Biological Activities of NHC–Pd(II) Complexes Based on Benzimidazolylidene N-heterocyclic Carbene (NHC) Ligands Bearing Aryl Substituents †
Abstract
:1. Introduction
2. Results and Discussion
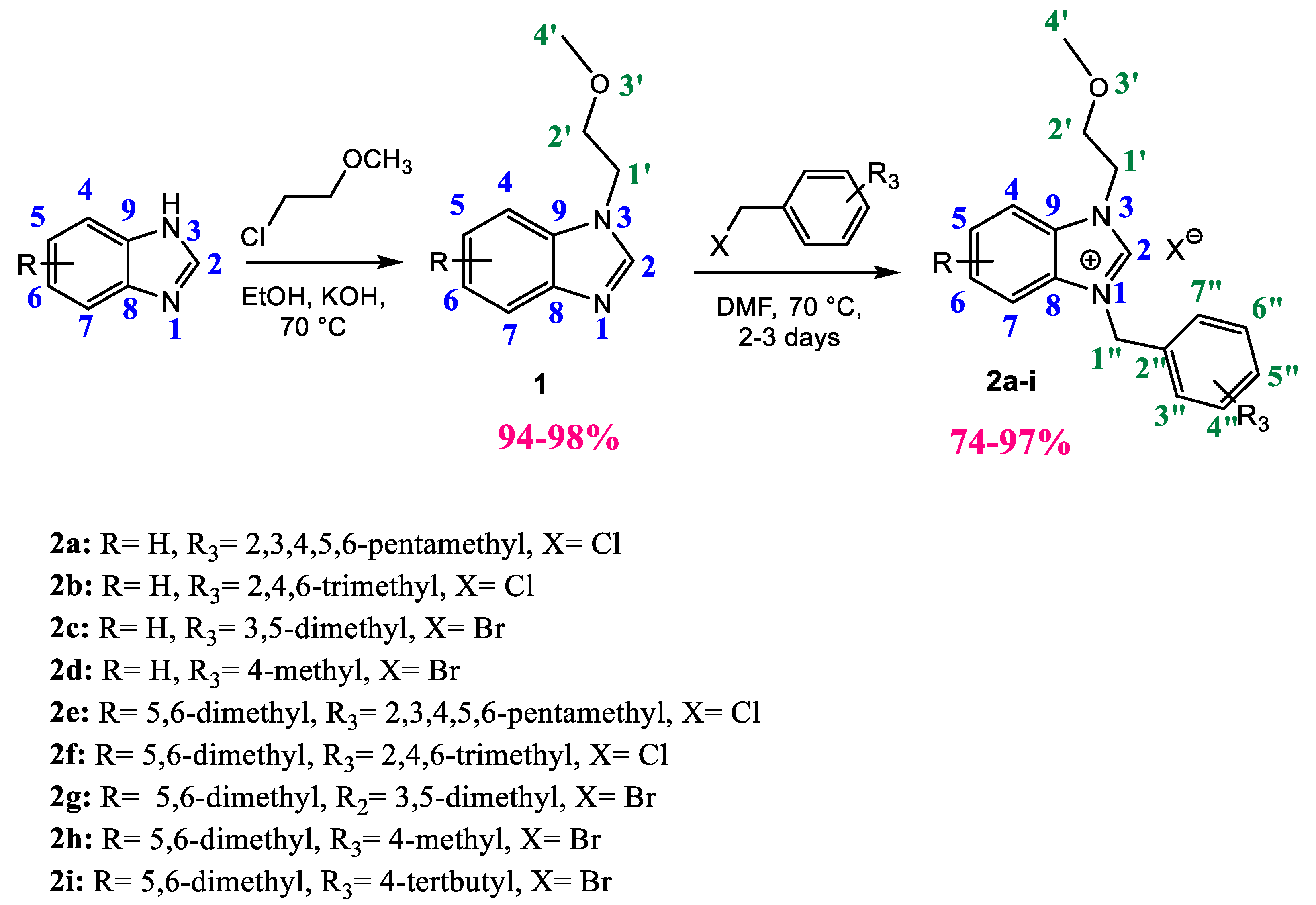
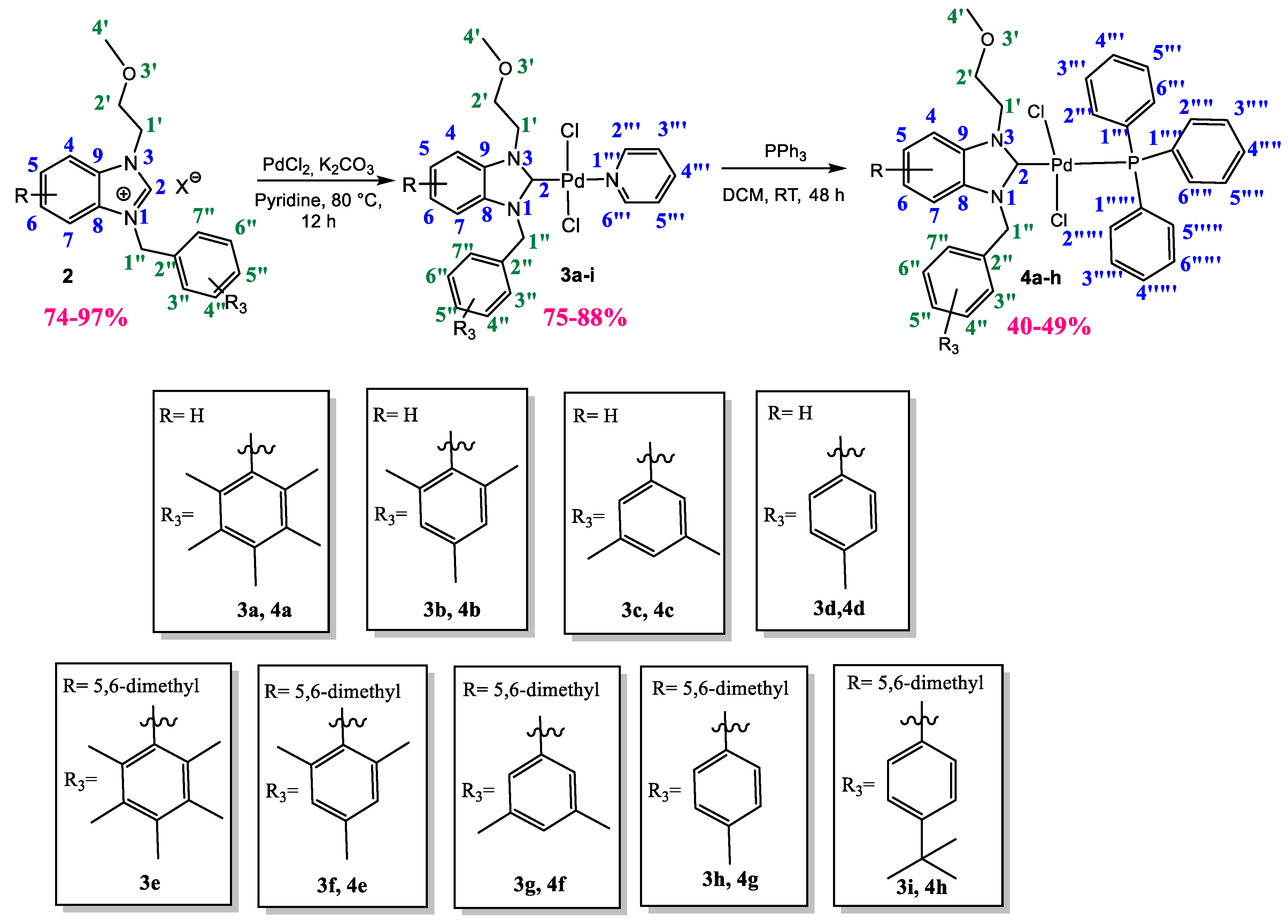
2.1. Synthesis and Characterization
2.2. Biological Evaluation
2.2.1. Anticancer Evaluation
2.2.2. Antimicrobial Activities
2.2.3. Antileishmanial Activities
2.2.4. Antitoxoplasmal Activities
3. Experimental Section
General Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Wurtz, S.; Glorius, F. Surveying Sterically Demanding N-Heterocyclic Carbene Ligands with Restricted Flexibility for Palladium-catalyzed Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef] [PubMed]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [PubMed]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with Poly(N-heterocyclic carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Nolan, S.P. The Development and Catalytic Uses of N-Heterocyclic Carbene Gold Complexes. Acc. Chem. Res. 2011, 44, 91–100. [Google Scholar] [CrossRef]
- Riener, K.; Haslinger, S.; Raba, A.; Hogerl, M.P.; Cokoja, M.; Herrmann, W.A.; Kuhn, F.E. Chemistry of Iron N-Heterocyclic Carbene Complexes: Syntheses, Structures, Reactivities, and Catalytic Applications. Chem. Rev. 2014, 114, 5215–5272. [Google Scholar] [CrossRef]
- Lebel, H.; Janes, M.K.; Charette, A.B.; Nolan, S.P. Structure and Reactivity of “Unusual” N-Heterocyclic Carbene (NHC) Palladium Complexes Synthesized from Imidazolium Salts. J. Am. Chem. Soc. 2004, 126, 5046–5047. [Google Scholar]
- Kong, Y.; Wen, L.; Song, H.; Xu, S.; Yang, M.; Liu, B.; Wang, B. Synthesis, Structures, and Norbornene Polymerization Behavior of Aryloxide-N-Heterocyclic Carbene Ligated Palladacycles. Organometallics 2011, 30, 153–159. [Google Scholar] [CrossRef]
- Guo, T.; Dechert, S.; Meyer, F. Dinuclear Palladium Complexes of Pyrazole-Bridged Bis(NHC) Ligands: A Delicate Balance between Normal and Abnormal Carbene Coordination. Organometallics 2014, 33, 5145–5155. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, J.-Y.; Chang, Y.-Y.; Hu, C.-H.; Wang, N.M.; Lee, H.M. Palladium Complexes with Tridentate N-Heterocyclic Carbene Ligands: Selective “Normal” and “Abnormal” Bindings and Their Anticancer Activities. Organometallics 2015, 34, 4359–4368. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Hadei, N.; Valente, C.; Chass, G.A.; Nasielski, J.C.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC = N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar]
