Specialized Olefin Metathesis Catalysts Featuring Unsymmetrical N-Heterocyclic Carbene Ligands Bearing N-(Fluoren-9-yl) Arm
Abstract
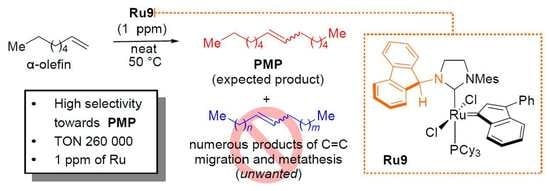
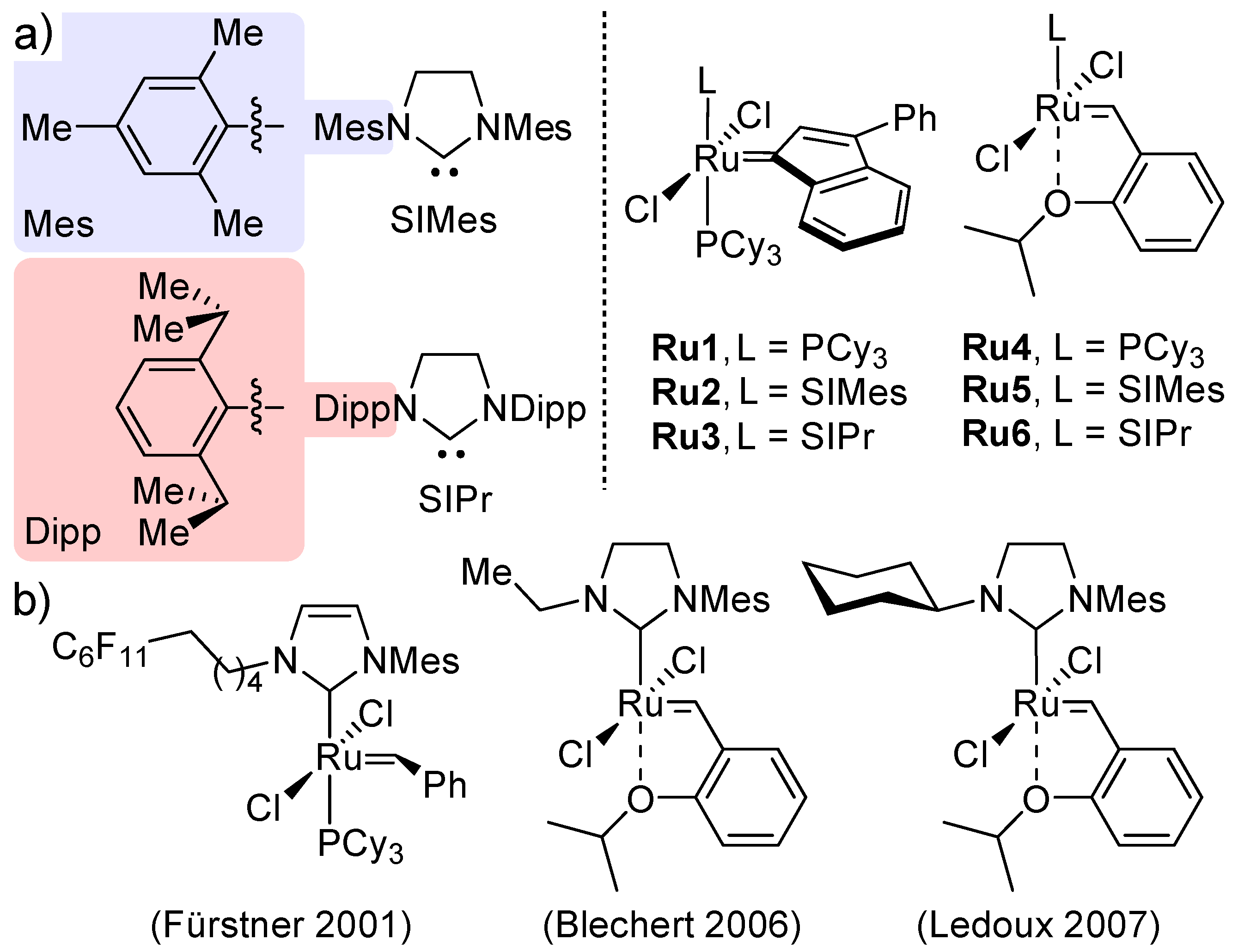
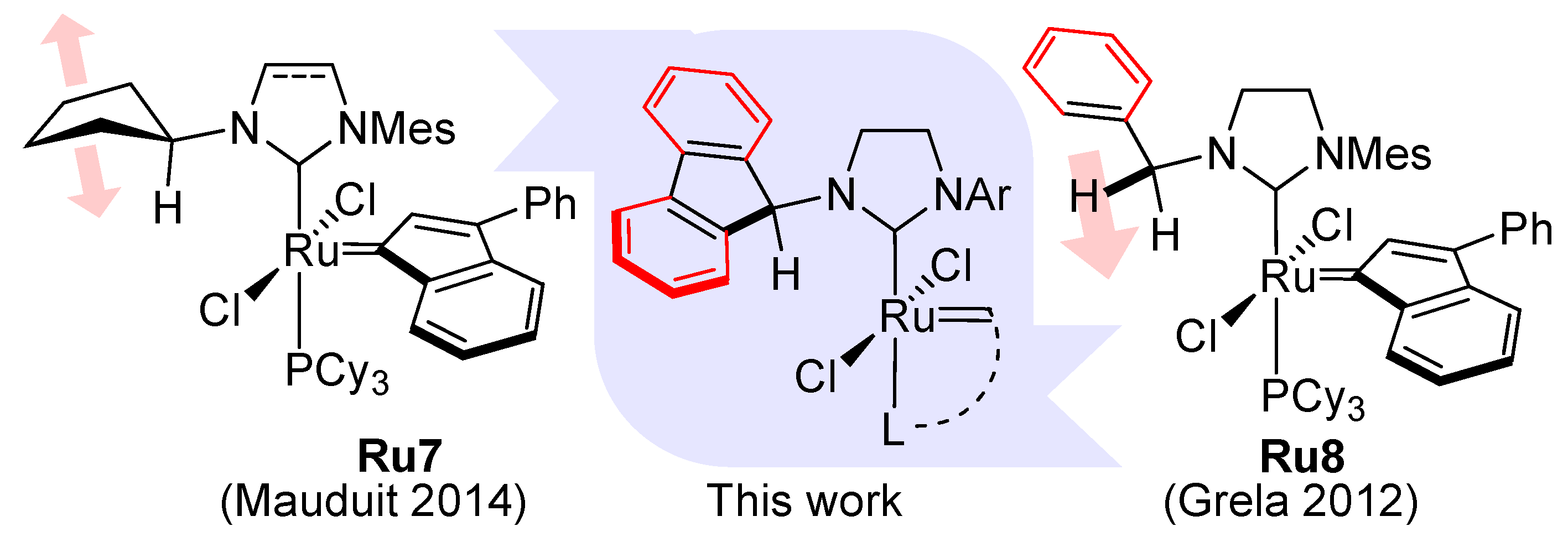
1. Introduction
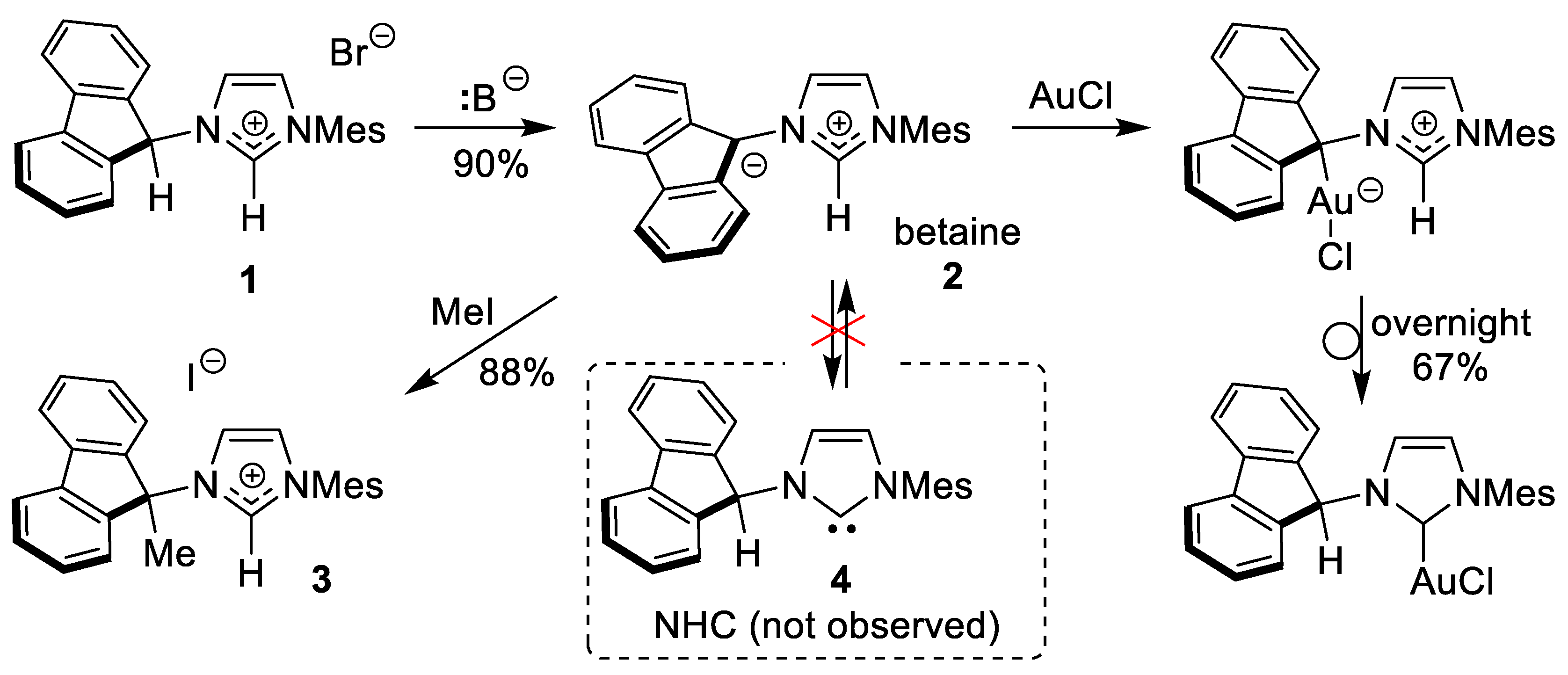
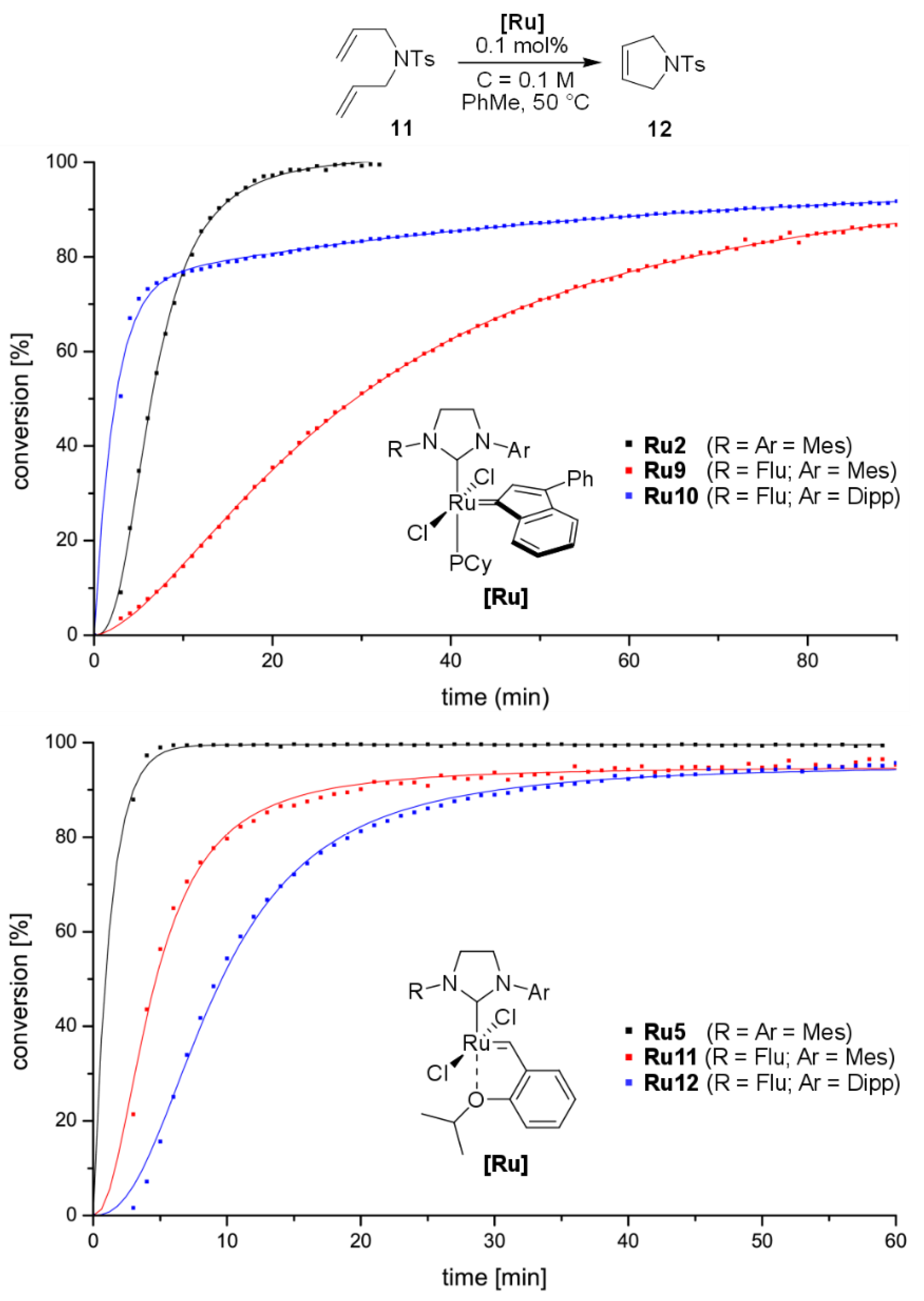
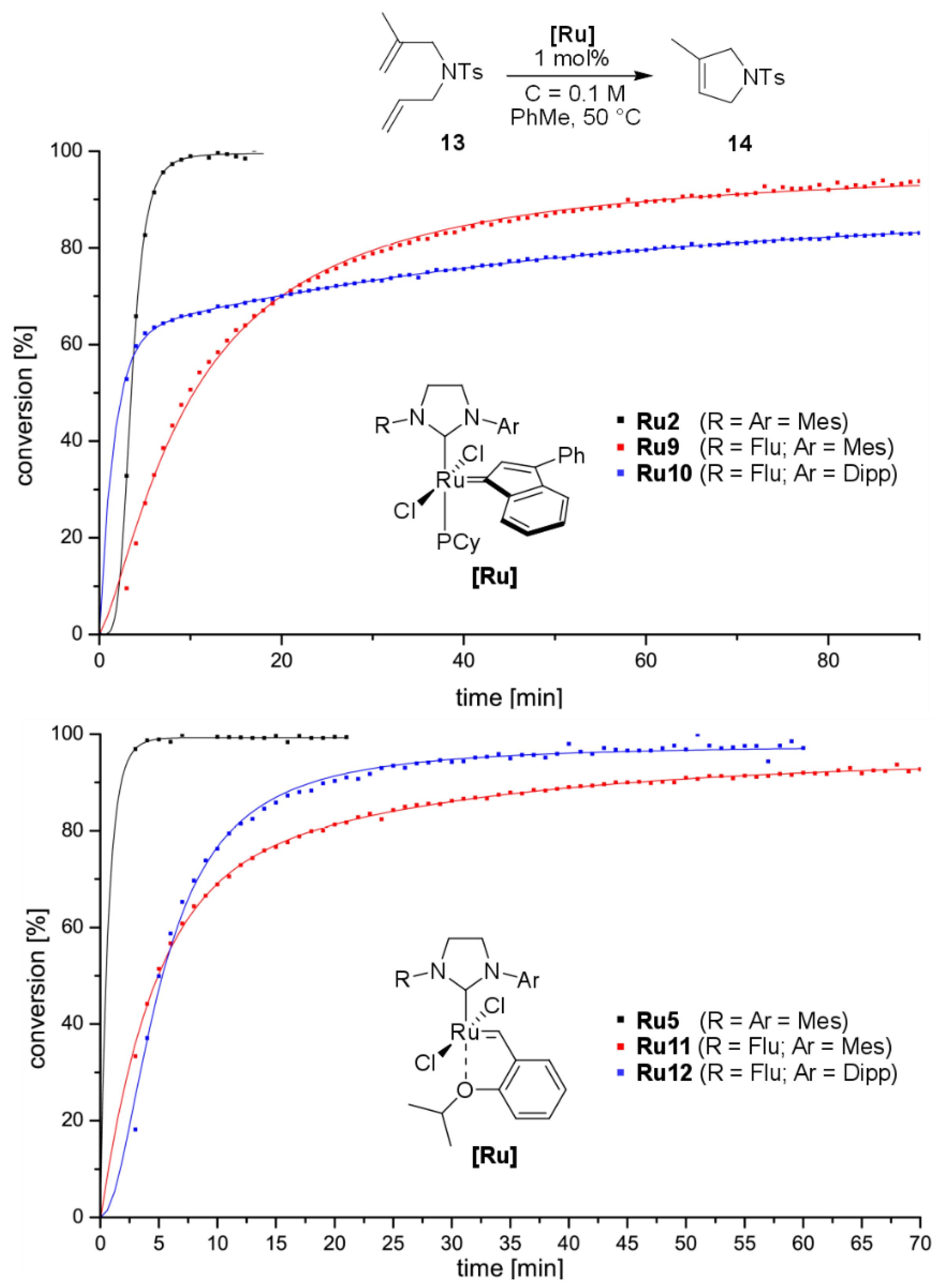
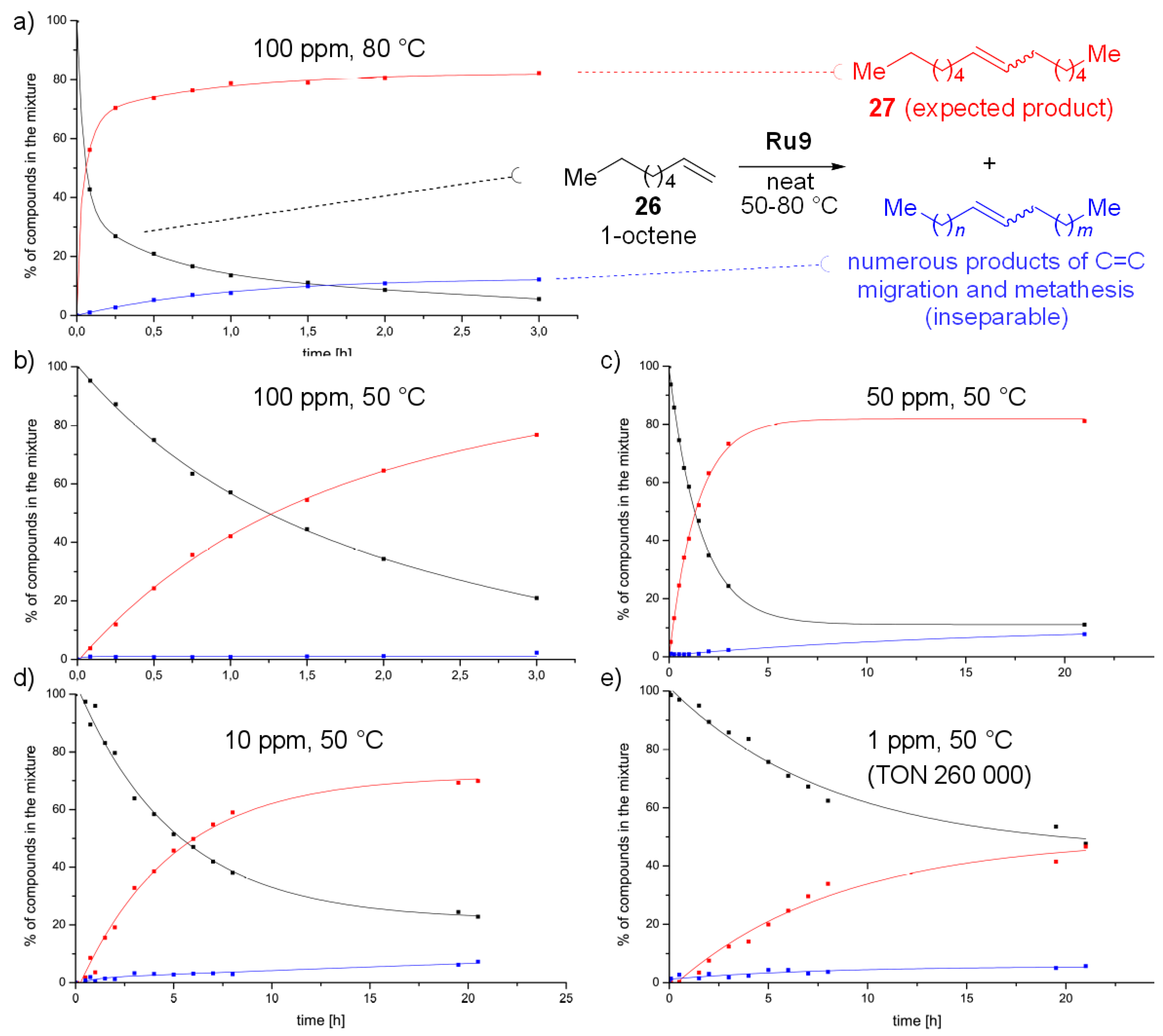
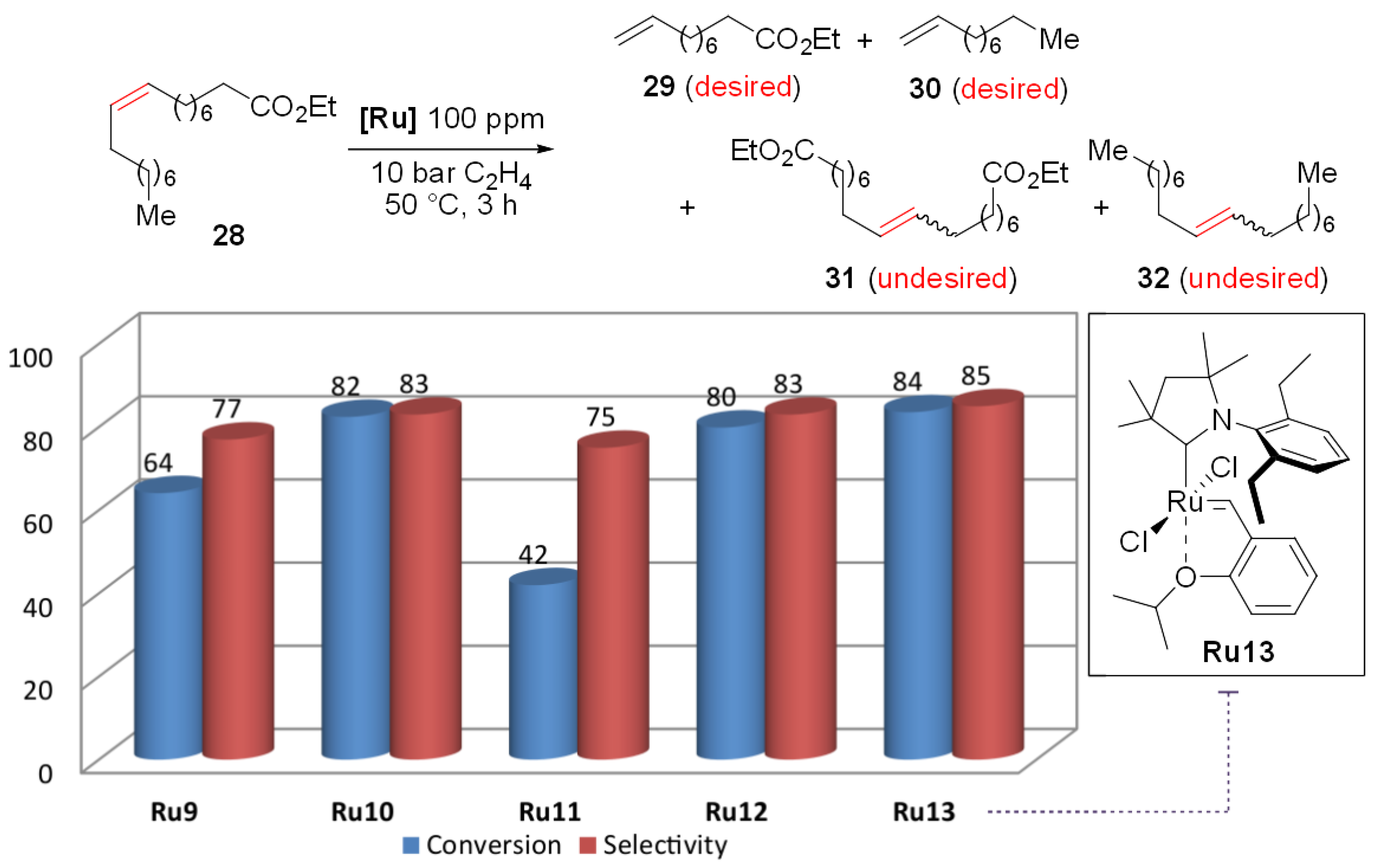
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of New Complexes
3.3. Catalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Grela, K. Olefin Metathesis: Theory and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- 12 Principles of Green Chemistry. Available online: https://www.acs.org/content/acs/en/greenchemistry/principles/12-principles-of-green-chemistry.html (accessed on 4 May 2020).
- Nguyen, S.T.; Johnson, L.K.; Grubbs, R.H.; Ziller, J.W. Ring-opening metathesis polymerization (ROMP) of norbornene by a group viii carbene complex in protic media. J. Am. Chem. Soc. 1992, 114, 3974–3975. [Google Scholar] [CrossRef]
