Multi-Combilipases: Co-Immobilizing Lipases with Very Different Stabilities Combining Immobilization via Interfacial Activation and Ion Exchange. The Reuse of the Most Stable Co-Immobilized Enzymes after Inactivation of the Least Stable Ones
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization and Stabilities of the Different Enzymes on Octyl Agarose
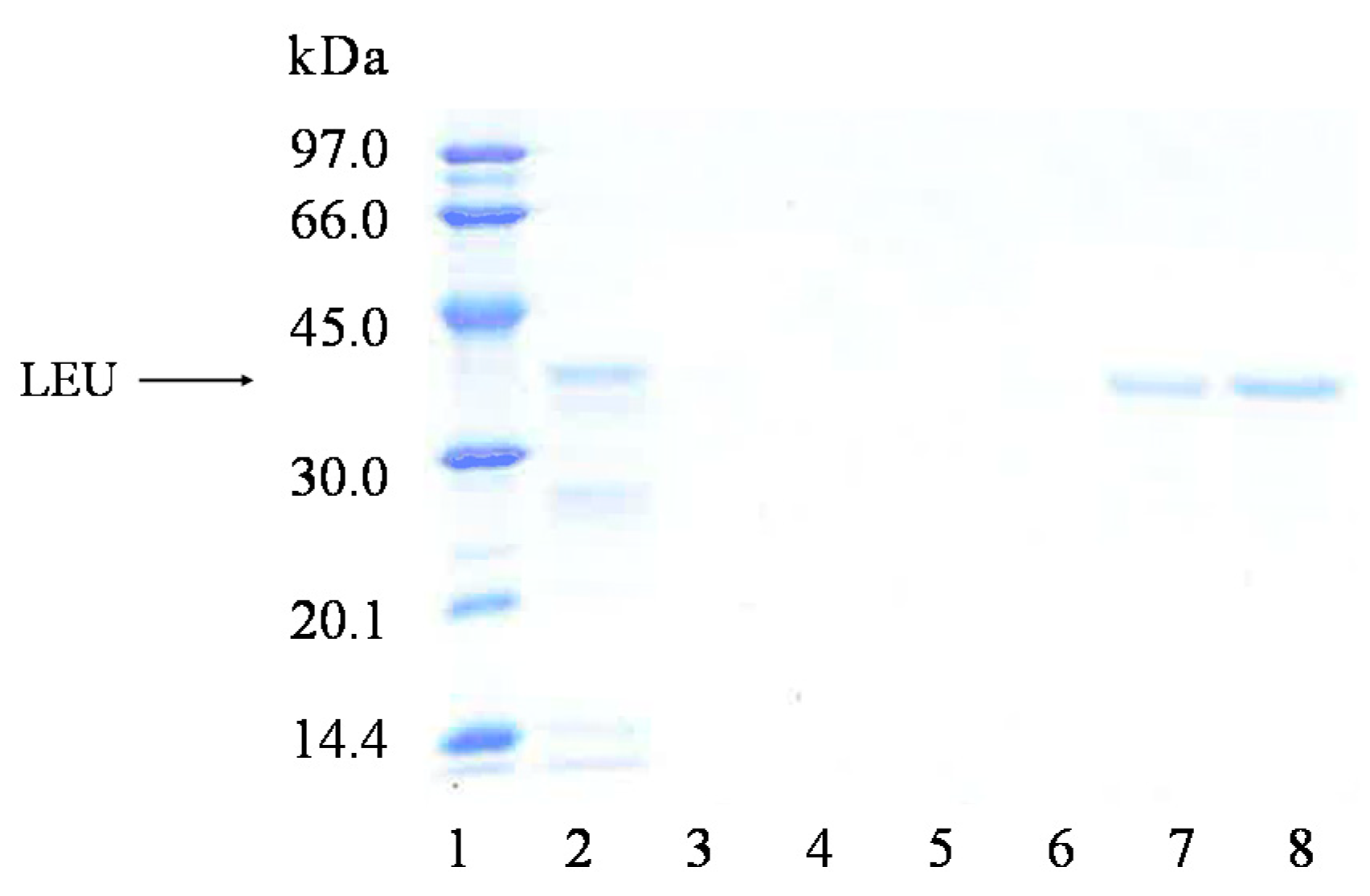
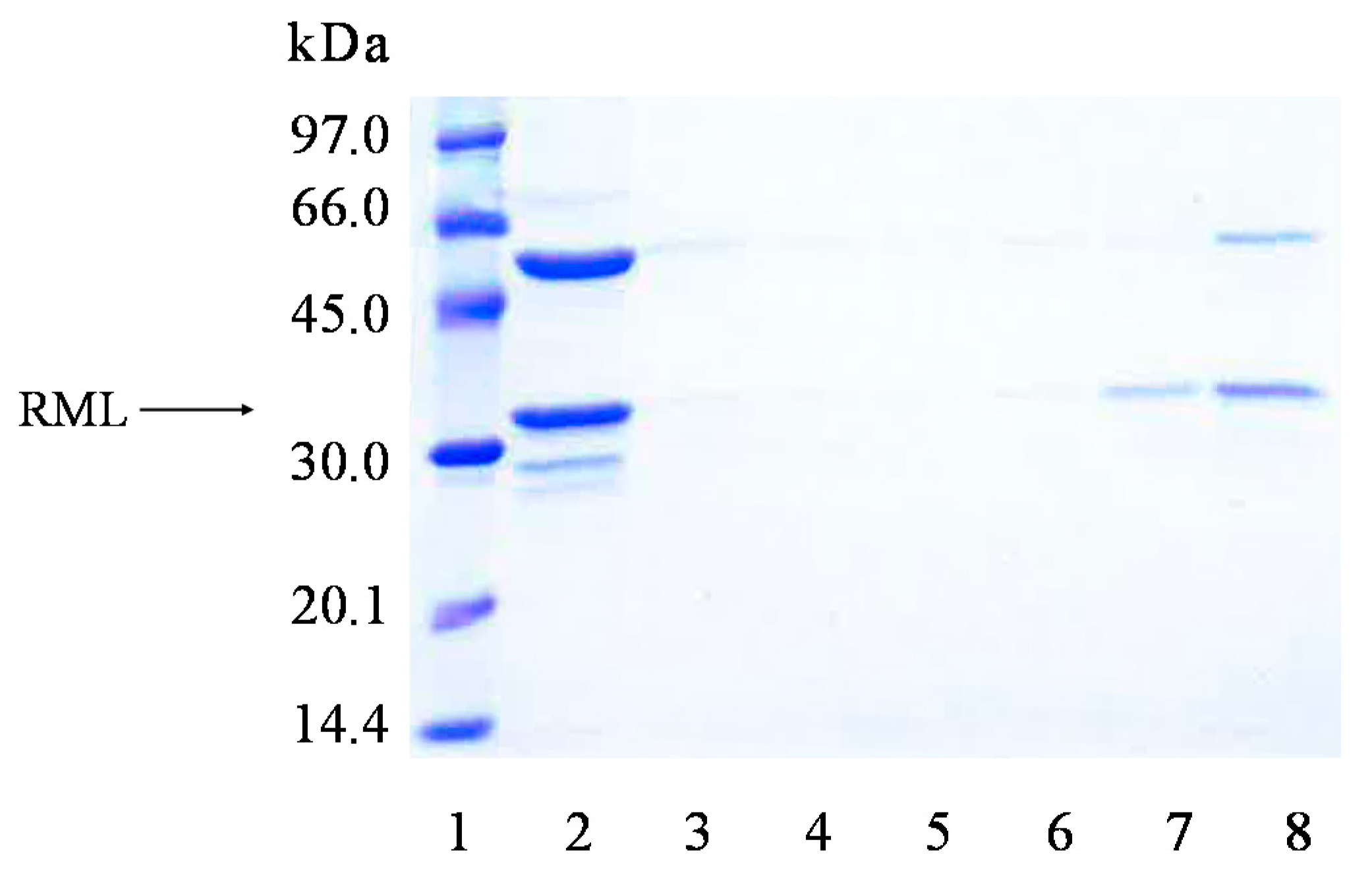
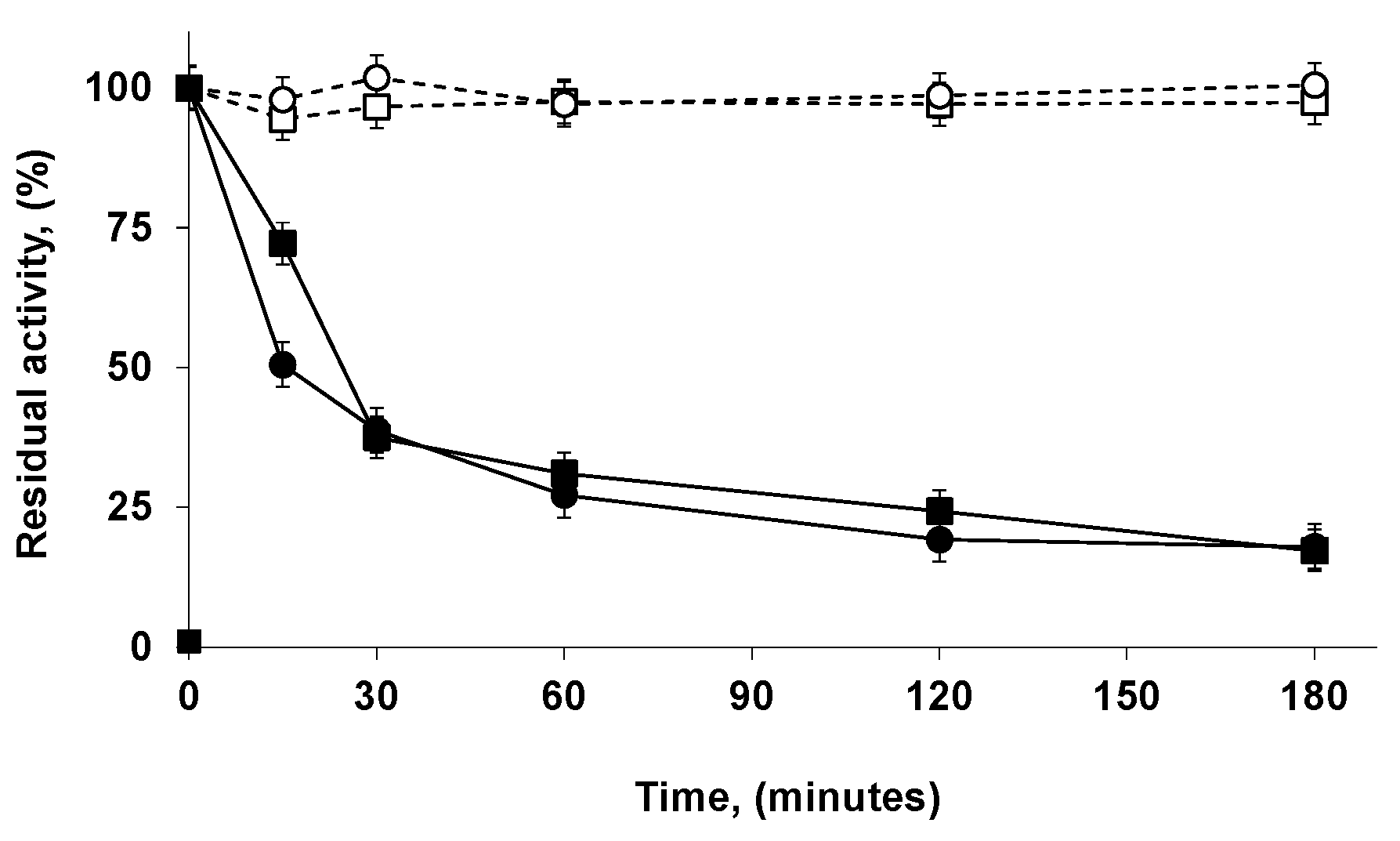
2.2. Immobilization and Stability of LEU and RML in a Support Coated with PEI (Octyl-VS-PEI Agarose Beads)
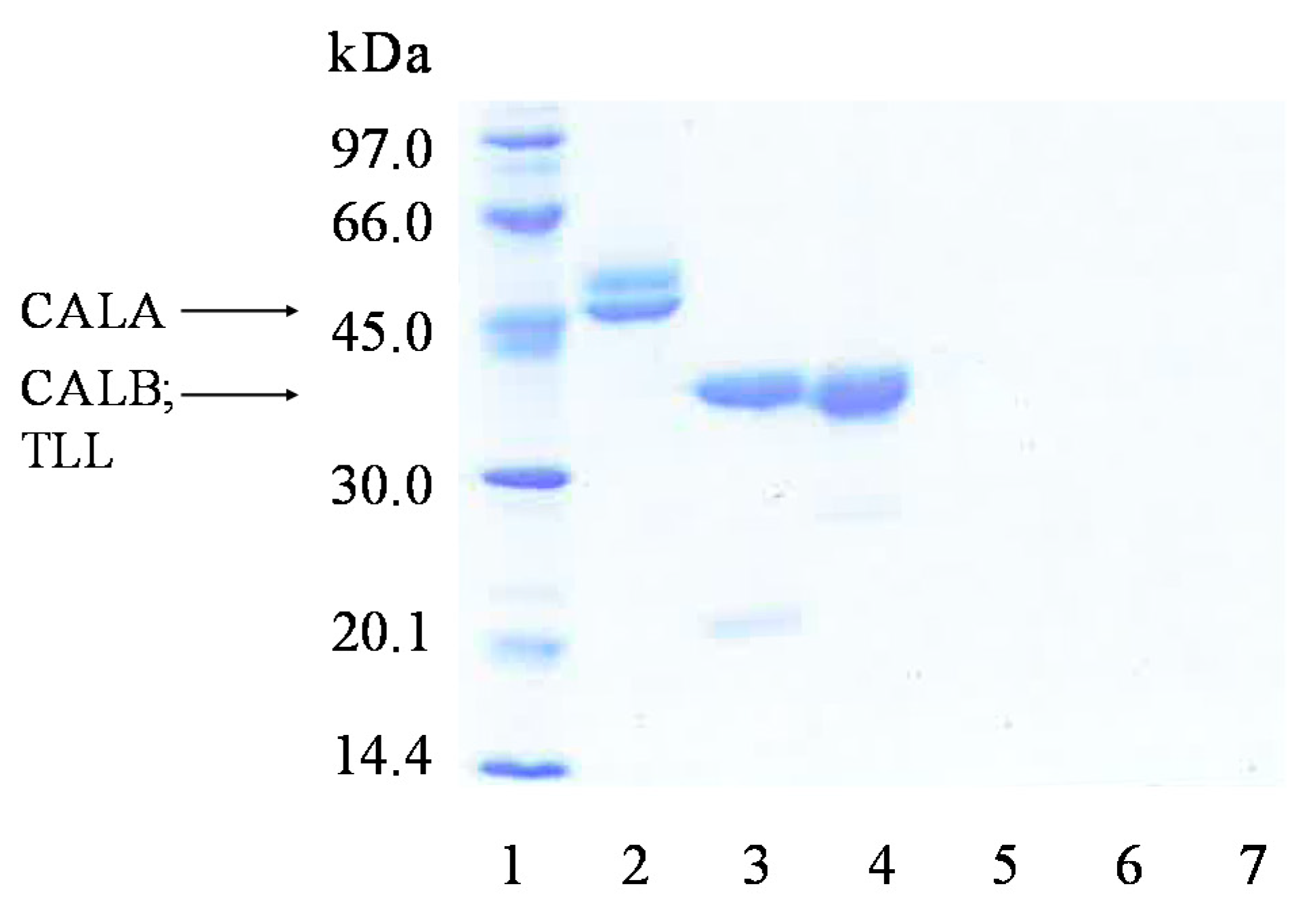
2.3. Covalent Immobilization of CALA, CALB and TLL on Octyl-VS
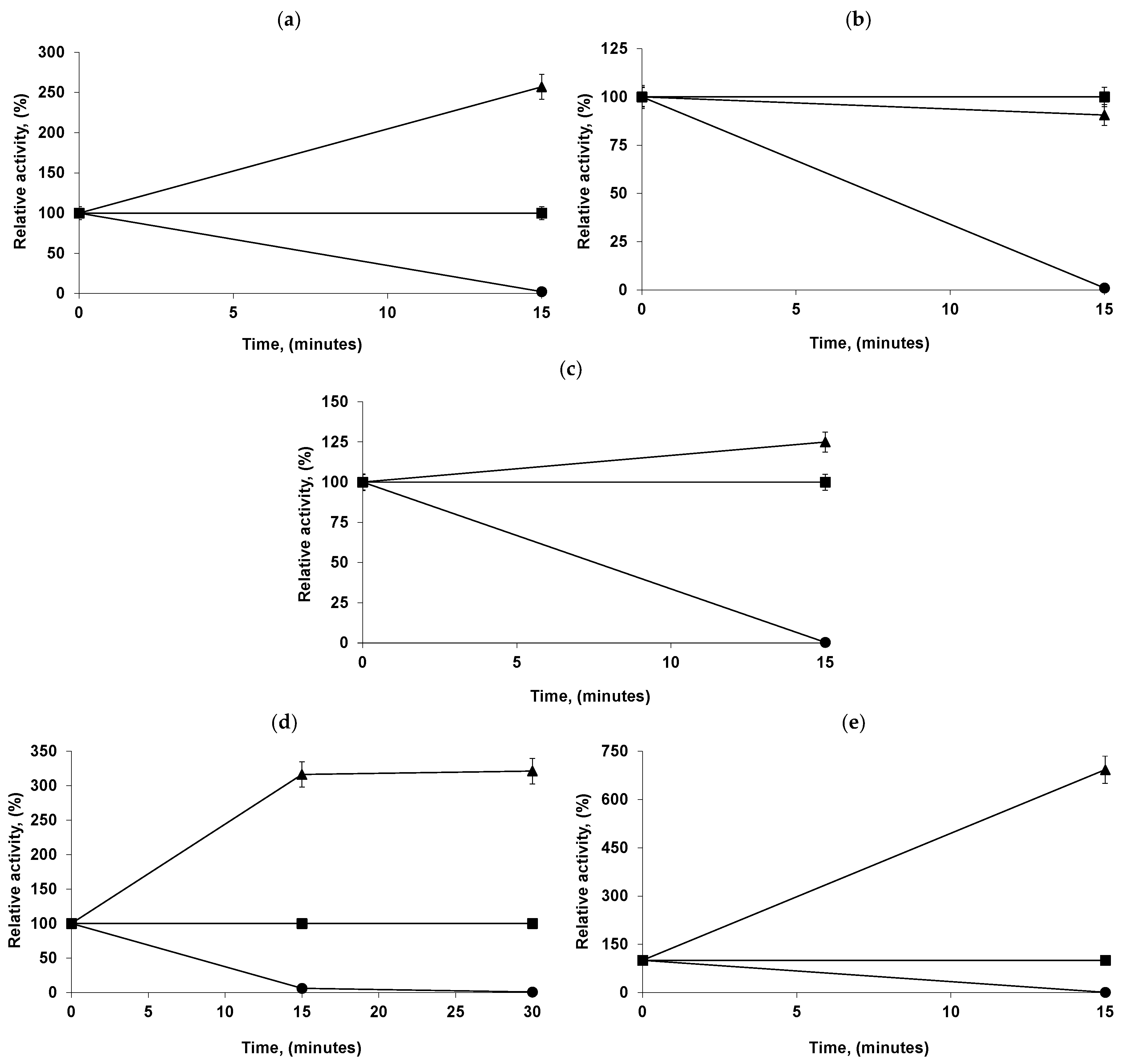
2.4. Immobilization and Desorption of LEU and RML from Octyl-VS-CALA-PEI
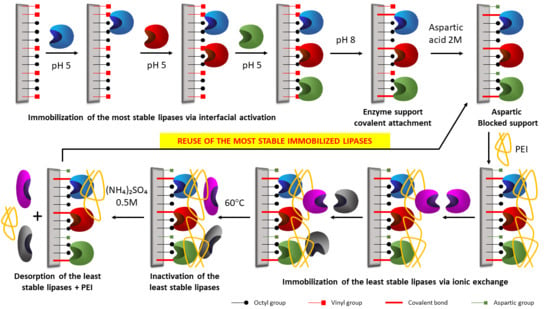
2.5. Building of the Combilipases
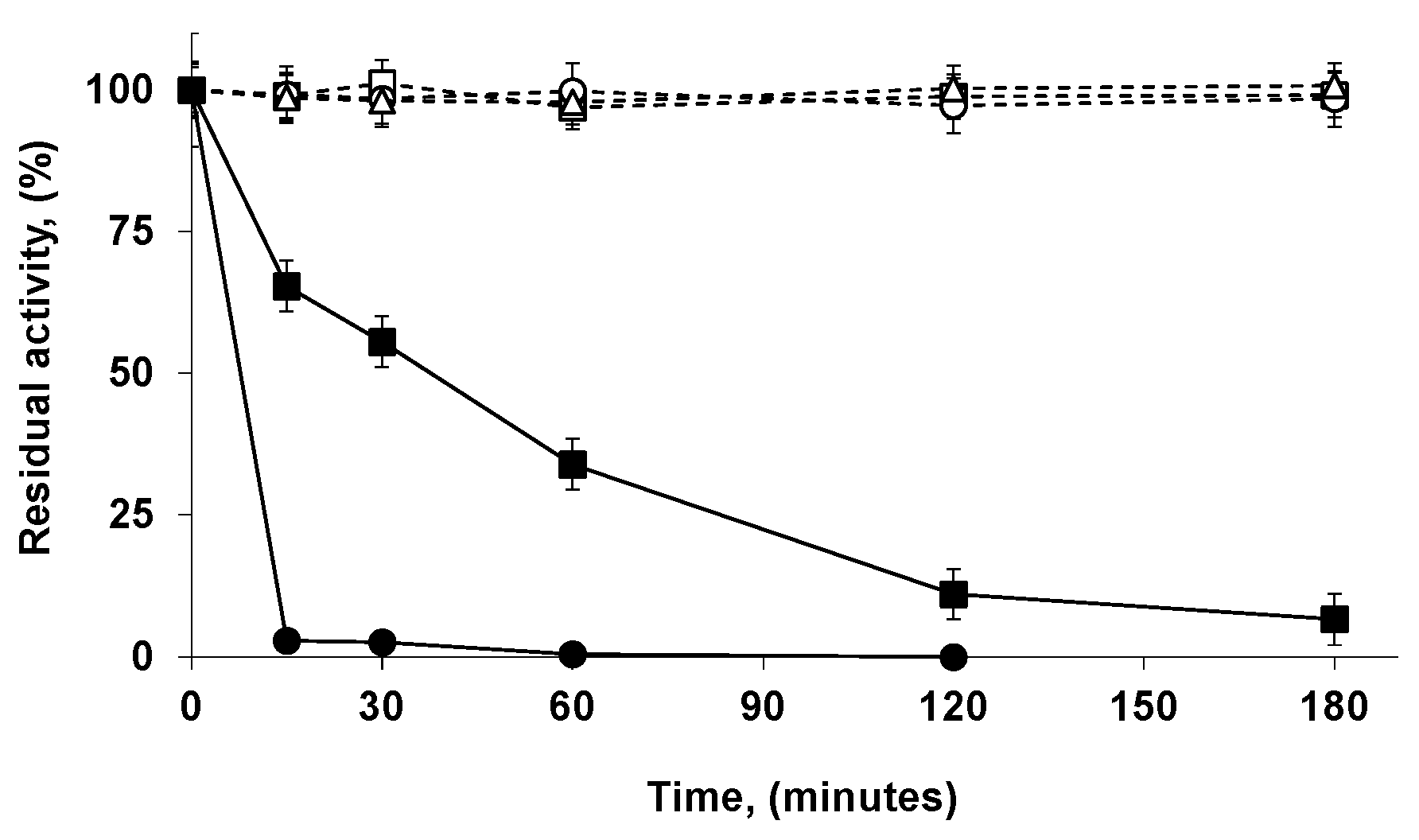
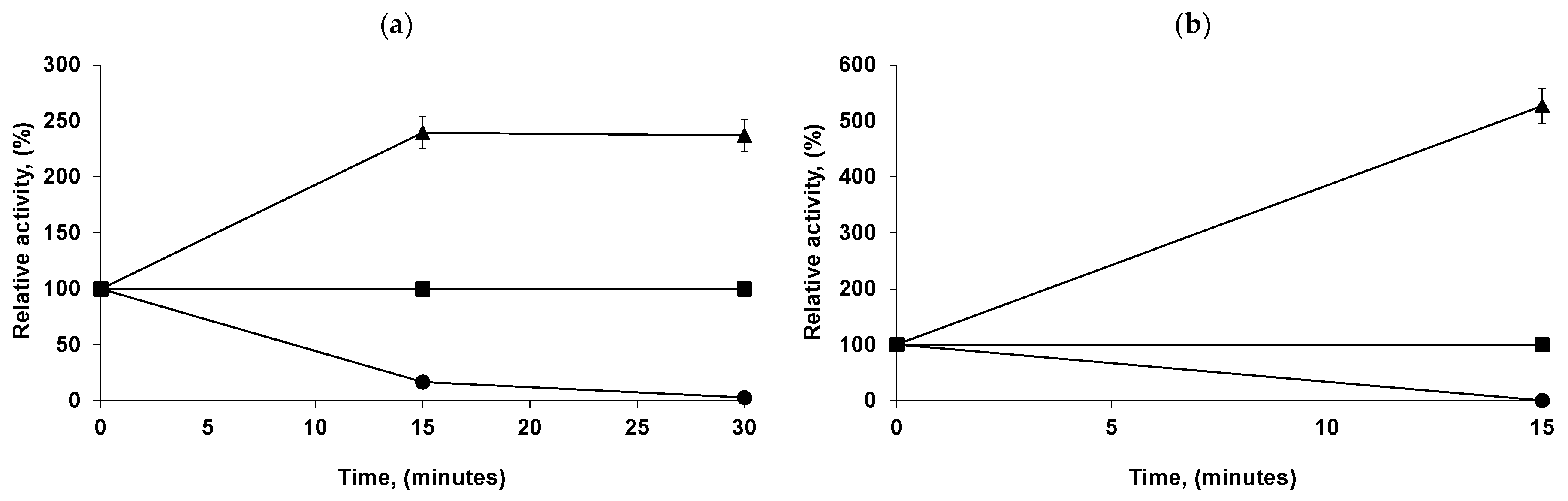
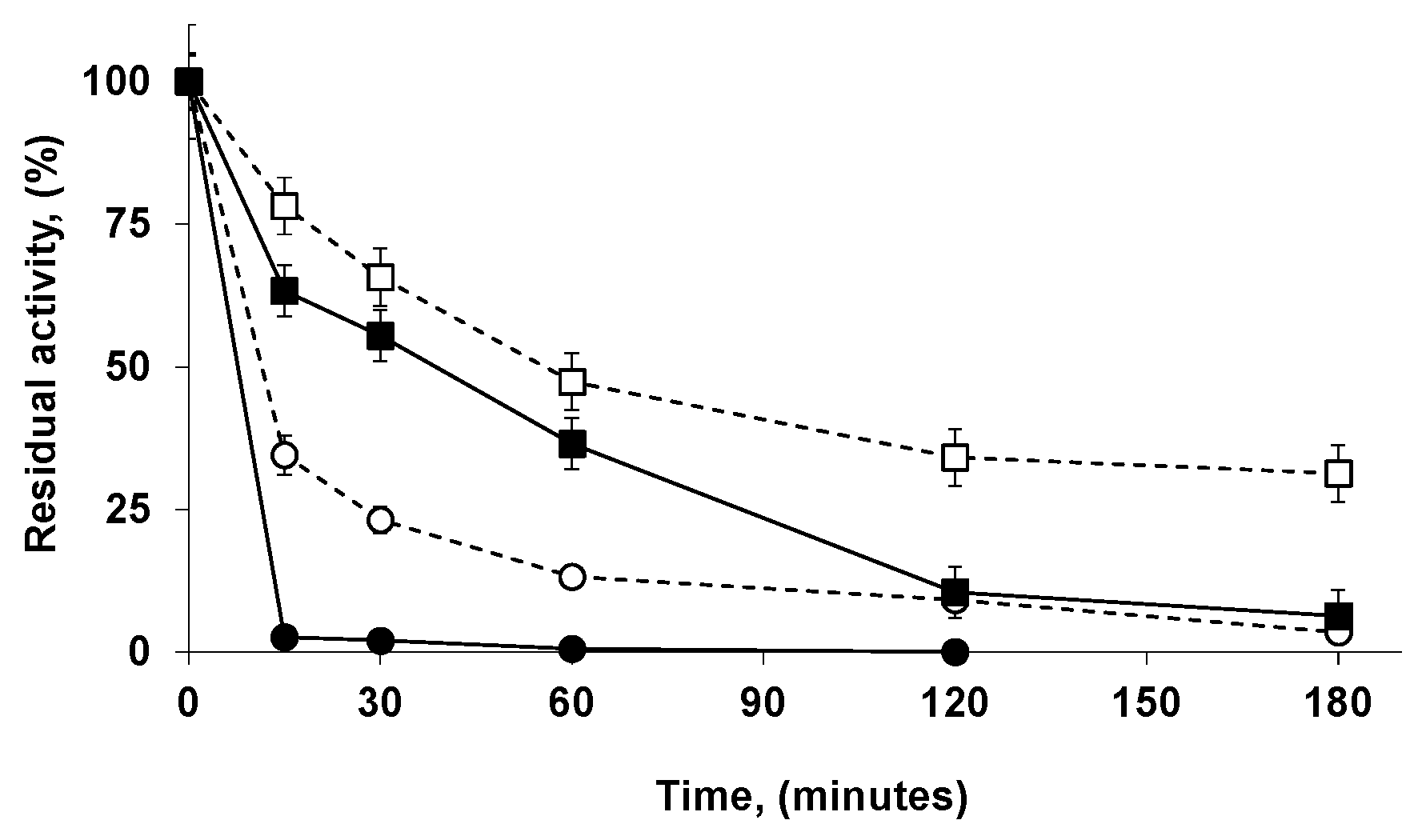
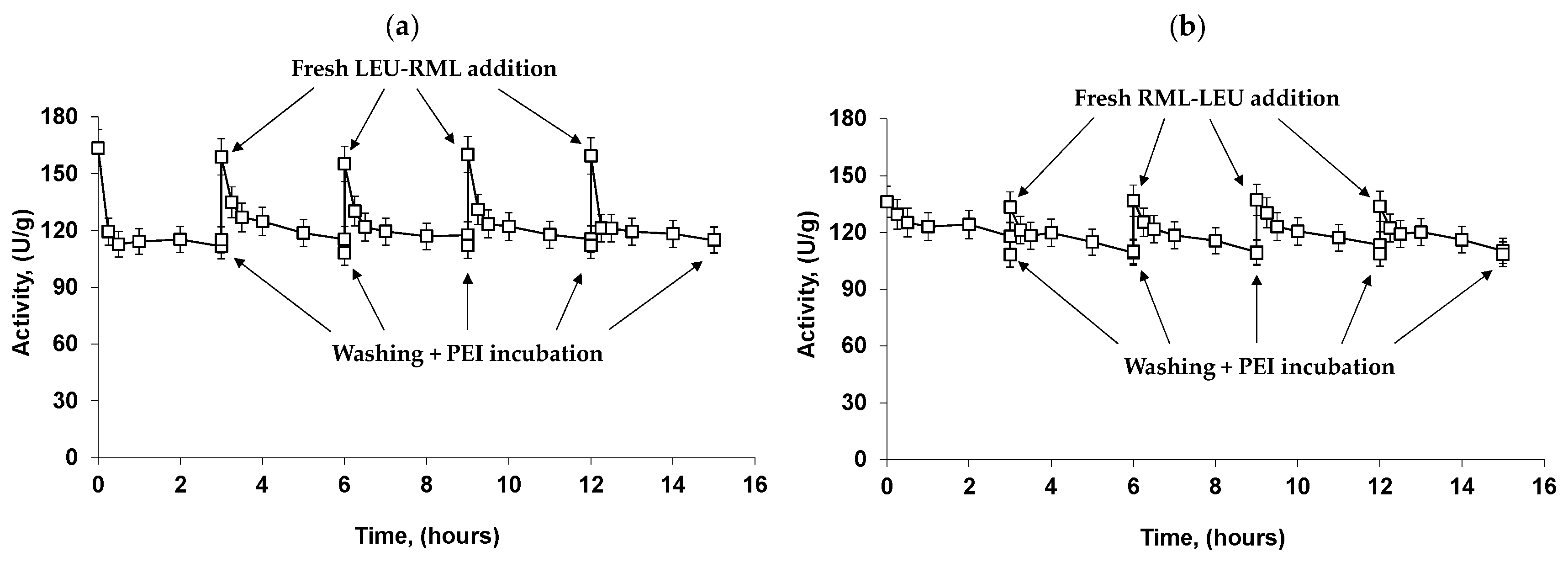
2.6. Reuse of the Most Stable Immobilized Enzymes after the Inactivation of the Least Stable Co-Immobilized Enzymes
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Octyl-Vinyl Sulfone Support (Octyl-VS)
3.2.2. Immobilization of Lipases on Octyl Agarose Beads
3.2.3. Immobilization of the Least Stable Enzymes on Octyl-VS-PEI Support
3.2.4. Co-Immobilized Biocatalysts Preparation
3.2.4.1. Immobilization of the Most Stable Lipases on Octyl-VS Support
3.2.4.2. Coating of Immobilized Enzymes with PEI
3.2.4.3. Immobilization of the Least Stable Lipases on Octyl-VS-Enzyme-PEI Biocatalysts
3.2.5. Determination of Enzyme Activity
3.2.5.1. Hydrolysis of p-NPB
3.2.5.2. Hydrolysis of Triacetin
3.2.5.3. Hydrolysis of (R)- or (S)-Methyl Mandelate
3.2.6. Lipase Biocatalysts Thermal Inactivations
3.2.7. Desorption of the Least Stable Lipases from the Supports
3.2.8. Analysis of the Immobilized Enzymes by SDS-PAGE
3.2.9. Titration of Primary Amino Groups in the Biocatalysts
3.2.10. Reuses of the Multi-Combilipases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasan, F.; Shah, A.; Javed, S.; Hameed, A. Enzymes used in detergents: Lipases. Afr. J. Biotechnol. 2010, 9, 4836–4844. [Google Scholar]
- Sharma, D.; Sharma, B.; Shukla, A. Biotechnological approach of microbial lipase: A review. Biotechnology 2011, 10, 23–40. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Kobayashi, S. Recent developments in lipase-catalyzed synthesis of polyesters. Macromol. Rapid Commun. 2009, 30, 237–266. [Google Scholar] [CrossRef]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Stead, D. Microbial lipases: Their characteristics, role in food spoilage and industrial uses. J. Dairy Res. 1986, 53, 481–505. [Google Scholar] [CrossRef] [PubMed]
- Yahya, A.; Anderson, W.; Moo-Young, M. Ester synthesis in lipase-catalyzed reactions. Enzym. Microb. Technol. 1998, 23, 438–450. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, S.S. Organic Solvent Tolerant Lipases and Applications. Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2018, 41, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; LeComte, J.; Villeneuve, P. Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Alvarez, E.; Rodriguez, J.; Villa, R.; Gomez, C.; Nieto, S.; Donaire, A.; Lozano, P. Clean enzymatic production of flavor esters in sponge-like ionic liquids. ACS Sustain. Chem. Eng. 2019, 7, 13307–13314. [Google Scholar] [CrossRef]
- Villa, R.; Alvarez, E.; Porcar, R.; García-Verdugo, E.; Luis, S.; Lozano, P. Ionic liquids as an enabling tool to integrate reaction and separation processes. Green Chem. 2019, 21, 6527–6544. [Google Scholar] [CrossRef]