- Moosun, S.B.; Bhowon, M.G.; Hosten, E.C.; Jhaumeer-Laulloo, S. Crystal structures, antibacterial, antioxidant and nucleic acid interactions of mononuclear and tetranuclear palladium(II) complexes containing Schiff base ligands. J. Coord. Chem. 2016, 69, 2736–2753. [Google Scholar] [CrossRef]
- Anitha, P.; Manikandan, R.; Viswanathamurthi, P. Palladium(II) 9,10-phenanthrenequinone N-substituted thiosemicarbazone/semicarbazone complexes as efficient catalysts for N-arylation of imidazole. J. Coord. Chem. 2015, 68, 3537–3550. [Google Scholar] [CrossRef]
- Hussain, S.; Bukhari, I.H.; Ali, S.; Shahzadi, S.; Shahid, M.; Munawar, K.S. Synthesis and spectroscopic and thermogravimetric characterization of heterobimetallic complexes with Sn(IV) and Pd(II); DNA binding, alkaline phosphatase inhibition and biological activity studies. J. Coord. Chem. 2015, 68, 662–677. [Google Scholar] [CrossRef]
- Ibrahim, M.B.; Hussain, S.M.S.; Fazal, A.; Fettouhi, M.; El Ali, B. Effective palladium(II)-bis(oxazoline) catalysts: Synthesis, crystal structure, and catalytic coupling reactions. J. Coord. Chem. 2015, 68, 432–448. [Google Scholar] [CrossRef]
- Yang, J. Synthesis and characterization of N-heterocyclic carbene–PdCl2–1H-benzotriazole complexes and their catalytic activities toward Mizoroki–Heck and Sonogashira reactions. J. Coord. Chem. 2017, 70, 441–450. [Google Scholar] [CrossRef]
- Nasielski, J.; Hadei, N.; Achonduh, G.; Kantchev, E.A.B.; O’Brien, C.J.; Lough, A.; Organ, M.G. Structure–Activity Relationship Analysis of Pd–PEPPSI Complexes in Cross-Couplings: A Close Inspection of the Catalytic Cycle and the Precatalyst Activation Mode. Chem. Eur. J. 2010, 16, 10844–10853. [Google Scholar] [CrossRef]
- Han, Y.; Huynh, H.V.; Tan, G.K. Syntheses and characterizations of Pd(II) complexes incorporating a N-heterocyclic carbene and aromatic N-heterocycles. Organometallics 2007, 26, 6447–6452. [Google Scholar]