- Piola, L.; Nahra, F.; Nolan, S.P. Olefin metathesis in air. Beilstein J. Org. Chem. 2015, 11, 2038–2056. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Stevens, E.D.; Nolan, S.P.; Petersen, J.L. Olefin metathesis-active ruthenium complexes bearing a nucleophilic carbene ligand. J. Am. Chem. Soc. 1999, 121, 2674–2678. [Google Scholar] [CrossRef]
- Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H. Increased ring closing metathesis activity of ruthenium-based olefin metathesis catalysts coordinated with imidazolin-2-ylidene ligands. Tetrahedron Lett. 1999, 40, 2247–2250. [Google Scholar] [CrossRef]
- Ackermann, L.; Fürstner, A.; Weskamp, T.; Kohl, F.J.; Herrmann, W.A. Ruthenium carbene complexes with imidazolin-2-ylidene ligands allow the formation of tetrasubstituted cycloalkenes by RCM. Tetrahedron Lett. 1999, 40, 4787–4790. [Google Scholar] [CrossRef]
- Paradiso, V.; Costabile, C.; Grisi, F. Ruthenium-based olefin metathesis catalysts with monodentate unsymmetrical NHC ligands. Beilstein J. Org. Chem. 2018, 14, 3122–3149. [Google Scholar] [CrossRef]
- Hamad, F.B.; Sun, T.; Xiao, S.; Verpoort, F. Olefin metathesis ruthenium catalysts bearing unsymmetrical heterocylic carbenes. Coord. Chem. Rev. 2013, 257, 2274–2292. [Google Scholar] [CrossRef]
