrossRef]
- Yang, T.; Campbell, L.; Dixon, D.J. A Au(I)-catalyzed N-acyl iminium ion cyclization cascade. J. Am. Chem. Soc. 2007, 129, 12070–12071. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhao, F.; Chen, X.; Zhang, L.; Jiang, H.; Liu, H. Gold-catalyzed tandem reaction in water: An efficient and convenient synthesis of fused polycyclic indoles. Green Chem. 2012, 14, 1888–1895. [Google Scholar] [CrossRef]
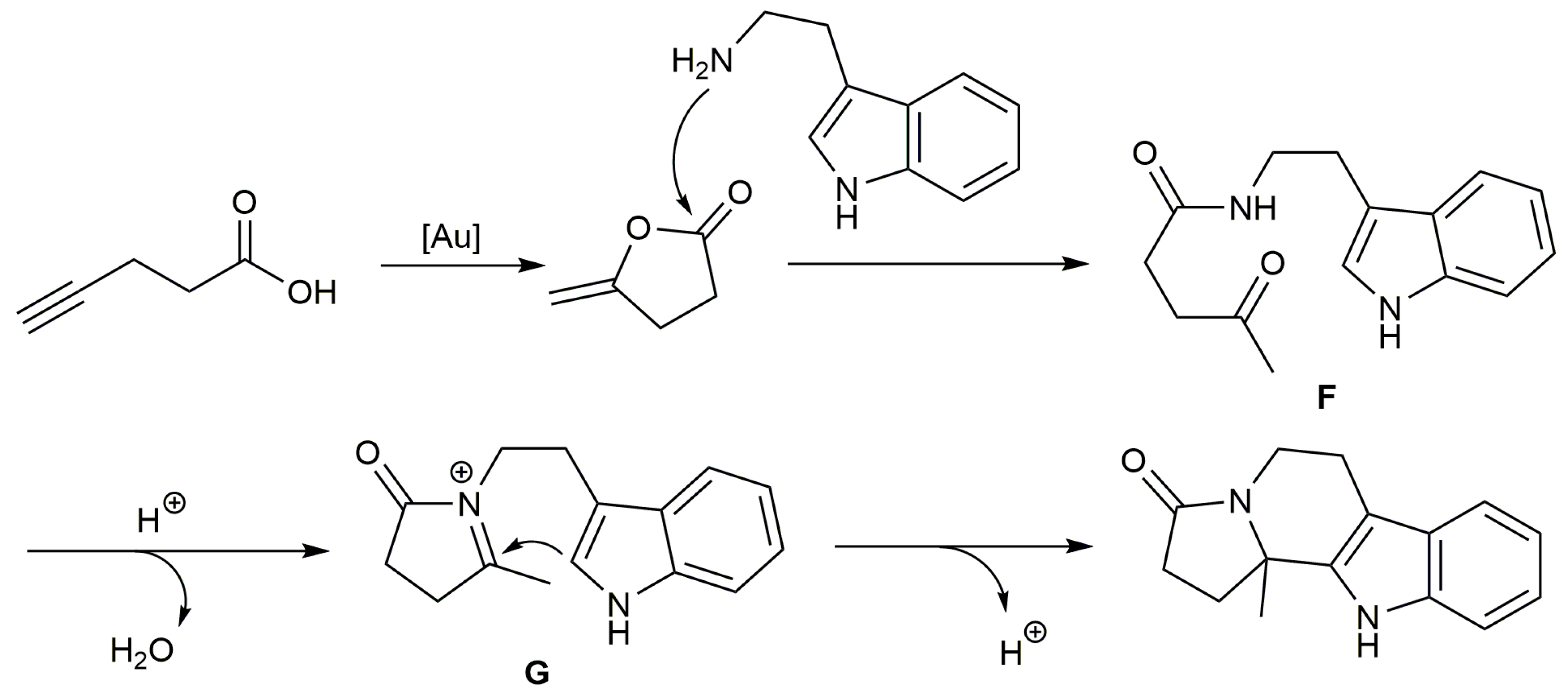
- Li, Z.; Li, J.; Yang, N.; Chen, Y.; Zhou, Y.; Ji, X.; Zhang, L.; Wang, J.; Xie, X.; Liu, H. Gold(I)-catalyzed cascade approach for the synthesis of tryptamine-based polycyclic scaffolds as α1-adrenergic receptor antagonists. J. Org. Chem. 2013, 78, 10802–10811. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, X.; Li, P.; Liu, X.; Zhao, J.; Zhou, Y.; Wang, J.; Liu, H.; Zhao, F. Gold-catalyzed rapid construction of nitrogen-containing heterocyclic compound library with scaffold diversity and molecular complexity. Adv. Synth. Catal. 2019, 361, 1419–1440. [Google Scholar] [CrossRef]
- Jia, X.; Li, P.; Liu, X.; Lin, J.; Chu, Y.; Yu, J.; Wang, J.; Liu, H.; Zhao, F. Green and facile assembly of diverse fused N-heterocycles using gold-catalyzed cascade reactions in water. Molecules 2019, 24, 988. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhai, Y.; Ji, X.; Liu, G.; Feng, E.; Ye, D.; Zhao, L.; Jiang, H.; Liu, H. Gold(I)-catalyzed one-pot tandem coupling/cyclization: An efficient synthesis of pyrrolo-/pyrido[2,1-b]benzo[d][1,3]oxazin-1-ones. Adv. Synth. Catal. 2010, 352, 373–378. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-catalyzed tandem transformation: A simple approach for the synthesis of pyrrolo/pyrido[2,1-a][1,3]benzoxazinones and pyrrolo/pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, Y.; Wang, J.; Zhao, L.; Jiang, H.; Liu, H. Au(I)/Ag(I)-catalyzed cascade approach for the synthesis of benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinones. J. Org. Chem. 2013, 78, 4312–4318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Ji, X.; Zhou, W.; Zhang, X.; Qian, W.; Jiang, H.; Liu, H. Silver- and gold-mediated domino transformation: A strategy for synthesizing benzo[e]indolo[1,2-a]pyrrolo/pyrido[2,1-c][1,4]-diazepine-3,9-diones. J. Org. Chem. 2011, 76, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ji, X.; Liu, G.; Zhang, D.; Zhao, L.; Jiang, H.; Liu, H. Gold(I)-catalyzed cascade for synthesis of pyrrolo[1,2-a:2´,1´-c]-/pyrido[2,1-c]pyrrolo[1,2-a]quinoxalinones. Adv. Synth. Catal. 2010, 352, 1711–1717. [Google Scholar] [CrossRef]
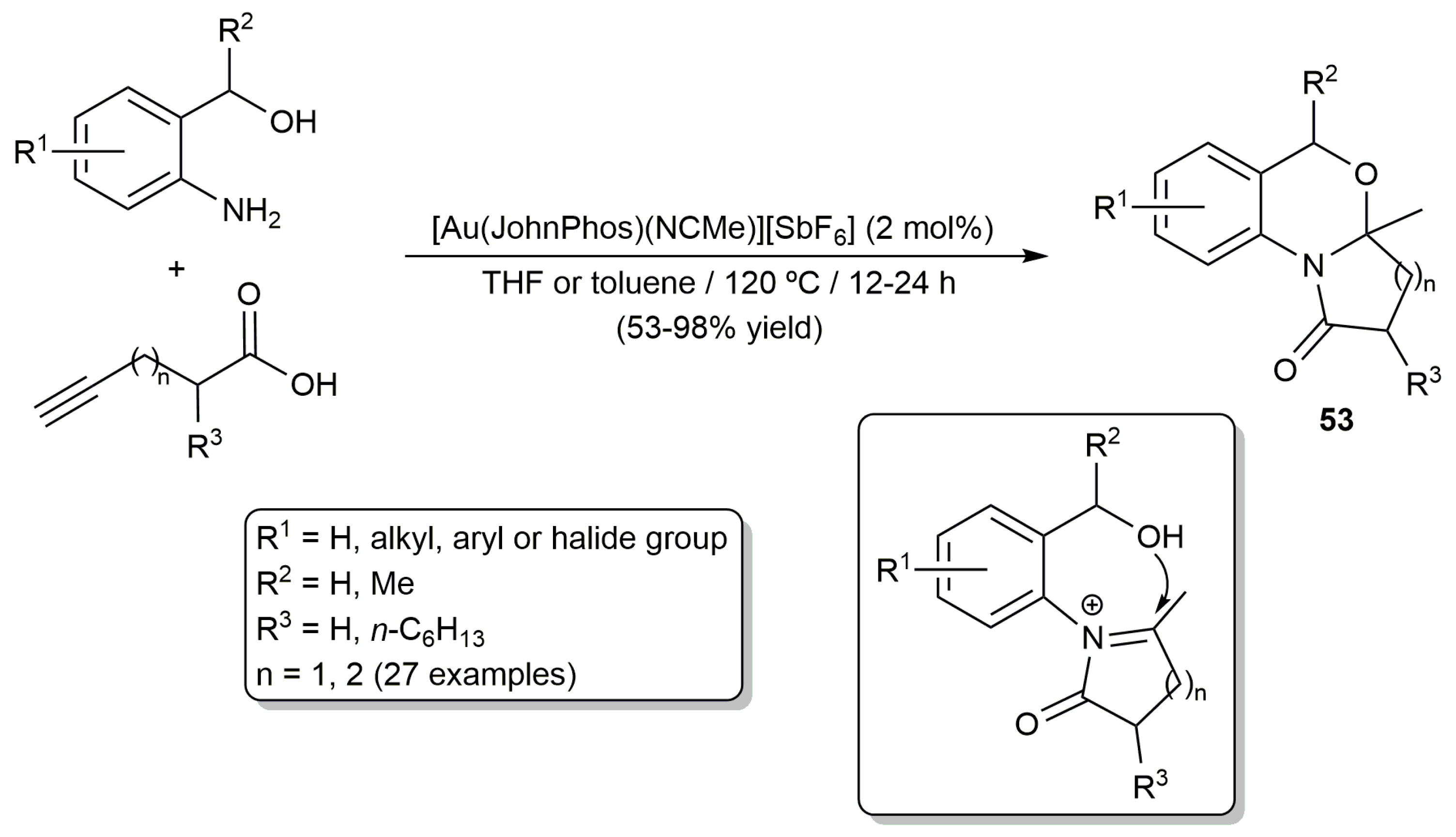
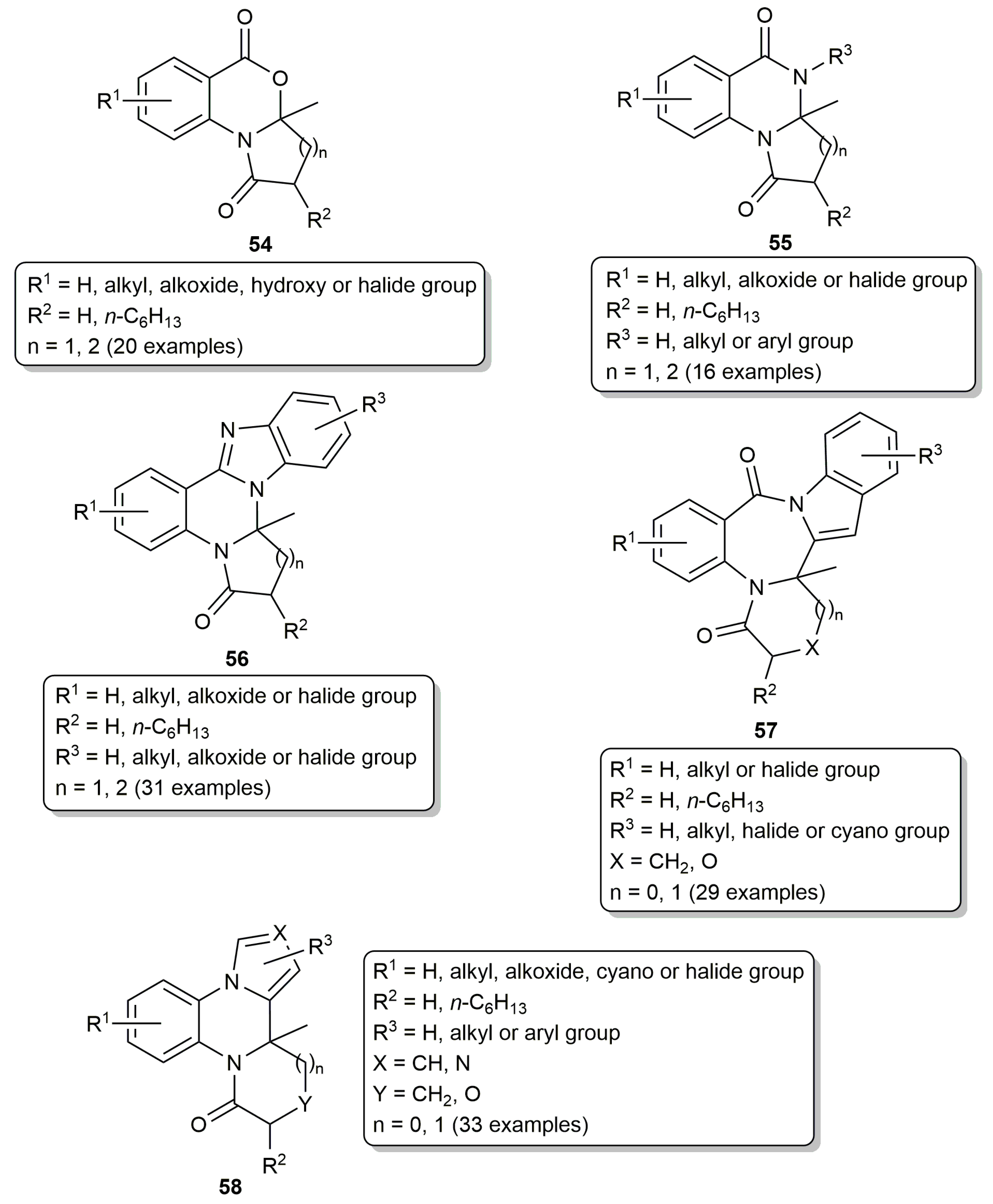
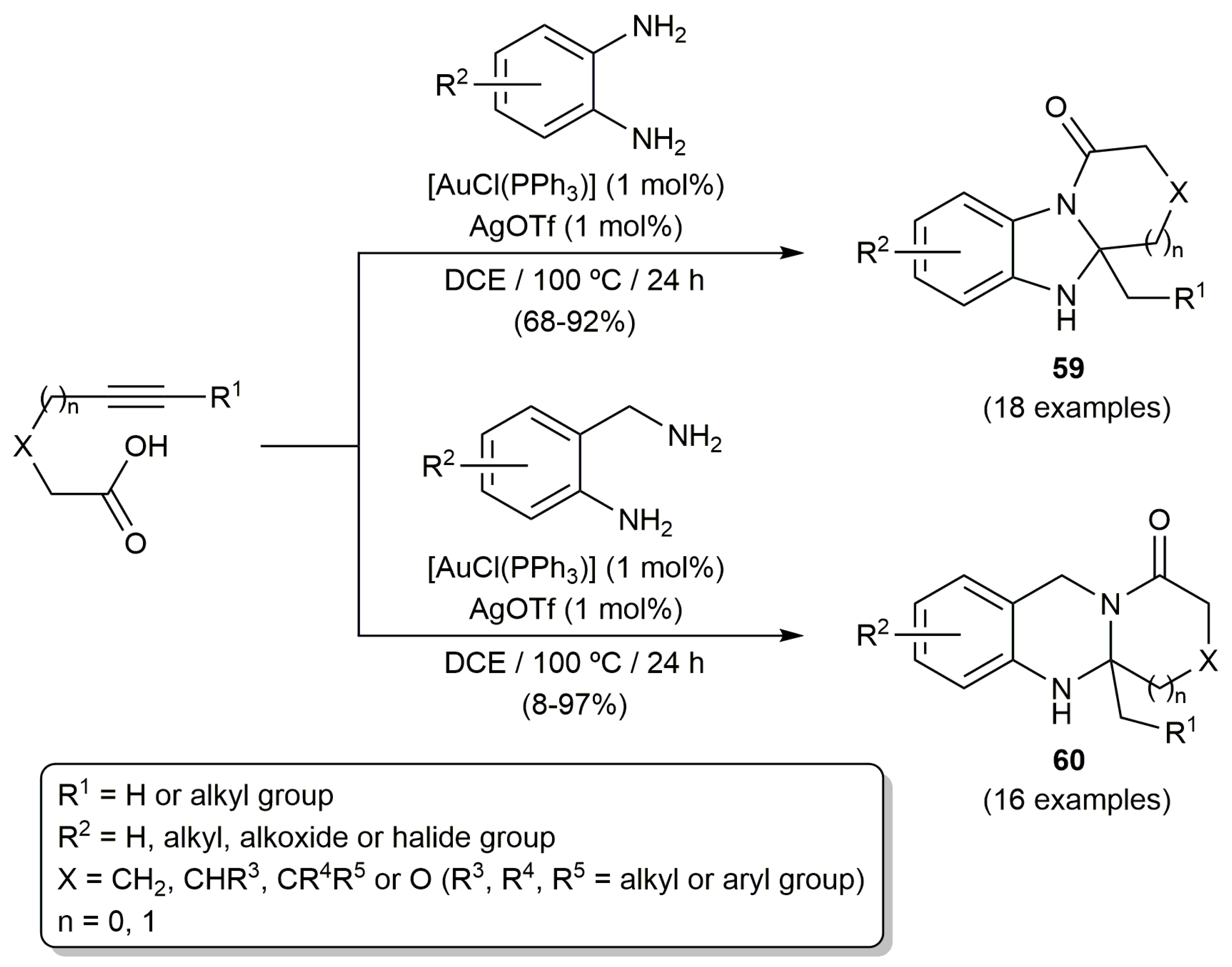
- Patil, N.T.; Mutyala, A.K.; Lakshmi, P.G.V.V.; Gajula, B.; Sridhar, B.; Pottireddygari, G.R.; Rao, T.P. Au(I)-catalyzed cascade reaction involving formal doble hydroamination of alkynes bearing tethered carboxylic groups: An easy access to fused dihydrobenzimidazoles and tetrahydroquinazolines. J. Org. Chem. 2010, 75, 5963–5975. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Lakshmi, P.G.V.V.; Sridhar, B.; Patra, S.; Bhadra, M.P.; Patra, C.R. New linearly and angularly fused quinazolinones: Synthesis through gold(I)-catalyzed cascade reactions and anticancer activities. Eur. J. Org. Chem. 2012, 2012, 1790–1799. [Google Scholar] [CrossRef]