- Lozano, P.; Alvarez, E.; Nieto, S.; Villa, R.; Bernal, J.M.; Donaire, A. Biocatalytic synthesis of panthenyl monoacyl esters in ionic liquids and deep eutectic solvents. Green Chem. 2019, 21, 3353–3361. [Google Scholar] [CrossRef]
- Porcar, R.; Lozano, P.; Burguete, M.I.; Garcia-Verdugo, E.; Luis, S.V. Dimethyl carbonate as a non-innocent benign solvent for the multistep continuous flow synthesis of amino alcohols. React. Chem. Eng. 2018, 3, 572–578. [Google Scholar] [CrossRef]
- De Diego, T.; Lozano, P.; Gmouh, S.; Vaultier, M.; Iborra, J.L. Understanding structure−stability relationships of Candida antartica lipase B in ionic liquids. Biomacromolecules 2005, 6, 1457–1464. [Google Scholar] [CrossRef]
- Lozano, P.; Puente, T.D.D.; Carrié, D.; Vaultier, M.; Iborra, J.L. Continuous green biocatalytic processes using ionic liquids and supercritical carbon dioxide. Chem. Commun. 2002, 692–693. [Google Scholar] [CrossRef]
- Tan, J.-N.; Dou, Y. Deep eutectic solvents for biocatalytic transformations: Focused lipase-catalyzed organic reactions. Appl. Microbiol. Biotechnol. 2020, 104, 1481–1496. [Google Scholar] [CrossRef]
- Dhake, K.P.; Thakare, D.D.; Bhanage, B.M. Lipase: A potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragr. J. 2013, 28, 71–83. [Google Scholar] [CrossRef]
- Ang, X.; Chen, H.; Xiang, J.-Q.; Wei, F.; Quek, S.Y. Preparation and functionality of lipase-catalysed structured phospholipid—A review. Trends Food Sci. Technol. 2019, 88, 373–383. [Google Scholar] [CrossRef]
- Utama, Q.D.; Sitanggang, A.B.; Adawiyah, D.R.; Hariyadi, P. lipase-catalyzed interesterification for the synthesis of medium-long-medium (MLM) structured lipids. Food Technol. Biotechnol. 2019, 57, 305–318. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Lipase-mediated chiral resolution of racemates in organic solvents. Tetrahedron: Asymmetry 2004, 15, 3331–3351. [Google Scholar] [CrossRef]
- Ghanem, A. Trends in lipase-catalyzed asymmetric access to enantiomerically pure/enriched compounds. Tetrahedron 2007, 63, 1721–1754. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Patel, N.; Rai, D.; Shivam; Shahane, S.; Mishra, U. Lipases: Sources, production, purification, and applications. Recent Patents Biotechnol. 2019, 13, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Melani, N.B.; Tambourgi, E.B.; Silveira, E. Lipases: From production to applications. Sep. Purif. Rev. 2019, 49, 143–158. [Google Scholar] [CrossRef]
- Nawal, G.; Irfan, M.; Ashfaq, S.; Shakir, H.A. An overview of bacterial lipases and their enormous applications. Punjab Univ. J. Zool. 2019, 34, 61–71. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial activation’ of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Aloulou, A.; Rodriguez, J.A.; Fernandez, S.; Van Oosterhout, D.; Puccinelli, D.; Carrière, F. Exploring the specific features of interfacial enzymology based on lipase studies. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2006, 1761, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nat. Cell Biol. 1991, 351, 491–494. [Google Scholar] [CrossRef]
- Van Tilbeurgh, H.; Egloff, M.-P.; Martinez, C.; Rugani, N.; Verger, R.; Cambillau, C. Interfacial activation of the lipase–procolipase complex by mixed micelles revealed by X-ray crystallography. Nat. Cell Biol. 1993, 362, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Derewenda, U.; Brzozowski, A.M.; Lawson, D.M.; Derewenda, Z.S. Catalysis at the interface: The anatomy of a conformational change in a triglyceride lipase. Biochemistry 1992, 31, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Suh, S.W. The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor. Structure 1997, 5, 173–185. [Google Scholar] [CrossRef]