- Fürstner, A.; Ackermann, L.; Gabor, B.; Goddard, R.; Lehmann, C.W.; Mynott, R.; Stelzer, F.; Thiel, O.R. Comparative investigation of ruthenium-based metathesis catalysts bearing N-heterocyclic carbene (NHC) ligands. Chemistry 2001, 7, 3236–3253. [Google Scholar] [CrossRef]
- Vehlow, K.; Gessler, S.; Blechert, S. Deactivation of ruthenium olefin metathesis catalysts through intramolecular carbene–arene bond formation. Angew. Chem. Int. Ed. 2007, 46, 8082–8085. [Google Scholar] [CrossRef]
- Ledoux, N.; Allaert, B.; Pattyn, S.; Mierde, H.V.; Vercaemst, C.; Verpoort, F. N,N′-dialkyl- and N-alkyl-N-mesityl-substituted n-heterocyclic carbenes as ligands in grubbs catalysts. Chemistry 2006, 12, 4654–4661. [Google Scholar] [CrossRef]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef] [PubMed]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2009, 110, 1746–1787. [Google Scholar] [CrossRef]
- Thomas, R.M.; Keitz, B.K.; Champagne, T.M.; Grubbs, R.H. Highly selective ruthenium metathesis catalysts for ethenolysis. J. Am. Chem. Soc. 2011, 133, 7490–7496. [Google Scholar] [CrossRef] [PubMed]