- Yen, S.K.; Koh, L.L.; Huynh, H.V.; Hor, T.S.A. Mono- and dinuclear palladium (II) N,S-heterocyclic carbene complexes with N spacers and their Suzuki coupling activities. Chem. Asian J. 2008, 3, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Huynh, H.V. Dinuclear and Tetranuclear Palladium(II) Complexes of a Thiolato-Functionalized, Benzannulated N-Heterocyclic Carbene Ligand and Their Activities toward Suzuki−Miyaura Coupling. Organometallics 2010, 29, 6020–6027. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L. Synthesis and characterization of dinuclear NHC–palladium complexes and their applications in the Hiyama reactions of aryltrialkyoxysilanes with aryl chlorides. Dalton Trans. 2012, 41, 2031–12037. [Google Scholar] [CrossRef] [PubMed]
- Guisado-Barrios, G.; Hiller, J.; Peris, E. Pyracene-Linked Bis-Imidazolylidene Complexes of Palladium and Some Catalytic Benefits Produced by Bimetallic Catalysts. Chem. Eur. J. 2013, 19, 10405–10411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-X.; Zhang, W.; Zhao, X.-J.; Zhao, Z.-X.; Shi, M.-C.; Wang, X.-G. NHC PdII Complex Bearing 1,6-Hexylene Linker: Synthesis and Catalytic Activity in the Suzuki–Miyaura and Heck–Mizoroki Reactions. Eur. J. Org. Chem. 2013, 2013, 1253–1261. [Google Scholar] [CrossRef]
- Yang, J.; Li, P.; Zhang, Y.; Wang, L. Dinuclear NHC–palladium complexes containing phosphine spacers: Synthesis, X-ray structures and their catalytic activities towards the Hiyama coupling reaction. Dalton Trans. 2014, 43, 7166–7175. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Krinsky, J.L.; Penafiel, I.; Castillon, S.; Loponov, K.; Lapkin, A.; Godard, C.; Claver, C. Heterogenization of Pd–NHC complexes onto a silica support and their application in Suzuki–Miyaura coupling under batch and continuous flow conditions. Cat. Sci. Technol. 2015, 5, 310–319. [Google Scholar] [CrossRef]
- Ghotbinejad, M.; Khosropour, A.R.; Poor-Baltork, I.M.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. SPIONs-bis(NHC)-palladium(II): A novel, powerful and efficient catalyst for Mizoroki–Heck and Suzuki–Miyaura C–C coupling reactions. J. Mol. Cat. A Chem. 2014, 385, 78–84. [Google Scholar] [CrossRef]
- Dehimat, Z.I.; Yasar, S.; Tebbani, D.; Ozdemir, I. Sonogashira cross-coupling reaction catalyzed by N -heterocyclic carbene-Pd(II)-PPh3 complexes under copper free and aerobic conditions. Inorg. Chim. Acta. 2018, 469, 325–334. [Google Scholar] [CrossRef]