- Derewenda, Z.S.; Derewenda, U.; Dodson, G.G. The crystal and molecular structure of the Rhizomucor miehei triacylglyceride lipase at 1.9 Å resolution. J. Mol. Biol. 1992, 227, 818–839. [Google Scholar] [CrossRef]
- Murty, V.R.; Bhat, J.; Muniswaran, P.K.A. Hydrolysis of oils by using immobilized lipase enzyme: A review. Biotechnol. Bioprocess Eng. 2002, 7, 57–66. [Google Scholar] [CrossRef]
- Mardani, M.; Farmani, J.; Kenari, R. Efficacy of some commercial lipases in hydrolysis of palm olein for production of free fatty acids and diacylglycerol oil. J. Oil Palm Res. 2015, 27, 250–260. [Google Scholar]
- Ferreira, M.M.; De Oliveira, G.F.; Basso, R.C.; Mendes, A.A.; Hirata, D.B. Optimization of free fatty acid production by enzymatic hydrolysis of vegetable oils using a non-commercial lipase from Geotrichum candidum. Bioprocess Biosyst. Eng. 2019, 42, 1647–1659. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J.K.; Matte, C.R.; Peralba, M.D.C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of ethyl ester production from olive and palm oils using mixtures of immobilized lipases. Appl. Catal. Gen. 2015, 490, 50–56. [Google Scholar] [CrossRef]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: A new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, N.H.; Lim, J.S.; Um, B.-H.; Park, C.; Kang, S.W.; Kim, S.W. Optimization of the process for biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. J. Microbiol. Biotechnol. 2008, 18, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Kim, J.M.; Shin, H.Y.; Kang, S.W.; Kim, S.W. Biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. Biotechnol. Bioprocess Eng. 2006, 11, 522–525. [Google Scholar] [CrossRef]
- Amoah, J.; Ho, S.-H.; Hama, S.; Yoshida, A.; Nakanishi, A.; Hasunuma, T.; Ogino, C.; Kondo, A. Lipase cocktail for efficient conversion of oils containing phospholipids to biodiesel. Bioresour. Technol. 2016, 211, 224–230. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernández-Lafuente, R. One pot use of combilipases for full modification of oils and fats: Multifunctional and heterogeneous substrates. Catalysts 2020, 10, 605. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, F.; Guan, W.; Zuo, J.; Feng, D. Optimisation of combi-lipases from Aspergillus niger for the synergistic and efficient hydrolysis of soybean oil. Anim. Sci. J. 2016, 88, 772–780. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A. transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; De Freitas, V.O.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Enzymatic synthesis of ethyl esters from waste oil using mixtures of lipases in a plug-flow packed-bed continuous reactor. Biotechnol. Prog. 2018, 34, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ayub, M.A. Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem. 2011, 46, 682–688. [Google Scholar] [CrossRef]
- Araki, C.A.; Marcucci, S.M.P.; Da Silva, L.S.; Maeda, C.H.; Arroyo, P.A.; Zanin, G.M. Effects of a combination of lipases immobilised on desilicated and thiol-modified ZSM-5 for the synthesis of ethyl esters from macauba pulp oil in a solvent-free system. Appl. Catal. A: Gen. 2018, 562, 241–249. [Google Scholar] [CrossRef]
- Pedro, K.; Parreira, J.; Correia, I.; Henriques, C.; Langone, M. Enzymatic biodiesel synthesis from acid oil using a lipase mixture. Química Nova 2017, 41, 284–291. [Google Scholar] [CrossRef]