- Patil, N.T.; Shinde, V.S.; Sridhar, B. Relay catalytic branching cascade: A technique to access diverse molecular scaffolds. Angew. Chem. Int. Ed. 2013, 52, 2251–2255. [Google Scholar] [CrossRef]
- García-García, P.; Fernández-Rodríguez, M.A.; Aguilar, E. Gold-catalyzed cycloaromatization of 2,4-dien-6-yne carboxylic acids: Synthesis of 2,3-disubstituted phenols and unsymmetrical bi- and terphenyls. Angew. Chem. Int. Ed. 2009, 48, 5534–5537. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Zhang, Z.; Kong, F.; Wang, S.; Wang, H.; Yao, Z.-J. Assembly of fused indenes via Au(I)-catalyzed C1-C5 cyclization of enediynes bearing an internal nucleophile. Chem. Commun. 2013, 49, 695–697. [Google Scholar]
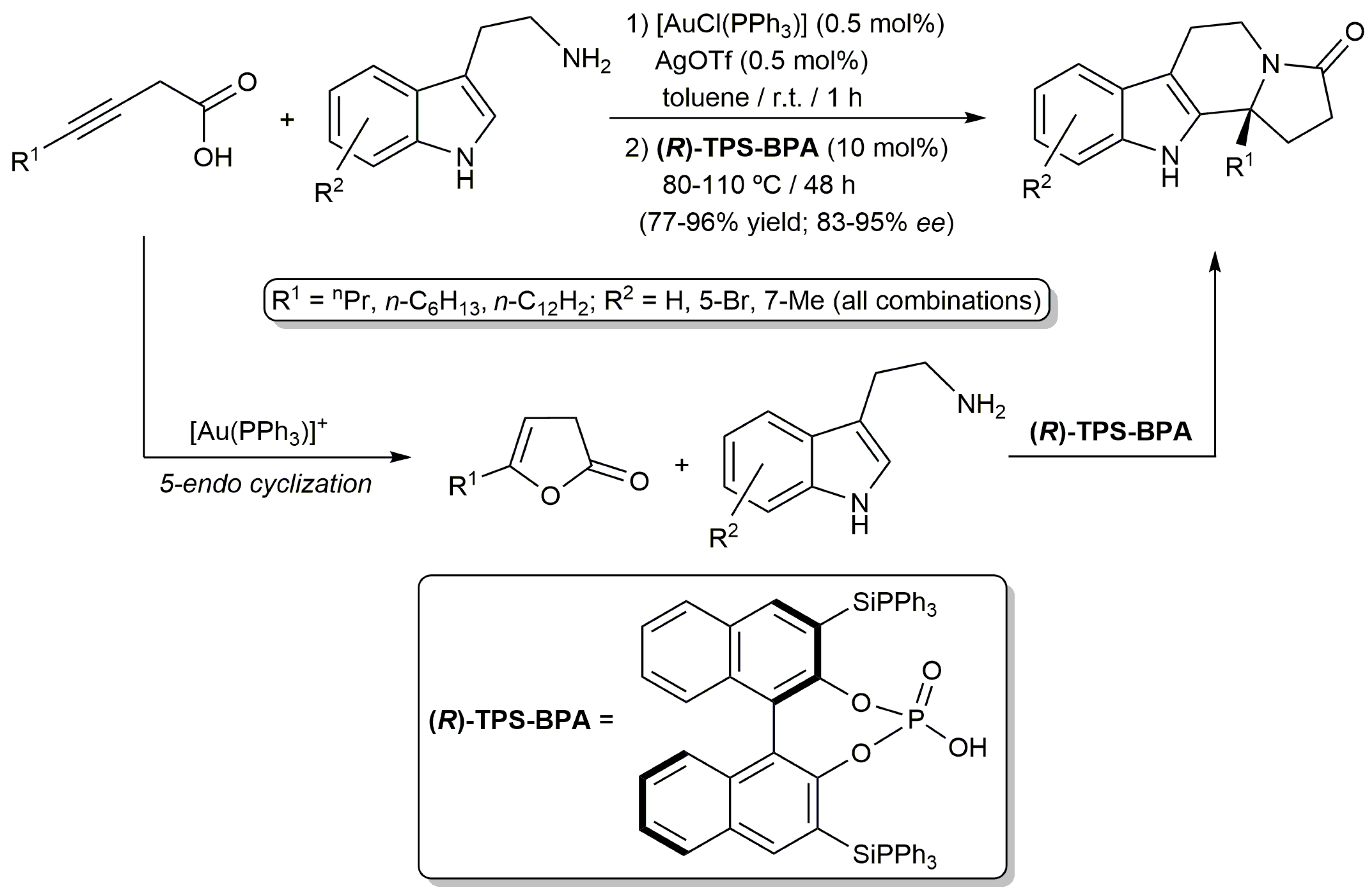
- Muratore, M.E.; Holloway, C.A.; Pilling, A.W.; Storer, R.I.; Trevitt, G.; Dixon, D.J. Enantioselective Brønsted acid-catalyzed N-acyliminium cyclization cascades. J. Am. Chem. Soc. 2009, 131, 10796–10797. [Google Scholar] [CrossRef]
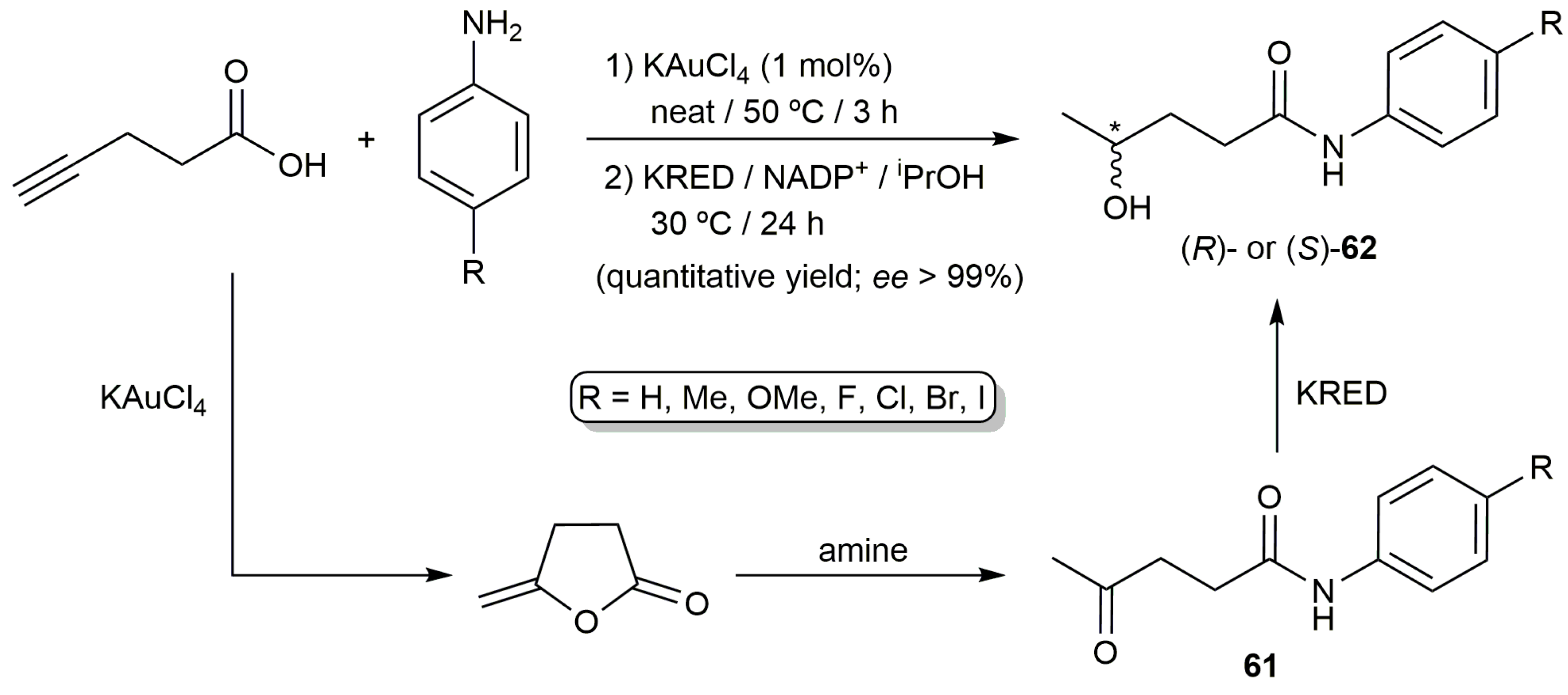
- Rodríguez-Álvarez, M.J.; Ríos-Lombardía, N.; Schumacher, S.; Pérez-Iglesias, D.; Morís, F.; Cadierno, V.; García-Álvarez, J.; González-Sabín, J. Combination of metal-catalyzed cycloisomerizations and biocatalysis in aqueous media: Asymmetric construction of chiral alcohols, lactones, and γ-hydroxy-carbonyl compounds. ACS Catal. 2017, 7, 7753–7759. [Google Scholar] [CrossRef]
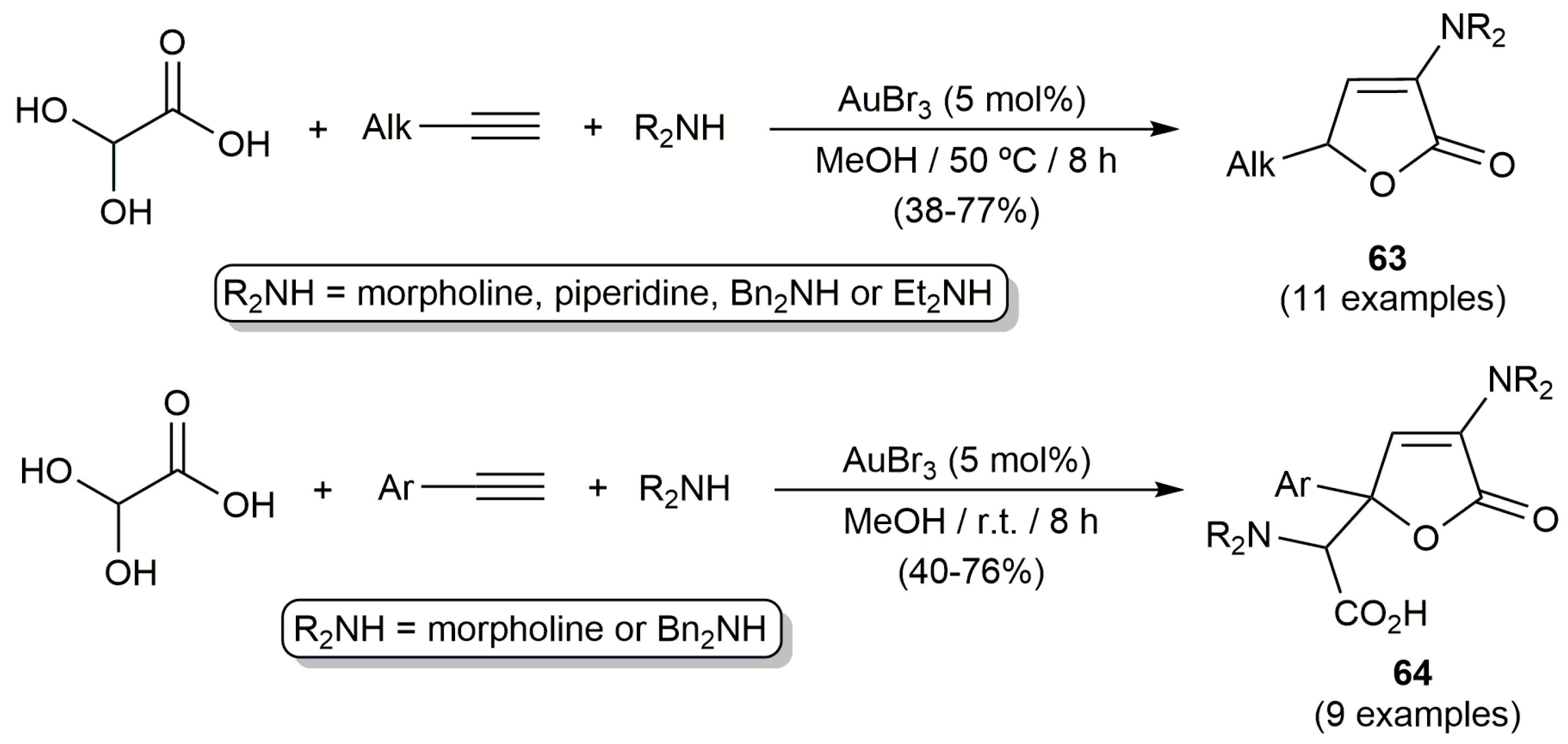
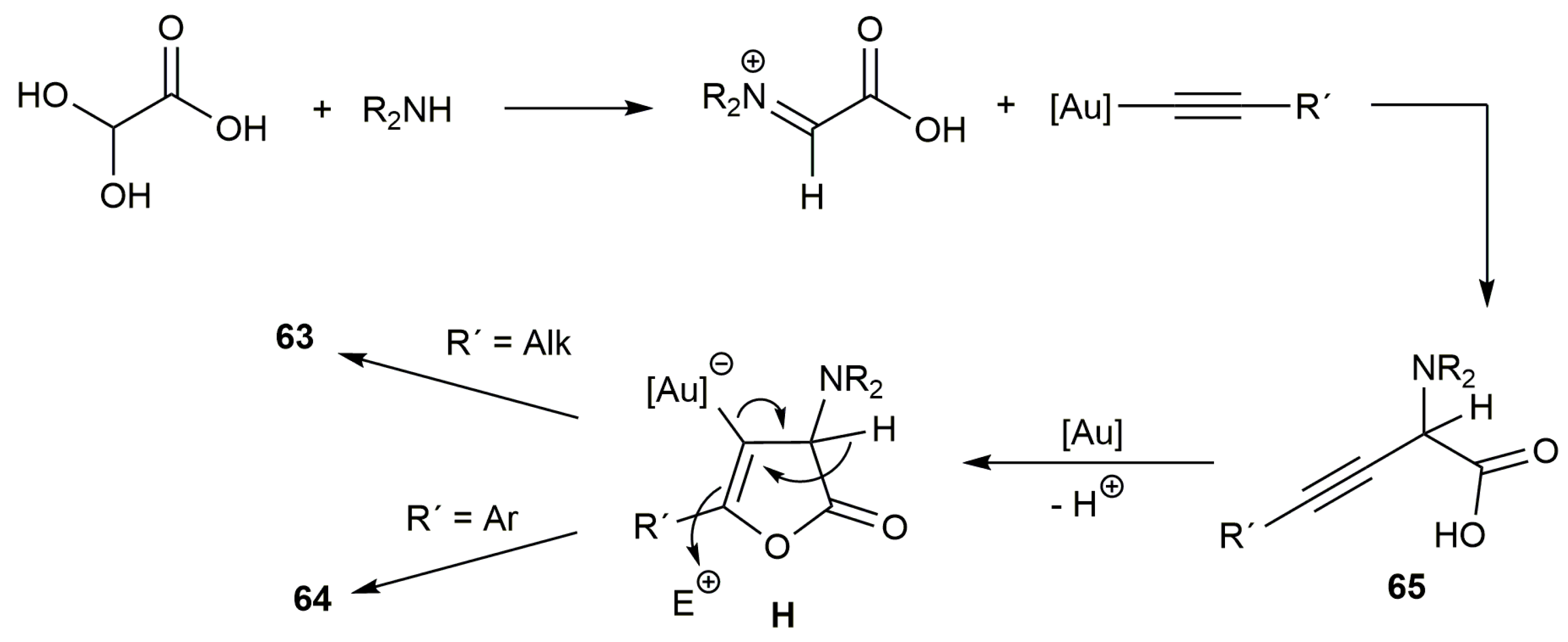
- Zhang, Q.; Cheng, M.; Hu, X.; Li, B.-G.; Ji, J.-X. Gold-catalyzed three-component tandem process: An efficient and facile assembly of complex butenolides from alkynes, amines and glyoxylic acid. J. Am. Chem. Soc. 2010, 132, 7256–7257. [Google Scholar] [CrossRef] [PubMed]