- Engl, P.S.; Fedorov, A.; Copéret, C.; Togni, A. N-trifluoromethyl NHC ligands provide selective ruthenium metathesis catalysts. Organometallics 2016, 35, 887–893. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Costabile, C.; Caruso, T.; Dąbrowski, M.; Grela, K.; Grisi, F. Expanding the family of Hoveyda–Grubbs catalysts containing unsymmetrical NHC ligands. Organometallics 2017, 36, 3692–3708. [Google Scholar] [CrossRef]
- Wyrębek, P.; Małecki, P.; Sytniczuk, A.; Kośnik, W.; Gawin, A.; Kostrzewa, J.; Kajetanowicz, A.; Grela, K. Looking for the noncyclic(amino)(alkyl)carbene ruthenium catalyst for ethenolysis of ethyl oleate: Selectivity is on target. ACS Omega 2018, 3, 18481–18488. [Google Scholar] [CrossRef]
- Thomas, R.M.; Fedorov, A.; Keitz, B.K.; Grubbs, R.H. Thermally stable, latent olefin metathesis catalysts. Organometallics 2011, 30, 6713–6717. [Google Scholar] [CrossRef]
- Rouen, M.; Queval, P.; Borré, E.; Falivene, L.; Poater, A.; Berthod, M.; Hugues, F.; Cavallo, L.; Baslé, O.; Olivier-Bourbigou, H.; et al. Selective metathesis of α-olefins from bio-sourced Fscher–Topsch feeds. ACS Catal. 2016, 6, 7970–7976. [Google Scholar] [CrossRef]