- Karmee, S.K. Preparation of biodiesel from nonedible oils using a mixture of used lipases. Energy Sources Part Recovery Util. Environ. Eff. 2016, 38, 2727–2733. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Kang, S.W.; Song, Y.S.; Park, C.; Han, S.O.; Kim, S.W. Biodiesel production by a mixture of Candida rugosa and Rhizopus oryzae lipases using a supercritical carbon dioxide process. Bioresour. Technol. 2011, 102, 2105–2108. [Google Scholar] [CrossRef]
- Toro, E.; Rodriguez, D.; Morales, N.; García, L.M.; Godoy, C.A. novel combi-lipase systems for fatty acid ethyl esters production. Catalysts 2019, 9, 546. [Google Scholar] [CrossRef]
- Bin Ibrahim, N.A.; Guo, Z.; Xu, X. Enzymatic interesterification of palm stearin and coconut oil by a dual lipase system. J. Am. Oil Chem. Soc. 2007, 85, 37–45. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Park, C.; Han, S.O.; Kim, S.W. Kinetic modeling of biodiesel production by mixed immobilized and co-immobilized lipase systems under two pressure conditions. Korean J. Chem. Eng. 2013, 30, 1272–1276. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Suh, Y.J.; Kang, G.B.; Jang, W.I.; Kang, J.; Park, C.; Kim, S.W. Biodiesel production by enzymatic process using Jatropha oil and waste soybean oil. Biotechnol. Bioprocess Eng. 2013, 18, 703–708. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Han, S.O.; Park, C.; Kim, S.W. Co-immobilization of Candida rugosa and Rhyzopus oryzae lipases and biodiesel production. Korean J. Chem. Eng. 2013, 30, 1335–1338. [Google Scholar] [CrossRef]
- Shahedi, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; As’Habi, M.A. Co-immobilization of Rhizomucor miehei lipase and Candida antarctica lipase B and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renew. Energy 2019, 141, 847–857. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, D.S.; Yoo, H.Y.; Park, C.; Kim, S.W. Biodiesel production by lipases co-immobilized on the functionalized activated carbon. Bioresour. Technol. Rep. 2019, 7, 100248. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Biodiesel production (FAEEs) by heterogeneous combi-lipase biocatalysts using wet extracted lipids from microalgae. Catalysts 2019, 9, 296. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. in press. [CrossRef]
- López-Serrano, P.; Cao, L.; Van Rantwijk, F.; Sheldon, R. Cross-linked enzyme aggregates with enhanced activity: Application to lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]
- Mingarro, I.; Abad, C.; Braco, L. Interfacial activation-based molecular bioimprinting of lipolytic enzymes. Proc. Natl. Acad. Sci. USA 1995, 92, 3308–3312. [Google Scholar] [CrossRef]
- Moreno-Perez, S.; Ghattas, N.; Filice, M.; Guisán, J.M.; Fernández-Lorente, G. Dramatic hyperactivation of lipase of Thermomyces lanuginosa by a cationic surfactant: Fixation of the hyperactivated form by adsorption on sulfopropyl-sepharose. J. Mol. Catal. Enzym. 2015, 122, 199–203. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W. surfactant imprinting hyperactivated immobilized lipase as efficient biocatalyst for biodiesel production from waste cooking oil. Catalysts 2019, 9, 914. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Mateo, C.; Munilla, R.; Ortiz, C.; Cabrera, Z.; Guisán, A.J.M.; Fernandez-Lafuente, R. Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules 2006, 7, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Garcia-Galan, C.; Rodrigues, R.C.; De Sant’Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R.; Dos Santos, J.C. Stabilizing hyperactivated Lecitase structures through physical treatment with ionic polymers. Process Biochem. 2014, 49, 1511–1515. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; Dos Santos, J.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Rueda, N.; Torres, R.T.R.; Barbosa, O.