- Roembke, P.; Schmidbauer, H.; Cronje, S.; Raubenheimer, H. Application of (phosphine)gold(I) carboxylates, sulfonates and related compounds as highly efficient catalysts for the hydration of alkynes. J. Mol. Catal. A: Chem. 2004, 212, 35–42. [Google Scholar] [CrossRef]
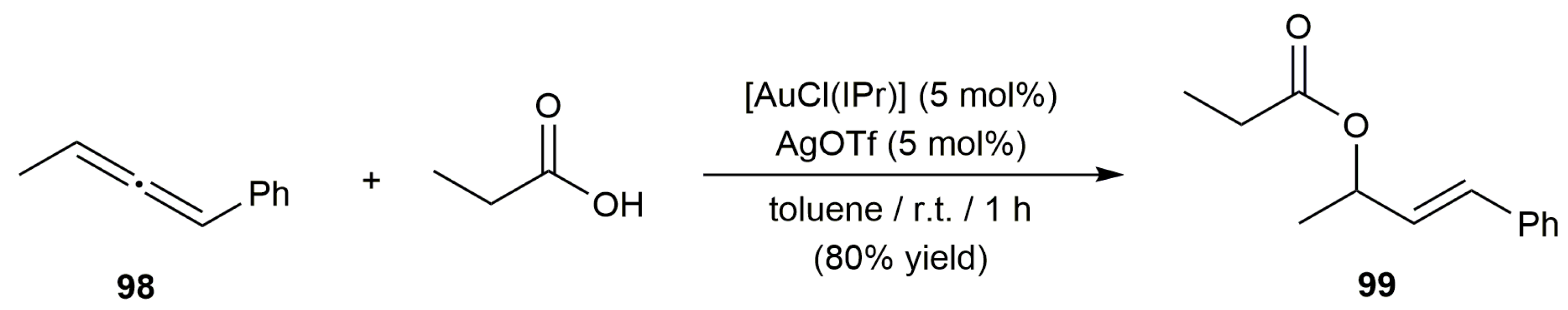
- Chary, B.C.; Kim, S. Gold(I)-catalyzed addition of carboxylic acids to alkynes. J. Org. Chem. 2010, 75, 7928–7931. [Google Scholar] [CrossRef]
- Luo, T.; Dai, M.; Zheng, S.-L.; Schreiber, S.L. Synthesis of α-pyrones using gold-catalyzed coupling reactions. Org. Lett. 2011, 13, 2834–2836. [Google Scholar] [CrossRef] [PubMed]
- González-Liste, P.J.; León, F.; Arribas, I.; Rubio, M.; García-Garrido, S.E.; Cadierno, V.; Pizzano, A. Highly stereoselective synthesis and hydrogenation of (Z)-1-alkyl-2-arylvinyl acetate: A wide scope procedure for the preparation of chiral homobenzylic esters. ACS Catal. 2016, 6, 3056–3060. [Google Scholar] [CrossRef]
- González-Liste, P.J.; Francos, J.; García-Garrido, S.E.; Cadierno, V. Gold-catalyzed regio- and stereoselective addition of carboxylic acids to iodoalkynes: Access to (Z)-β-iodoenol esters and 1,4-disubstituted (Z)-enynyl esters. J. Org. Chem. 2017, 82, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- León, F.; González-Liste, P.J.; García-Garrido, S.E.; Arribas, I.; Rubio, M.; Cadierno, V.; Pizzano, A. Broad scope synthesis of esters precursors of nonfunctionalized chiral alcohols based on the asymmetric hydrogenation of α,β-dialkyl-, α,β-diaryl-, and α-alkyl-β-aryl-vinyl esters. J. Org. Chem. 2017, 82, 5852–5867. [Google Scholar] [CrossRef]