- Małecki, P.; Gajda, K.; Ablialimov, O.; Malińska, M.; Gajda, R.; Woźniak, K.; Kajetanowicz, A.; Grela, K. Hoveyda–Grubbs-type precatalysts with unsymmetrical n-heterocyclic carbenes as effective catalysts in olefin metathesis. Organometallics 2017, 36, 2153–2166. [Google Scholar] [CrossRef]
- Kavitake, S.; Samantaray, M.K.; Dehn, R.; Deuerlein, S.; Limbach, M.; Schachner, J.A.; Jeanneau, E.; Coperet, C.; Thieuleux, C. Unsymmetrical Ru-NHC catalysts: A key for the selective tandem ring opening-ring closing alkene metathesis (RO-RCM) of cyclooctene. Dalton Trans. 2011, 40, 12443–12446. [Google Scholar] [CrossRef]
- Małecki, P.; Gajda, K.; Gajda, R.; Woźniak, K.; Trzaskowski, B.; Kajetanowicz, A.; Grela, K. Specialized ruthenium olefin metathesis catalysts bearing bulky unsymmetrical nhc ligands: Computations, synthesis, and application. ACS Catal. 2019, 9, 587–598. [Google Scholar] [CrossRef]
- Ablialimov, O.; Kędziorek, M.; Malińska, M.; Woźniak, K.; Grela, K. Synthesis, structure, and catalytic activity of new ruthenium(II) indenylidene complexes bearing unsymmetrical N-heterocyclic carbenes. Organometallics 2014, 33, 2160–2171. [Google Scholar] [CrossRef]