- Fong, T.T.H.; Lok, C.N.; Chung, C.Y.S.; Fung, Y.M.E.; Chow, P.K.; Wan, P.K.; Che, C.M. Cyclometalated Palladium(II) N-Heterocyclic Carbene Complexes: Anticancer Agents for Potent In Vitro Cytotoxicity and In Vivo Tumor Growth Suppression. Angew. Chem. Int. Ed. 2016, 55, 11935–11939. [Google Scholar] [CrossRef]
- Haque, R.A.; Salman, A.W.; Budagumpi, S.; Abdullah, A.A.; Majid, A.M.A. Sterically tuned Ag(i)- and Pd(ii)-N-heterocyclic carbene complexes of imidazol-2-ylidenes: Synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Metallomics 2013, 5, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Teyssot, M.-L.; Jarrousse, A.-S.; Manin, M.; Chevry, A.; Roche, S.; Norre, F.; Beaudoin, C.; Morel, L.; Boyer, D.; Mahiou, R.; et al. Metal-NHC complexes: A survey of anti-cancer properties. Dalton Trans. 2009. [Google Scholar] [CrossRef]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The medicinal applications of imidazolium carbene-metal complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef] [Green Version]
- Gautier, A.; Cisnetti, F. Advances in metal-carbene complexes as potent anti-cancer agents. Metallomics 2012, 4, 23–32. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Fatima, T.; Agha, T.M.; Abdul Majid, A.M.S.; Abdullah, H.H.; Razali, M.R. Nitrile functionalized silver(I) N-heterocyclic carbene complexes: DFT calculations and antitumor studies. Transit. Met. Chem. 2008, 43, 301–312. [Google Scholar] [CrossRef]
- Slimani, I.; Chakchouk-Mtibaa, A.; Mansour, L.; Mellouli, L.; Ozdemir, I.; Gurbuz, N.; Hamdi, N. Synthesis, characterization, biological determination and catalytic evaluation of ruthenium(II) complexes bearing benzimidazole-based NHC ligands in transfer hydrogenation Catalysis. New J. Chem. 2020, 44, 5309–5323. [Google Scholar] [CrossRef]
- Özdemir, İ.; Çiftçi, O.; Evren, E.; Gürbüz, N.; Kaloğlu, N.; Türkmen, N.B.; Yaşar, Ş.; Üstün, E.; Hamdi, N.; Mansour, L.; et al. Synthesis, characterization and antitumor properties of novel silver(I) and gold(I) N-heterocyclic carbene complexes. Inorg. Chim. Acta. 2020, 506, 119530. [Google Scholar] [CrossRef]