- León, F.; Francos, J.; López-Serrano, J.; García-Garrido, S.E.; Cadierno, V.; Pizzano, A. Double asymmetric hydrogenation of conjugated dienes: A self-breeding chirality route to C2 symmetric 1,4-diols. Chem. Commun. 2019, 55, 786–789. [Google Scholar] [CrossRef]
- Francos, J.; Cadierno, V. Nickel-catalyzed homocoupling of (Z)-β-iodoenol esters: Stereoselective access to (Z,Z)-buta-1,3-diene-1,4-diyl diesters. Synthesis 2019, 51, 3117–3126. [Google Scholar] [CrossRef]
- Muthusamy, G.; Pansare, S.V. Stereoselective synthesis of E-3-(arylmethylidene)-5-(alkyl/aryl)-2(3H)-furanones by sequential hydroacyloxylation-Mizoroki-Heck reactions of iodoalkynes. Org. Biomol. Chem. 2018, 16, 7971–7983. [Google Scholar] [CrossRef]
- Francos, J.; Díaz-Álvarez, A.E.; Cadierno, V. Catalytic addition of indole-2-carboxylic acid to 1-hexyne. Molbank 2020, 2020, M1145. [Google Scholar] [CrossRef]
- González-Liste, P.J.; García-Garrido, S.E.; Cadierno, V. Gold(I)-catalyzed addition of carboxylic acids t internal alkynes in aqueous medium. Org. Biomol. Chem. 2017, 15, 1670–1679. [Google Scholar] [CrossRef]
- Francos, J.; Moreno-Narváez, M.E.; Cadierno, V.; Sierra, D.; Ariz, K.; Gómez, J. Gold(I) complexes with ferrocenylphosphino sulfonate ligands: Synthesis and application in the catalytic addition of carboxylic acids to internal alkynes in water. Catalysts 2019, 9, 955. [Google Scholar] [CrossRef]
- Dupuy, S.; Gasperini, D.; Nolan, S.P. Highly efficient gold(I)-catalyzed regio and stereoselective hydrocarboxylation of internal alkynes. ACS Catal. 2015, 5, 6918–6921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Li, Y.; Wu, G.; Cao, Z.; Zhang, L. A general ligand design for gold catalysis allowing ligand-directed anti-nucleophilic attack of alkynes. Nat. Commun. 2014, 5, 3470. [Google Scholar] [CrossRef]