- Smoleń, M.; Kośnik, W.; Gajda, R.; Woźniak, K.; Skoczeń, A.; Kajetanowicz, A.; Grela, K. Ruthenium complexes bearing thiophene-based unsymmetrical N-heterocyclic carbene ligands as selective catalysts for olefin metathesis in toluene and environmentally friendly 2-methyltetrahydrofuran. Chemistry 2018, 24, 15372–15379. [Google Scholar] [CrossRef] [PubMed]
- Sytniczuk, A.; Dąbrowski, M.; Banach, Ł.; Urban, M.; Czarnocka-Śniadała, S.; Milewski, M.; Kajetanowicz, A.; Grela, K. At long last: Olefin metathesis macrocyclization at high concentration. J. Am. Chem. Soc. 2018, 140, 8895–8901. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Wenzel, A.G.; Salguero, T.T.; Day, M.W.; Grubbs, R.H. Decomposition of ruthenium olefin metathesis catalysts. J. Am. Chem. Soc. 2007, 129, 7961–7968. [Google Scholar] [CrossRef]
- Higman, C.S.; Plais, L.; Fogg, D.E. Isomerization during olefin metathesis: An assessment of potential catalyst culprits. ChemCatChem 2013, 5, 3548–3551. [Google Scholar] [CrossRef]