- Touj, N.; Al Ayed, A.S.; Suathier, M.; Mansour, L.; Harrath, A.A.; Al Tamimi, J.; Özdemir, I.; Yasar, S.; Hamdi, N. Efficient in situ N-heterocyclic carbene palladium(II) generated from Pd(OAc)2 catalysts for carbonylative Suzuki coupling reactions of arylboronic acids with 2-bromopyridine under inert conditions leading to unsymmetrical arylpyridine ketones: Synthesis, characterization and cytotoxic activities. RSC Adv. 2018, 8, 40000–40015. [Google Scholar]
- Touj, N.; Gürbüz, N.; Hamdi, N.; Yaşar, S.; Özdemir, İ. Palladium PEPPSI complexes: Synthesis and catalytic activity on the Suzuki-Miyaura coupling reactions for aryl bromides at room temperature in aqueous media. Inorg. Chim. Acta. 2018, 478, 187–219. [Google Scholar] [CrossRef]
- Touj, N.; Yaşar, S.; Özdemir, N.; Hamdi, N.; Özdemir, İ. Sonogashira cross-coupling reaction catalysed by mixed NHC-Pd-PPh3 complexes under copper free condition. J. Organomet. Chem. 2018, 860, 59–71. [Google Scholar] [CrossRef]
- Boubakri, L.; Mansour, L.; Harrath, A.H.; Özdemir, I.; Yaşar, S.; Hamdi, N. N-heterocyclic carbene-Pd (II)-PPh3 complexes a new highly efficient catalyst system for the Sonogashira cross-coupling reaction: Synthesis, characterization and biological activities. J. Coord. Chem. 2018, 71, 183–199. [Google Scholar] [CrossRef]
- Touj, N.; Chakchouk-Mtibaa, A.; Mansour, L.; Harrath, A.H.; Al-Tamimi, J.H.; Özdemir, I.; Mellouli, L.; Yaşar, S.; Hamdi, N. An efficient one-pot synthesis of triazoles by copper (I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction under mild condition in water: Synthesis, catalytic application, DFT study and biological activities. J. Organomet. Chem. 2017, 853, 49–63. [Google Scholar] [CrossRef]
- Touj, N.; Özdemir, I.; Yaşar, S.; Hamdi, N. An efficient (NHC) Copper (I)-catalyst for azide–alkyne cycloaddition reactions for the synthesis of 1,2,3-trisubstituted triazoles: Click chemistry. Inorg. Chim. Acta. 2017, 467, 21–32. [Google Scholar] [CrossRef]
- Boubakri, L.; Yasar, S.; Dorcet, V.; Roisnel, T.; Bruneau, C.; Hamdi, N.; Ozdemir, I. Synthesis and catalytic applications of palladium N-heterocyclic carbene complexes: As efficient pre-catalyst for Suzuki Miyaura and Sonogashira coupling reactions. New J. Chem. 2017, 41, 5105–5113. [Google Scholar] [CrossRef]
- Kızrak, Ü.; Çiftçi, O.; Özdemir, İ.; Gürbüz, N.; Demir Düşünceli, S.; Kaloğlu, M.; Mansour, L.; Zaghrouba, F.; Hamdi, N.; Özdemir, İ. Amine-functionalized silver and gold N-heterocyclic carbene complexes: Synthesis, characterization and antitumor properties. J. Organomet. Chem. 2019, 882, 26–32. [Google Scholar] [CrossRef]