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Evaluation of divinylsulfone activated agarose to immobilize lipases and to tune their catalytic properties. Process Biochem. 2015, 50, 918–927. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; López, C.C.O.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Suescun, A.; Rueda, N.; Dos Santos, J.C.; Castillo, J.J.; López, C.C.O.; Torres, R.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of lipases on glyoxyl–octyl supports: Improved stability and reactivation strategies. Process Biochem. 2015, 50, 1211–1217. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Rios, N.S.; Arana-Peña, S.; Mendez-Sanchez, C.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Reuse of lipase from Pseudomonas fluorescens via its step-by-step coimmobilization on glyoxyl-octyl agarose beads with least stable lipases. Catalysts 2019, 9, 487. [Google Scholar] [CrossRef]
- Manoel, E.A.; Dos Santos, J.C.; Freire, D.M.; Rueda, N.; Fernández-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef]
- Abian, O.; Wilson, L.; Mateo, C.; Fernandez-Lorente, G.; Palomo, J.; Fernández-Lafuente, R.; Guisán, J.M.; Re, D.; Tam, A.; Daminatti, M. Preparation of artificial hyper-hydrophilic micro-environments (polymeric salts) surrounding enzyme molecules. J. Mol. Catal. B: Enzym. 2002, 295–303. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Rocha-Martin, J.; Mateo, C.; Cava, F.; Berenguer, J.; Fernandez-Lafuente, R.; Guisan, J.M. Coating of soluble and immobilized enzymes with ionic polymers: Full stabilization of the quaternary structure of multimeric enzymes. Biomacromolecules 2009, 10, 742–747. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Virgen-Ortíz, J.J.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers. Process Biochem. 2017, 54, 81–88. [Google Scholar] [CrossRef]
- Pedroche, J.; Yust, M.D.M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme–support linkages and thermal stability. Enzym. Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Mateo, C.; Fernandes, B.; Van Rantwijk, F.; Stolz, A.; A Sheldon, R. Stabilisation of oxygen-labile nitrilases via co-aggregation with poly(ethyleneimine). J. Mol. Catal. Enzym. 2006, 38, 154–157. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzym. Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Virgen-Ortíz, J.J.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Optimization of the coating of octyl-CALB with ionic polymers to improve stability and decrease enzyme leakage. Biocatal. Biotransform. 2017, 36, 47–56. [Google Scholar] [CrossRef]
- Rios, N.S.; Arana-Peña, S.; Mendez-Sanchez, C.; Lokha, Y.; Cortes-Corberan, V.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Increasing the enzyme loading capacity of porous supports by a layer-by-layer immobilization strategy using PEI as glue. Catalysts 2019, 9, 576. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Coimmobilization of different lipases: Simple layer by layer enzyme spatial ordering. Int. J. Biol. Macromol. 2020, 145, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Use of polyethylenimine to produce immobilized lipase multilayers biocatalysts with very high volumetric activity using octyl-agarose beads: Avoiding enzyme release during multilayer production. Enzym. Microb. Technol. 2020, 137, 109535. [Google Scholar] [CrossRef]