- Higman, C.S.; Lanterna, A.E.; Marin, M.L.; Scaiano, J.C.; Fogg, D.E. Catalyst decomposition during olefin metathesis yields isomerization-active ruthenium nanoparticles. ChemCatChem 2016, 8, 2446–2449. [Google Scholar] [CrossRef]
- Ablialimov, O.; Kędziorek, M.; Torborg, C.; Malińska, M.; Woźniak, K.; Grela, K. New ruthenium(II) indenylidene complexes bearing unsymmetrical N-heterocyclic carbenes. Organometallics 2012, 31, 7316–7319. [Google Scholar] [CrossRef]
- Rouen, M.; Borre, E.; Falivene, L.; Toupet, L.; Berthod, M.; Cavallo, L.; Olivier-Bourbigou, H.; Mauduit, M. Cycloalkyl-based unsymmetrical unsaturated (U2)-NHC ligands: Flexibility and dissymmetry in ruthenium-catalysed olefin metathesis. Dalton Trans. 2014, 43, 7044–7049. [Google Scholar] [CrossRef]
- For an alternative approach where a sterically reduced NHC ligand, bearing a CF3 wing gave a very selective Ru catalyst, see ref [17].
- Benhamou, L.; Bastin, S.; Lugan, N.; Lavigne, G.; César, V. Metal-assisted conversion of an N-ylide mesomeric betaine into its carbenic tautomer: Generation of N-(fluoren-9-yl)imidazol-2-ylidene complexes. Dalton Trans. 2014, 43, 4474–4482. [Google Scholar] [CrossRef]