- Karataş, M.O.; Özdemir, N.; Alıcı, B.; Özdemir, İ. N-heterocyclic carbene palladium complexes with different N-coordinated ligands: Comparison of their catalytic activities in Suzuki-Miyaura and Mizoroki-Heck reactions. Polyhedron 2020, 176, 114271. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Icsel, C.; Turgut, O.R.; Aygun, M.; Evren, E.; Ozdemir, I. Synthesis, structures and catalytic activity of Pd(II) saccharinate complexes with monophosphines in direct arylation of five-membered heteroarenes with aryl bromides. Inorg. Chim. Acta. 2020, 500, 119220. [Google Scholar] [CrossRef]
- İmik, F.; Yaşar, S.; Özdemir, İ. Synthesis and investigation of catalytic activity of phenylene- and biphenylene bridged bimetallic Palladium-PEPPSI complexes. J. Organomet. Chem. 2019, 896, 162–167. [Google Scholar] [CrossRef]
- Şahin-Bölükbaşı, S.; Şahin, N. Novel Silver-NHC complexes: Synthesis and anticancer properties. J. Organomet. Chem. 2019, 891, 78–84. [Google Scholar] [CrossRef]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef]
- Sitalu, K.; Babu, B.H.; Latha, J.N.L.; Rao, A.L. Synthesis, Characterization and Antimicrobial Activities of Copper Derivatives of NHC-II Complexes. Pak. J. Biol. Sci. 2017, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Delmas, S.B.; Álvarez, Á.F.; Valentin, A.; Hemmert, C.; Gornitzka, H. Synthesis, characterization, and antileishmanial activity of neutral N-heterocyclic carbenes gold(I) complexes. Eur. J. Med. Chem. 2018, 143, 1635–1643. [Google Scholar] [CrossRef]
- Hemmert, C.; Fabié, A.; Fabre, A.; Benoit-Vical, F.; Gornitzka, H. Synthesis, structures, and antimalarial activities of some silver(I), gold(I) and gold(III) complexes involving N-heterocyclic carbene ligands. Eur. J. Med. Chem. 2013, 60, 64–75. [Google Scholar] [CrossRef] [PubMed]
| Pd(II)–NHC Complexes 3–4 | Anticancer Activity IC50 in µM | |
|---|---|---|
| MCF7 | MDA-MB-231 | |
| 3a | 1.180 | 1.011 |
| 3b | 1.416 | 0.885 |
| 3c | 1.270 | 1.452 |
| 3d | 1.677 | 1.304 |
| 3e | 1.288 | 1.127 |
| 3f | 0.675 | 1.012 |
| 3g | 0.518 | 1.036 |
| 3h | 1.062 | 0.708 |
| 3i | 1.812 | 1.318 |
| 4a | 1.160 | 1.546 |
| 4b | 1.871 | 0.936 |