- Hamilton, G.L.; Kang, E.J.; Mba, M.; Toste, F.D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 2007, 317, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.H.; Veguillas, M.; Toste, F.D. Gold(I)-catalyzed enantioselective bromocyclization of allenes. Chem. Sci. 2013, 4, 3427–3431. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Loos, A.; Littmann, A.; Braun, I.; Knight, J.; Doherty, S.; Rominger, F. Gold(I) complexes of KITPHOS monophosphines: Efficient cycloisomerisation catalysts. Adv. Synth. Catal. 2009, 351, 576–582. [Google Scholar] [CrossRef]
- Cauteruccio, S.; Loos, A.; Bossi, A.; Jaimes, M.C.B.; Dova, D.; Rominger, F.; Prager, S.; Dreuw, A.; Licandro, E.; Hashmi, A.S.K. Gold(I) complexes of tetrathiaheterohelicene phosphanes. Inorg. Chem. 2013, 52, 7995–8004. [Google Scholar] [CrossRef]
- Handa, S.; Lippincott, D.J.; Aue, D.H.; Lipshutz, B.H. Asymmetric gold-catalyzed lactonizations in water at room temperature. Angew. Chem. Int. Ed. 2014, 53, 10658–10662. [Google Scholar] [CrossRef]
- Ye, R.; Zhukhovitskiy, A.V.; Kazantsev, R.V.; Fakra, S.C.; Wickemeyer, B.B.; Toste, F.D.; Somorjai, G.A. Supported Au nanoparticles with N-heterocyclic carbene ligands as active and heterogeneous catalysts for lactonization. J. Am. Chem. Soc. 2018, 140, 4144–4149. [Google Scholar] [CrossRef] [PubMed]
- Klumphu, P.; Desfeux, C.; Zhang, Y.; Handa, S.; Gallou, F.; Lipshutz, B.H. Micellar catalysis-enabled sustainable ppm Au-catalyzed reactions at room temperature. Chem. Sci. 2017, 8, 6354–6358. [Google Scholar] [CrossRef]