- Albuquerque, T.L.; Rueda, N.; Dos Santos, J.C.; Barbosa, O.; López, C.C.O.; Binay, B.; Özdemir, E.; Gonçalves, L.R.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Zaak, H.; Kornecki, J.F.; Siar, E.-H.; Fernandez-Lopez, L.; Cortes Corberán, V.; Sassi, M.; Fernandez-Lafuente, R. Coimmobilization of enzymes in bilayers using PEI as a glue to reuse the most stable enzyme: Preventing PEI release during inactivated enzyme desorption. Process Biochem. 2017, 61, 95–101. [Google Scholar] [CrossRef]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One Biocatalyst–Many Applications: The use of Candida antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Da Rocha, T.N.; Dos Santos, J.C.; Alcántara, A.R.; Fernandez-Lafuente, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today. in press. [CrossRef]
- Gotor-Fernández, V.; Busto, E.; Gotor, V. Candida antarctica lipase B: An ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv. Synth. Catal. 2006, 348, 797–812. [Google Scholar] [CrossRef]
- De María, P.D.; Carboni-Oerlemans, C.; Tuin, B.; Bargeman, G.; Van Der Meer, A.; Van Gemert, R. Biotechnological applications of Candida antarctica lipase A: State-of-the-art. J. Mol. Catal. B: Enzym. 2005, 37, 36–46. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B: Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J. Mol. Catal. B: Enzym. 2010, 64, 1–22. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modification. J. Mol. Catal. B: Enzym. 2010, 66, 15–32. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Lecitase ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Garcia-Galan, C.; Rodrigues, R.C.; Ana, H.B.D.S.; Gonçalves, L.R.B.; Fernandez-Lafuente, R.; Dos Santos, J.C. Improving the catalytic properties of immobilized Lecitase via physical coating with ionic polymers. Enzym. Microb. Technol. 2014, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zaak, H.; Siar, E.-H.; Kornecki, J.F.; Fernandez-Lopez, L.; Pedrero, S.G.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of immobilization rate and enzyme crowding on enzyme stability under different conditions. The case of lipase from Thermomyces lanuginosus immobilized on octyl agarose beads. Process Biochem. 2017, 56, 117–123. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Z. Critical review of the impact of tortuosity on diffusion. Chem. Eng. Sci. 2007, 62, 3748–3755. [Google Scholar] [CrossRef]
- Pronk, S.; Lindahl, E.; Kasson, P.M. Dynamic heterogeneity controls diffusion and viscosity near biological interfaces. Nat. Commun. 2014, 5, 3034. [Google Scholar] [CrossRef]
- Regan, D.L.; Lilly, M.D.; Dunnill, P. Influence of intraparticle diffusional limitation on the observed kinetics of immobilized enzymes and on catalyst design. Biotechnol. Bioeng. 1974, 16, 1081–1093. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Consolati, T.; Mayr, T.; Nidetzky, B. Shine a light on immobilized enzymes: Real-time sensing in solid supported biocatalysts. Trends Biotechnol. 2013, 31, 194–203. [Google Scholar] [CrossRef]
- Boniello, C.; Mayr, T.; Klimant, I.; Koenig, B.; Riethorst, W.; Nidetzky, B. Intraparticle concentration gradients for substrate and acidic product in immobilized cephalosporin C amidase and their dependencies on carrier characteristics and reaction parameters. Biotechnol. Bioeng. 2010, 106, 528–540. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Rivas, B.D.L.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. rational co-immobilization of bi-enzyme cascades on porous supports and their applications in bio-redox reactions with in situ recycling of soluble cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fernandez-Lorente, G.; Mateo, C.; Ortiz, C.; Fernandez-Lafuente, R.; Guisán, J.M. Modulation of the enantioselectivity of lipases via controlled immobilization and medium engineering: Hydrolytic resolution of mandelic acid esters. Enzym. Microb. Technol. 2002, 31, 775–783. [Google Scholar] [CrossRef]