| 4c | 1.499 | 1.226 |
| 4d | 1.111 | 1.25 |
| 4e | 1.417 | 1.031 |
| 4f | 1.181 | 1.05 |
| 4g | 0.802 | 0.936 |
| 4h | 1.139 | 0.886 |
| 4i | 0.6 ± 0.04 | 0.4 ± 0.03 |
| Pd(II)–NHC Complexes 3–4 a | Antimicrobial Activity (50 µg/disc) | ||
|---|---|---|---|
| E.coli | MRSA | C.albicans | |
| 3a | 20.3 ± 1.1 | 17.4 ± 0.3 | 18.0 ± 0.2 |
| 3b | 18.3 ± 1.6 | 17.5 ± 0.4 | 32.0 ± 0.3 |
| 3c | 19.3 ± 0.6 | 19.0 ± 0.1 | 12.0 ± 0.3 |
| 3d | 18.3 ± 0.6 | 15.0 ± 1.0 | 19.0 ± 0.6 |
| 3e | 12.0 ± 0.6 | 18.0 ± 0.8 | 20.0 ± 0.7 |
| 3f | 25.0 ± 0.4 | 28.5 ± 2.5 | 26.0 ± 0.0 |
| 3g | 26.3 ± 1.8 | 26.5 ± 1.4 | 29.5 ± 1.4 |
| 3h | 22.4 ± 0.6 | 23.0 ± 0.1 | 28.0 ± 0.0 |
| 3i | 19.0 ± 1.2 | 18.5 ± 0.6 | 20.0 ± 0.8 |
| 4a | 25.0 ± 0.5 | 22.0 ± 0.4 | 26.0 ± 0.9 |
| 4b | 23.0 ± 0.5 | 26.0 ± 0.5 | 27.0 ± 0.5 |
| 4c | 25.0 ± 0.6 | 27.0 ± 0.5 | 28.0 ± 0.3 |
| 4d | 18.5 ± 2.2 | 19.5 ± 0.6 | 19.0 ± 0.4 |
| 4e | 19.3 ± 1.5 | 18.5 ± 0.6 | 29.0 ± 0.7 |
| 4f | 26.3 ± 0.6 | 28.0 ± 0.0 | 15.0 ± 0.9 |
| 4g | 18.3 ± 0.6 | 15.0 ± 1.5 | 22.0 ± 0.8 |
| 4h | 24.0 ± 0.6 | 20.0 ± 0.3 | 23.0 ± 0.9 |
| 4i | 22.0 ± 1.0 | 24.5 ± 2.5 | 26.0 ± 0.0 |
| Tetracycline | 22.3 ± 1.5 | 26.5 ± 1.5 | - |
| Fluconazole | - | - | 28.0 ± 0.8 |
| Pd(II)–(NHC) Complexes 3–4 | CC50 of Vero Cells at µg mL−1 | Amastigote IC50 at µg mL−1 | Promastigotes IC50 at µg mL−1 | Amastigote SI | Promastigote SI |
|---|---|---|---|---|---|
| 3a | 6.1 ± 1.8 | 0.5 ± 0.07 | 0.5 ± 0.09 | 12.2 | 12.2 |
| 3b | 3.6 ± 1.2 | 0.3 ± 0.04 | 0.6 ± 0.07 | 12.0 | 6.0 |
| 3c | 6.6 ± 1.7 | 0.4 ± 0.05 | 0.6 ± 0.11 | 16.4 | 11.0 |
| 3d | 28.0 ± 3.6 | 0.6 ± 0.09 | 0.7 ± 0.13 | 46.6 | 39.9 |
| 3e | 22.8 ± 3.3 | 17.4 ± 3.8 | 7.6 ± 1.9 | 1.3 | 3.0 |
| 3f | 16.4 ± 2.8 | 0.7 ± 0.12 | 0.6 ± 0.09 | 23.4 | 27.3 |
| 3g | 29.8 ± 6.4 | 2.7 ± 0.6 | 3.2 ± 0.7 | 11.1 | 9.3 |
| 3h | 1.8 ± 0.7 | 0.7 ± 0.09 | 1.6 ± 0.3 | 2.6 | 1.1 |
| 3i | 15.9 ± 3.0 | 2.9 ± 0.8 | 6.3 ± 2.0 | 5.5 | 2.5 |
| 4a | 8.9 ± 2.9 | 0.3 ± 0.07 | 0.4 ± 0.07 | 29. | 22.4 |
| 4b | 4.8 ± 1.5 | <0.2 | 0.4 ± 0.08 | >24 | 12.0 |
| 4c | 4.9 ± 1.1 | 1.1 ± 0.6 | 0.8 ± 0.06 | 4.5 | 6.2 |
| 4d | 6.1 ± 1.6 | 1.6 ± 0.7 | 0.4 ± 0.03 | 3.8 | 15.2 |
| 4e | 13.1 ± 2.7 | 2.4 ± 0.9 | 1.6 ± 0.5 | 5.5 | 8.2 |
| 4f | 19.9 ± 3.2 | 1.7 ± 0.7 | 0.5 ± 0.07 | 11.7 | 39.8 |
| 4g | 34.4 ± 6.6 | 2.9 ± 0.9 | 15.4 ± 2.8 | 11.9 | 2.2 |