- Okada, T.; Sakaguchi, K.; Shinada, T.; Ohfune, Y. Au-catalyzed cyclization of allenylsilanes. Regioselective conversion to 2-amino-4-silylmethylene γ-butyrolactone. Tetrahedron Lett. 2011, 52, 5740–5743. [Google Scholar] [CrossRef]
- Okada, T.; Sakaguchi, K.; Shinada, T.; Ohfume, Y. Total synthesis of (-)-funebrine via Au-catalyzed regio- and stereoselective γ-butyrolactonization of allenylsilane. Tetrahedron Lett. 2011, 52, 5744–5746. [Google Scholar] [CrossRef]
- Zhou, J.; Fu, C.; Ma, S. Gold-catalyzed stereoselective cycloisomerization of allenoic acids for two types of common natural γ-butyrolactones. Nat. Commun. 2018, 9, 1654. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhou, J.; Fu, C.; Ma, S. Catalytic enantioselective construction of axial chirality in 1,3-disubstituted allenes. Nat. Commun. 2019, 10, 507. [Google Scholar] [CrossRef] [PubMed]
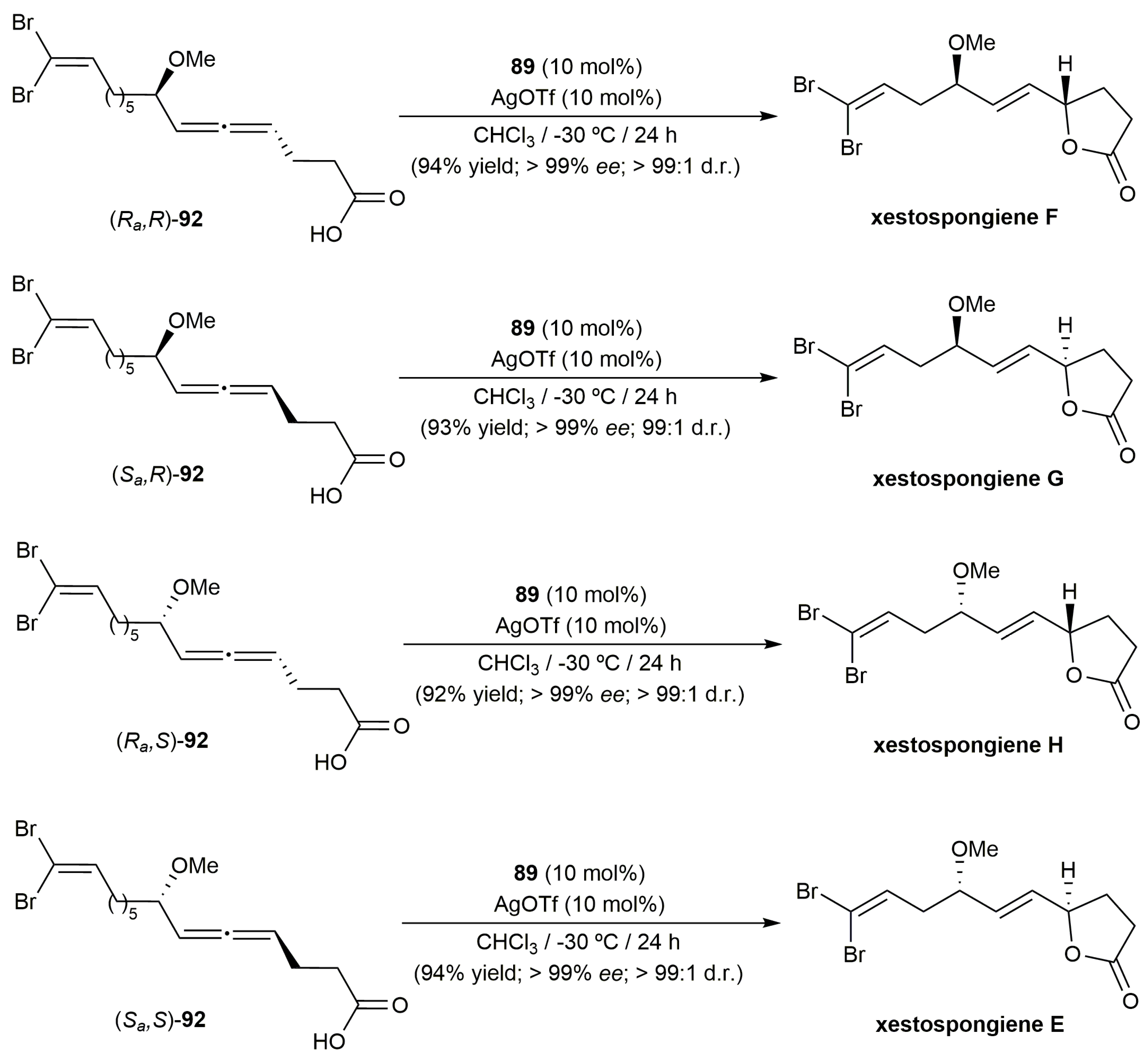
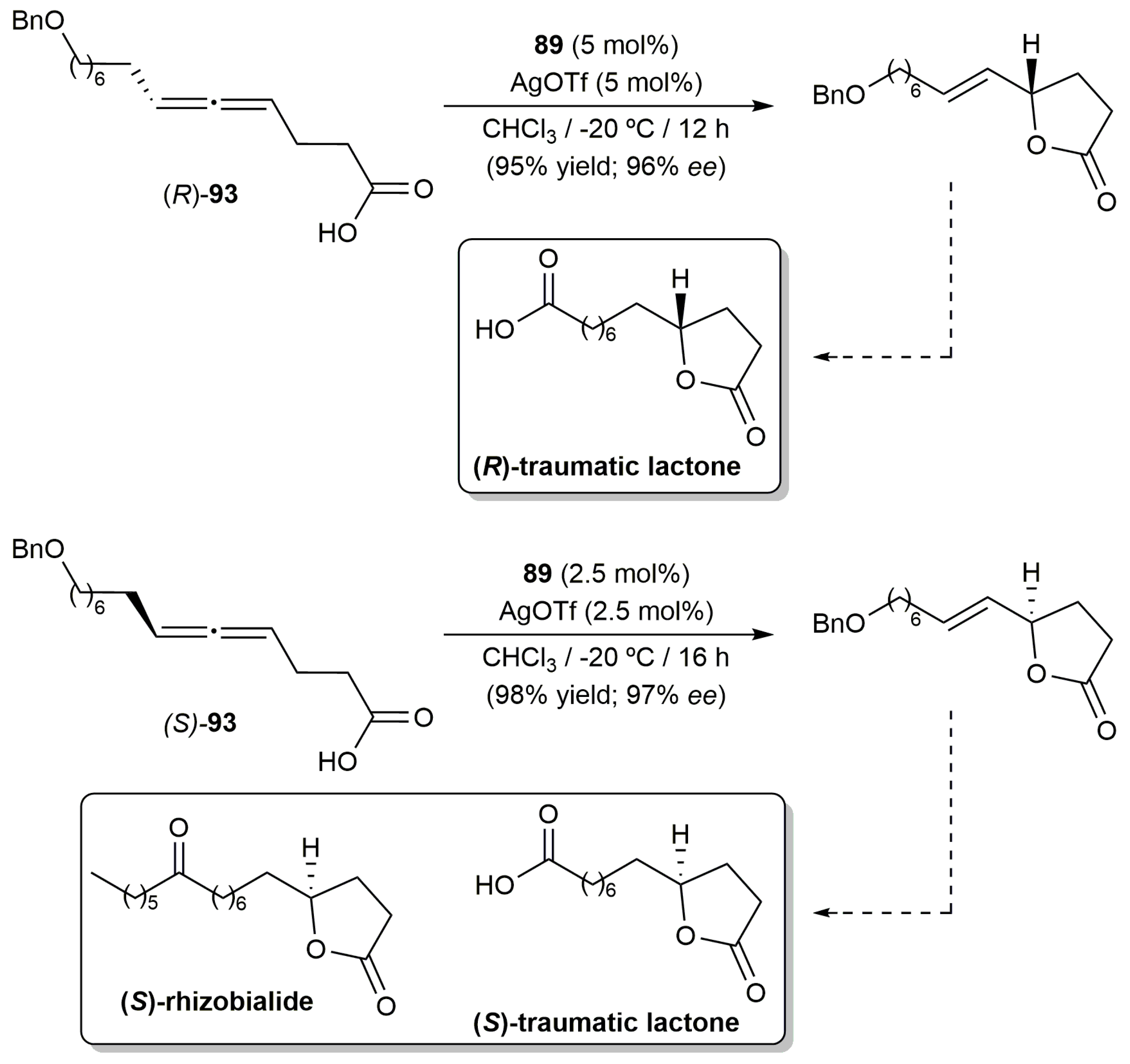
- Zhou, J.; Song, S.; Jiang, F.; Fu, C.; Ma, S. Efficient synthesis of traumatic lactone and rhizobialide. Chem. Eur. J. 2019, 25, 9948–9958. [Google Scholar] [CrossRef] [PubMed]
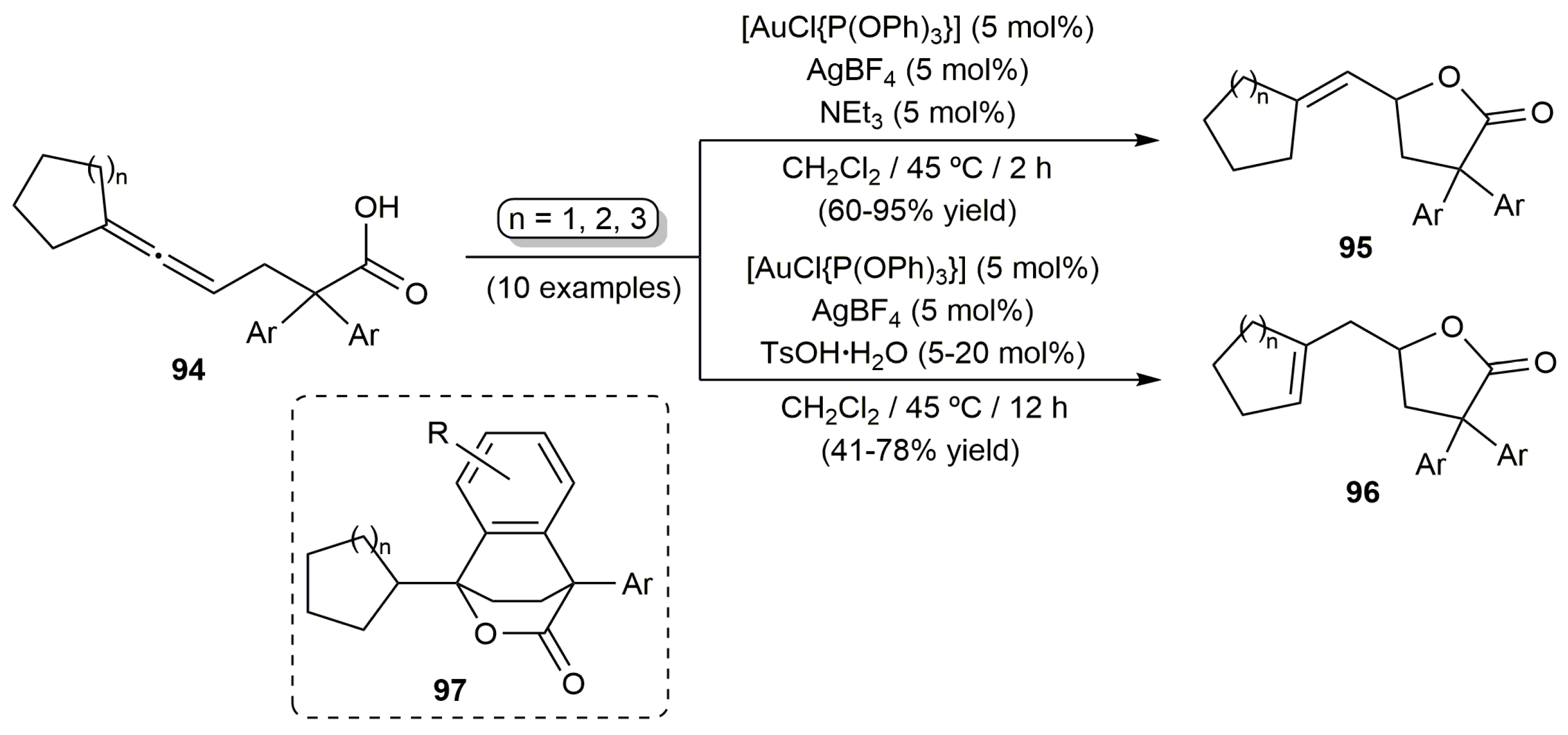
- Handa, S.; Subramanium, S.S.; Ruch, A.A.; Tanski, J.M.; Slaughter, L.M. Ligand- and Brønsted acid/base-switchable reaction pathways in gold(I)-catalyzed cycloisomerizations of allenoic acids. Org. Biomol. Chem. 2015, 13, 3936–3949. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.P. Transition metal-catalysed intermolecular reaction of allenes with oxygen nucleophiles: A perspective. Org. Biomol. Chem. 2012, 10, 3584–3594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Widenhoefer, R.A. Regio- and stereoselective synthesis of alkyl allylic ethers via gold(I)-catalyzed intermolecular hydroalkoxylation of allenes with alcohols. Org. Lett. 2008, 10, 2079–2081. [Google Scholar] [CrossRef]
- Fuchibe, K.; Abe, M.; Sasaki, M.; Ichikawa, J. Gold-catalyzed electrophilic activation of 1,1-difluoroallenes: α- and γ-selective addition of heteroatom nucleophiles. J. Fluorine Chem. 2020, 232, 109452. [Google Scholar] [CrossRef]






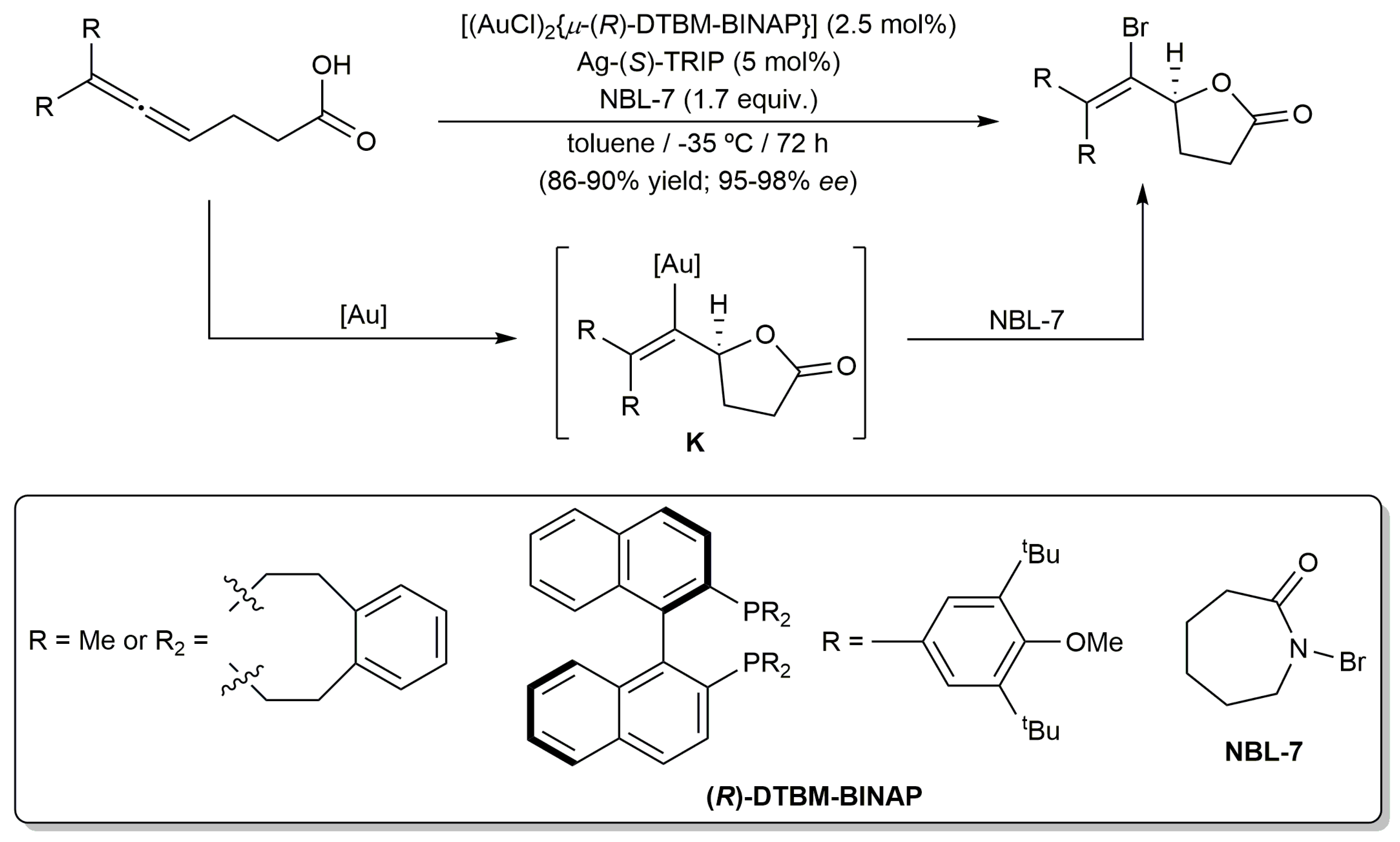
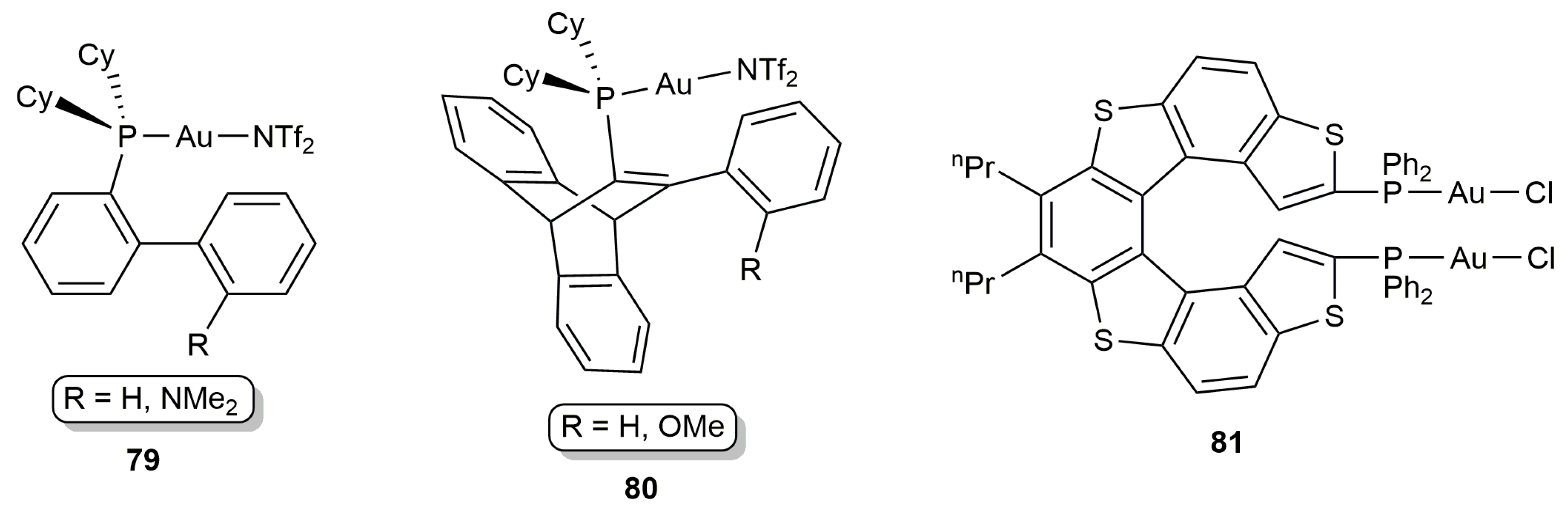
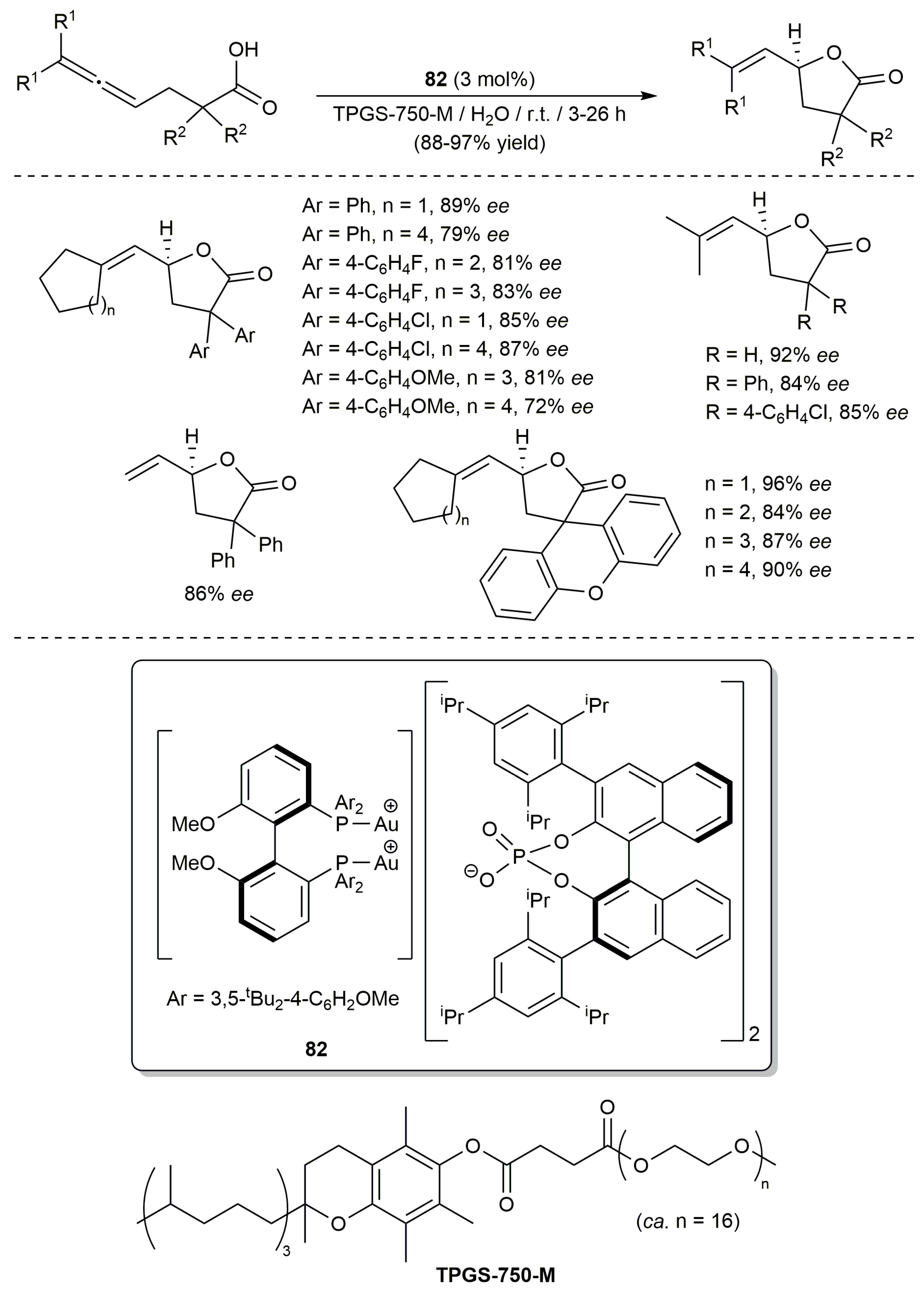
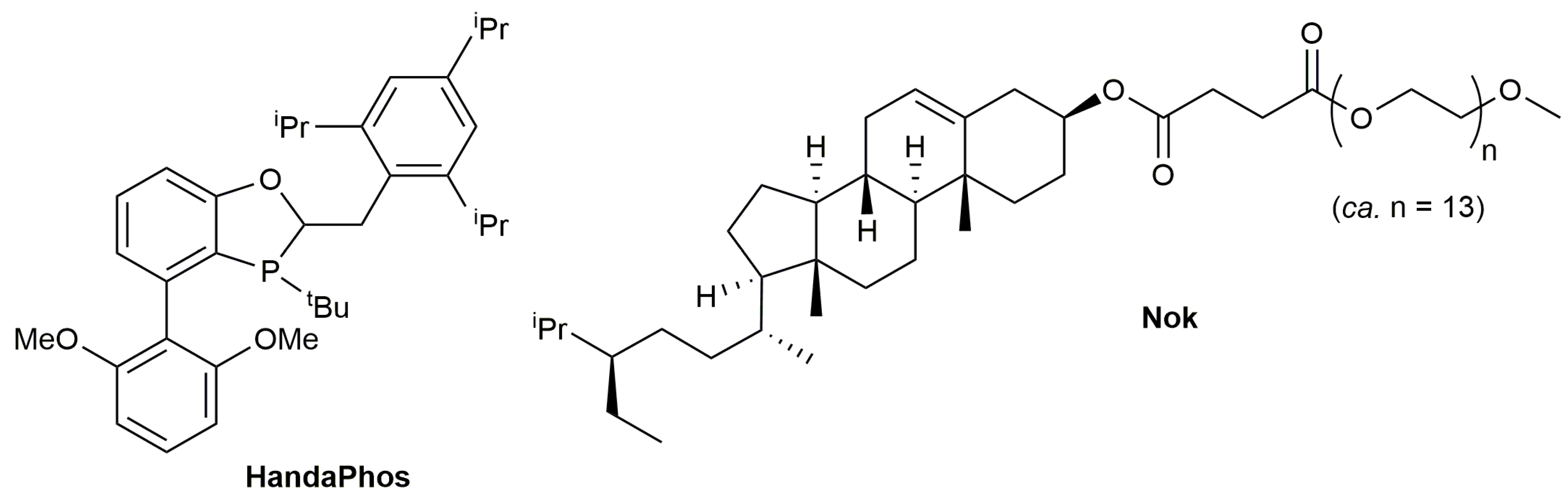
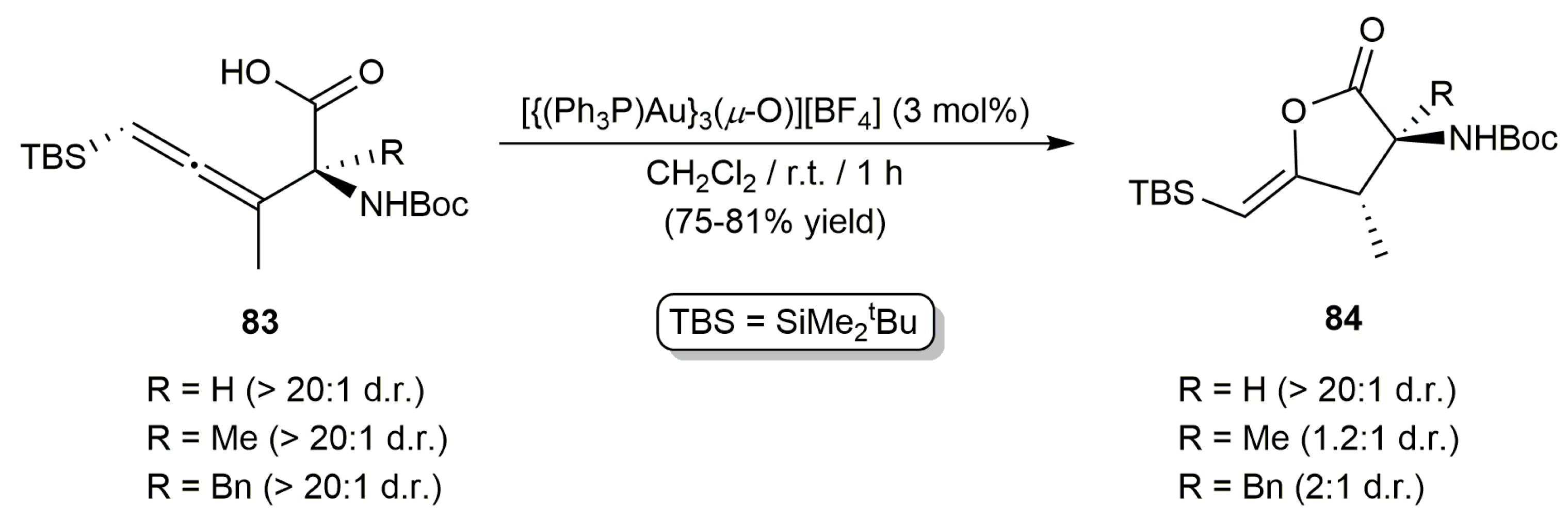
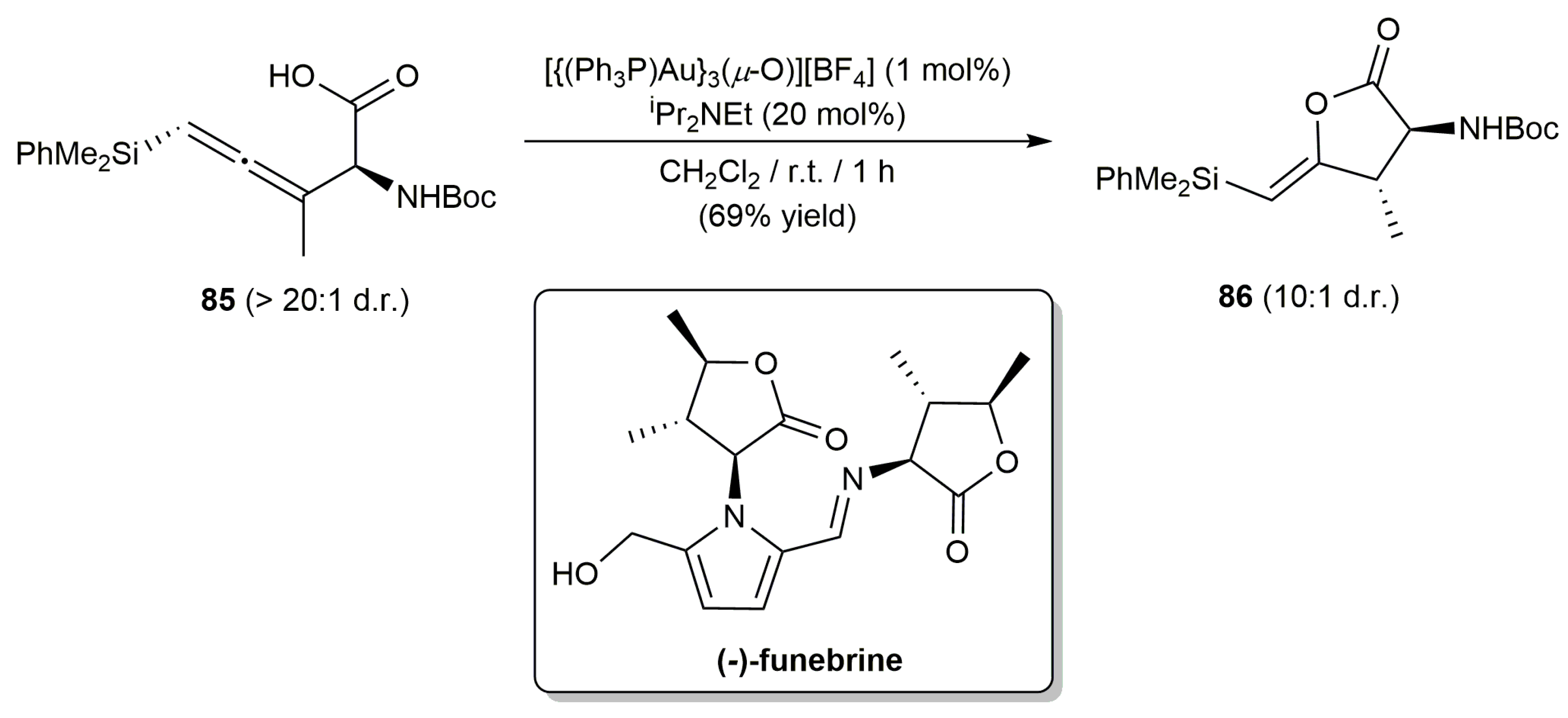

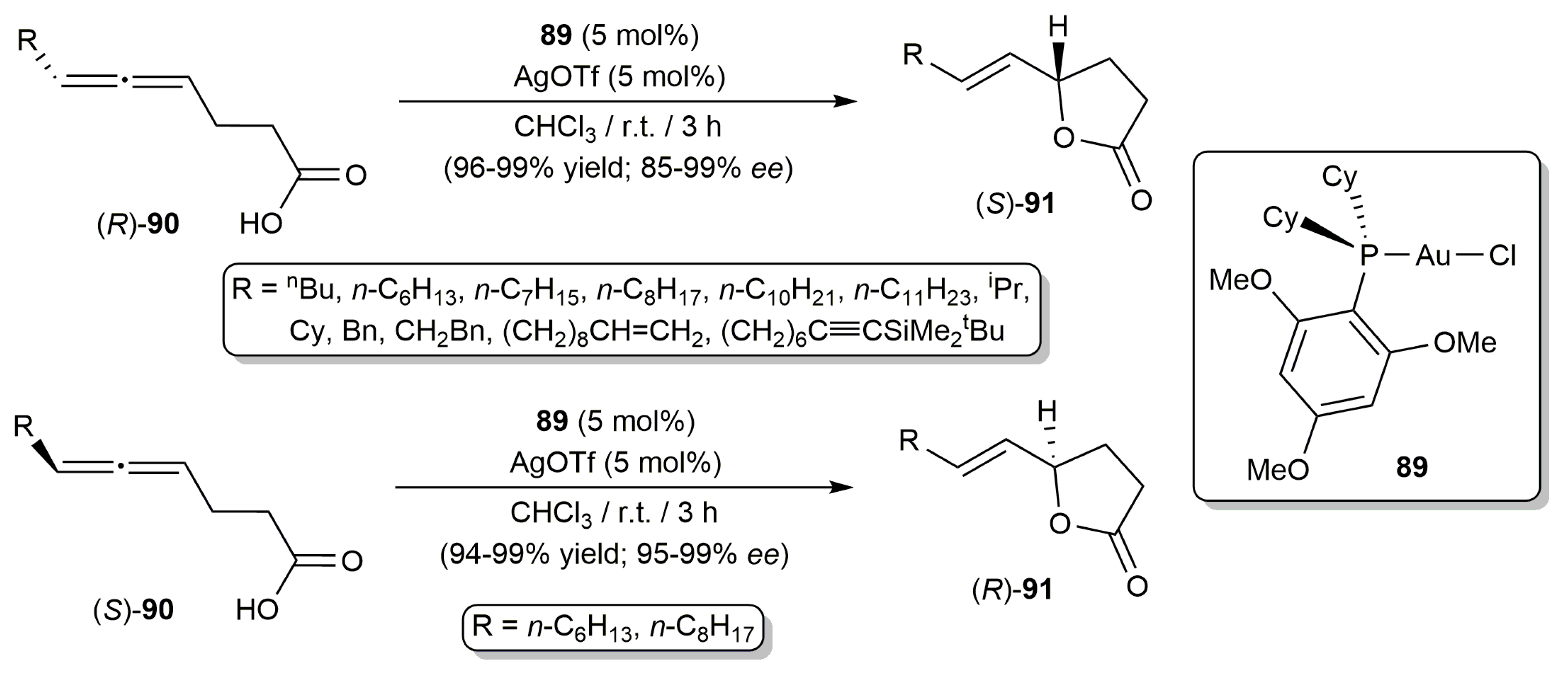
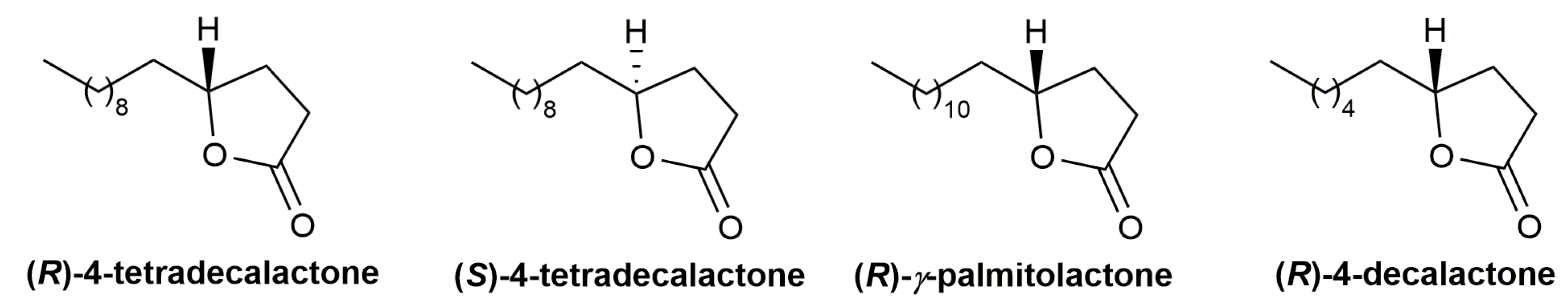
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadierno, V. Gold-Catalyzed Addition of Carboxylic Acids to Alkynes and Allenes: Valuable Tools for Organic Synthesis. Catalysts 2020, 10, 1206. https://doi.org/10.3390/catal10101206
Cadierno V. Gold-Catalyzed Addition of Carboxylic Acids to Alkynes and Allenes: Valuable Tools for Organic Synthesis. Catalysts. 2020; 10(10):1206. https://doi.org/10.3390/catal10101206
Chicago/Turabian StyleCadierno, Victorio. 2020. "Gold-Catalyzed Addition of Carboxylic Acids to Alkynes and Allenes: Valuable Tools for Organic Synthesis" Catalysts 10, no. 10: 1206. https://doi.org/10.3390/catal10101206
APA StyleCadierno, V. (2020). Gold-Catalyzed Addition of Carboxylic Acids to Alkynes and Allenes: Valuable Tools for Organic Synthesis. Catalysts, 10(10), 1206. https://doi.org/10.3390/catal10101206