- Chaubey, A.; Parshad, R.; Koul, S.; Taneja, S.C.; Qazi, G.N. Enantioselectivity modulation through immobilization of Arthrobacter sp. lipase: Kinetic resolution of fluoxetine intermediate. J. Mol. Catal. B: Enzym. 2006, 42, 39–44. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Ashjari, M.; Mohammadi, M.; Badri, R. Chemical amination of Rhizopus oryzae lipase for multipoint covalent immobilization on epoxy-functionalized supports: Modulation of stability and selectivity. J. Mol. Catal. B: Enzym. 2015, 115, 128–134. [Google Scholar] [CrossRef]
- Lokha, Y.; Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Gonçalves, L.R.B.; López-Gallego, F.; Fernandez-Lafuente, R. Modulating the properties of the lipase from Thermomyces lanuginosus immobilized on octyl agarose beads by altering the immobilization conditions. Enzym. Microb. Technol. 2020, 133, 109461. [Google Scholar] [CrossRef]
- Silveira, E.A.; Moreno-Perez, S.; Basso, A.; Serban, S.; Pestana-Mamede, R.; Tardioli, P.W.; Farinas, C.S.; Castejon, N.; Fernandez-Lorente, G.; Rocha-Martin, J.; et al. Biocatalyst engineering of Thermomyces lanuginosus lipase adsorbed on hydrophobic supports: Modulation of enzyme properties for ethanolysis of oil in solvent-free systems. J. Biotechnol. 2019, 289, 126–134. [Google Scholar] [CrossRef]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized biocatalysts of Eversa® Transform 2.0 and lipase from Thermomyces lanuginosus: Comparison of some properties and performance in biodiesel production. Catalysts 2020, 10, 738. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Lombardo, D.; Guy, O. Effect of alcohols on the hydrolysis catalyzed by human pancreatic carboxylic-ester hydrolase. Biochim. Biophys. Acta (BBA)-Enzym. 1981, 657, 425–437. [Google Scholar] [CrossRef]
- Hernandez, K.; Porcar, R.; Porcar, R.; Fernandez-Lafuente, R. Hydrolysis of triacetin catalyzed by immobilized lipases: Effect of the immobilization protocol and experimental conditions on diacetin yield. Enzym. Microb. Technol. 2011, 48, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, J.F.; Carballares, D.; Morellon-Sterling, R.; Siar, E.H.; Kashefi, S.; Chafiaa, M.; Arana-Peña, S.; Rios, N.S.; Gonçalves, L.R.; Fernandez-Lafuente, R. Influence of phosphate anions on the stability of immobilized enzymes. Effect of enzyme nature, immobilization protocol and inactivation conditions. Process Biochem. 2020, 95, 288–296. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Velasco-Lozano, S.; Alcaraz-Fructuoso, M.T.; Sassi, M.; Lopez-Gallego, F.; Fernandez-Lafuente, R. Effect of high salt concentrations on the stability of immobilized lipases: Dramatic deleterious effects of phosphate anions. Process Biochem. 2017, 62, 128–134. [Google Scholar] [CrossRef]
- Laemmli, U.K. cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. Cell Biol. 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Trobo-Maseda, L.; Orrego, A.H.; Guisán, J.M.; Rocha-Martin, J. Coimmobilization and colocalization of a glycosyltransferase and a sucrose synthase greatly improves the recycling of UDP-glucose: Glycosylation of resveratrol 3-O-β-D-glucoside. Int. J. Biol. Macromol. 2020, 157, 510–521. [Google Scholar] [CrossRef]

| Ammonium Sulfate, (M) | PEI on the Support, (%) |
|---|---|
| 0.1 | 98 |
| 0.25 | 86 |
| 0.5 | 83 |
| 1 | 84 |
| 2 | 79 |
| 4 | 78 |
| Substrate | ||||
|---|---|---|---|---|
| Biocatalyst | p-NPB | Triacetin | (R)-Methyl Mandelate | (S)-Methyl Mandelate |
| Octyl-CALA | 102.67 ± 5.14 | 0.90 ± 0.04 | 0.255 ± 0.013 | 0.251 ± 0.013 |
| Octyl-VS-CALA | 106.00 ± 5.35 | 1.29 ± 0.07 | 0.092 ± 0.005 | 0.064 ± 0.003 |