| 4h | 35.4 ± 5.9 | 14.3 ± 2.6 | 32.8 ± 6.1 | 2.5 | 1.1 |
| 4i | 9.9 ± 2.8 | 0.5 ± 0.03 | 0.9 ± 0.1 | 19.8 | 11.0 |
| AmB | 7.4 ± 2.64 | 0.46 ± 0.07 | 0.78 ± 0.09 | 16.09 | 9.49 |
| Pd(II)–NHC Complexes 3–4 | CC50 of Vero Cells at µg mL−1 | Antitoxoplasma IC50 at µg mL−1 | SI |
|---|---|---|---|
| 3a | 6.1 ± 1.8 | 4.2 ± 0.9 | 1.5 |
| 3b | 3.6 ± 1.2 | 3.9 ± 0.9 | 0.9 |
| 3c | 6.6 ± 1.7 | 4.6 ± 1.1 | 1.4 |
| 3d | 28.0 ± 3.6 | 18 ± 3.6 | 1.6 |
| 3e | 22.8 ± 3.3 | 8.1 ± 1.9 | 2.8 |
| 3f | 16.4 ± 2.8 | 13.8 ± 2.7 | 1.2 |
| 3g | 29.8 ± 6.4 | 18.1 ± 2.8 | 1.6 |
| 3h | 1.8 ± 0.7 | 1.2 ± 0.2 | 1.5 |
| 3i | 15.9 ± 3.0 | 8.5 ± 1.7 | 1.9 |
| 4a | 8.9 ± 2.9 | 4.8 ± 1.1 | 1.9 |
| 4b | 4.8 ± 1.5 | 3.6 ± 0.9 | 1.3 |
| 4c | 4.9 ± 1.1 | 3.9 ± 0.8 | 1.3 |
| 4d | 6.1 ± 1.6 | 11.9 ± 2.0 | 0.5 |
| 4e | 13.1 ± 2.7 | 6.3 ± 1.8 | 2.1 |
| 4f | 19.9 ± 3.2 | 38.4 ± 6.5 | 0.5 |
| 4g | 34.4 ± 6.6 | 25.3 ± 4.1 | 1.4 |
| 4h | 35.4 ± 5.9 | 21.7 ± 4.3 | 1.6 |
| 4i | 9.9 ± 2.8 | 7.8 ± 1.7 | 1.3 |
| ATO Atovaquone is an antitoxoplasma reference drug | 9.3 ± 2.08 | 0.09 ± 0.02 | 103.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Nasr, I.; Touj, N.; Koko, W.; Khan, T.; Özdemir, I.; Yaşar, S.; Hamdi, N. Biological Activities of NHC–Pd(II) Complexes Based on Benzimidazolylidene N-heterocyclic Carbene (NHC) Ligands Bearing Aryl Substituents. Catalysts 2020, 10, 1190. https://doi.org/10.3390/catal10101190
Al Nasr I, Touj N, Koko W, Khan T, Özdemir I, Yaşar S, Hamdi N. Biological Activities of NHC–Pd(II) Complexes Based on Benzimidazolylidene N-heterocyclic Carbene (NHC) Ligands Bearing Aryl Substituents. Catalysts. 2020; 10(10):1190. https://doi.org/10.3390/catal10101190
Chicago/Turabian StyleAl Nasr, Ibrahim, Nedra Touj, Waleed Koko, Tariq Khan, Ismail Özdemir, Sedat Yaşar, and Naceur Hamdi. 2020. "Biological Activities of NHC–Pd(II) Complexes Based on Benzimidazolylidene N-heterocyclic Carbene (NHC) Ligands Bearing Aryl Substituents" Catalysts 10, no. 10: 1190. https://doi.org/10.3390/catal10101190
APA StyleAl Nasr, I., Touj, N., Koko, W., Khan, T., Özdemir, I., Yaşar, S., & Hamdi, N. (2020). Biological Activities of NHC–Pd(II) Complexes Based on Benzimidazolylidene N-heterocyclic Carbene (NHC) Ligands Bearing Aryl Substituents. Catalysts, 10(10), 1190. https://doi.org/10.3390/catal10101190