| Octyl-CALB | 20.57 ± 1.03 | 8.60 ± 0.43 | 35.797 ± 1.790 | 4.680 ± 0.234 |
| Octyl-VS-CALB | 22.79 ± 1.15 | 8.16 ± 0.41 | 34.708 ± 1.738 | 4.215 ± 0.211 |
| Octyl-TLL | 148.31 ± 7.55 | 34.27 ± 1.72 | 0.041 ± 0.002 | 0.045 ± 0.002 |
| Octyl-VS-TLL | 114.16 ± 5.72 | 32.13 ± 1.71 | 0.016 ± 0.001 | 0.025 ± 0.001 |
| Octyl-LEU | 97.22 ± 4.82 | 6.47 ± 0.31 | 0.026 ± 0.001 | 0.041 ± 0.020 |
| Octyl-VS-PEI-LEU | 114.67 ± 5.75 | 20.97 ± 1.08 | 0.126 ± 0.006 | 0.135 ± 0.006 |
| Octyl-RML | 69.36 ± 3.52 | 21.49 ± 1.20 | 0.038 ± 0.002 | 0.038 ± 0.002 |
| Octyl-VS-PEI-RML | 65.68 ± 3.28 | 27.94 ± 1.35 | 0.292 ± 0.015 | 0.261 ± 0.013 |
| Substrate | ||||
|---|---|---|---|---|
| Biocatalyst | p-NPB | Triacetin | (R)-Methyl Mandelate | (S)-Methyl Mandelate |
| Octyl-VS-CALA | 108.82 ± 5.10 | 1.21 ± 0.06 | 0.094 ± 0.004 | 0.061 ± 0.003 |
| Octyl-VS-CALA-TLL | 136.37 ± 6.82 | 37.21 ± 1.86 | 0.109 ± 0.005 | 0.099 ± 0.005 |
| Octyl-VS-CALA-TLL-CALB | 134.49 ± 5.53 | 40.22 ± 1.90 | 31.447 ± 1.472 | 4.080 ± 0.215 |
| Octyl-VS-CALA-TLL-CALB-PEI | 138.36 ± 6.81 | 40.92 ± 2.12 | 39.901 ± 2.015 | 4.253 ± 0.223 |
| Octyl-VS-CALA-TLL-CALB-PEI-LEU | 153.64 ± 7.70 | 47.54 ± 2.23 | 36.643 ± 1.865 | 4.320 ± 0.224 |
| Octyl-VS-CALA-TLL-CALB-PEI-LEU-RML | 176.52 ± 8.83 | 60.74 ± 3.10 | 37.078 ± 1.855 | 4.602 ± 0.235 |
| Substrate | ||||
|---|---|---|---|---|
| Biocatalyst | p-NPB | Triacetin | (R)-Methyl Mandelate | (S)-Methyl Mandelate |
| Octyl-VS-CALB | 21.26 ± 1.08 | 7.88 ± 0.45 | 34.708 ± 1.738 | 4.104 ± 0.224 |
| Octyl-VS-CALB-TLL | 93.83 ± 4.72 | 34.36 ± 1.79 | 35.605 ± 1.753 | 3.928 ± 0.195 |
| Octyl-VS-CALB-TLL-CALA | 115.45 ± 5.83 | 34.09 ± 1.35 | 34.942 ± 1.497 | 3.909 ± 0.190 |
| Octyl-VS-CALB-TLL-CALA-PEI | 118.75 ± 5.95 | 30.75 ± 1.40 | 35.331 ± 1.698 | 4.144 ± 0.210 |
| Octyl-VS-CALB-TLL-CALA-PEI-RML | 126.23 ± 6.32 | 39.54 ± 2.89 | 33.978 ± 1.707 | 4.131 ± 0.205 |
| Octyl-VS-CALB-TLL-CALA-PEI-RML-LEU | 166.73 ± 8.50 | 56.46 ± 3.05 | 34.790 ± 2.005 | 4.310 ± 0.221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arana-Peña, S.; Carballares, D.; Cortés Corberan, V.; Fernandez-Lafuente, R. Multi-Combilipases: Co-Immobilizing Lipases with Very Different Stabilities Combining Immobilization via Interfacial Activation and Ion Exchange. The Reuse of the Most Stable Co-Immobilized Enzymes after Inactivation of the Least Stable Ones. Catalysts 2020, 10, 1207. https://doi.org/10.3390/catal10101207
Arana-Peña S, Carballares D, Cortés Corberan V, Fernandez-Lafuente R. Multi-Combilipases: Co-Immobilizing Lipases with Very Different Stabilities Combining Immobilization via Interfacial Activation and Ion Exchange. The Reuse of the Most Stable Co-Immobilized Enzymes after Inactivation of the Least Stable Ones. Catalysts. 2020; 10(10):1207. https://doi.org/10.3390/catal10101207
Chicago/Turabian StyleArana-Peña, Sara, Diego Carballares, Vicente Cortés Corberan, and Roberto Fernandez-Lafuente. 2020. "Multi-Combilipases: Co-Immobilizing Lipases with Very Different Stabilities Combining Immobilization via Interfacial Activation and Ion Exchange. The Reuse of the Most Stable Co-Immobilized Enzymes after Inactivation of the Least Stable Ones" Catalysts 10, no. 10: 1207. https://doi.org/10.3390/catal10101207
APA StyleArana-Peña, S., Carballares, D., Cortés Corberan, V., & Fernandez-Lafuente, R. (2020). Multi-Combilipases: Co-Immobilizing Lipases with Very Different Stabilities Combining Immobilization via Interfacial Activation and Ion Exchange. The Reuse of the Most Stable Co-Immobilized Enzymes after Inactivation of the Least Stable Ones. Catalysts, 10(10), 1207. https://doi.org/10.3390/catal10101207