- Jolly, P.I.; Marczyk, A.; Małecki, P.; Ablialimov, O.; Trzybiński, D.; Woźniak, K.; Osella, S.; Trzaskowski, B.; Grela, K. Azoliniums, adducts, NHCs and azomethine ylides: Divergence in Wanzlick equilibrium and olefin metathesis catalyst formation. Chemistry 2018, 24, 4785–4789. [Google Scholar] [CrossRef]
- Blum, A.P.; Ritter, T.; Grubbs, R.H. Synthesis of N-heterocylic carbene-containing metal complexes from 2-(pentafluorophenyl)imidazolidines. Organometallics 2007, 26, 2122–2124. [Google Scholar] [CrossRef]
- Nyce, G.W.; Csihony, S.; Waymouth, R.M.; Hedrick, J.L. A general and versatile approach to thermally generated n-heterocyclic carbenes. Chemistry 2004, 10, 4073–4079. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Barbasiewicz, M.; Bieniek, M.; Michrowska, A.; Szadkowska, A.; Makal, A.; Woźniak, K.; Grela, K. Probing of the ligand anatomy: Effects of the chelating alkoxy ligand modifications on the structure and catalytic activity of ruthenium carbene complexes. Adv. Synth. Catal. 2007, 349, 193–203. [Google Scholar] [CrossRef]
- Ledoux, N.; Linden, A.; Allaert, B.; Mierde, H.V.; Verpoort, F. Comparative investigation of Hoveyda–Grubbs catalysts bearing modified N-heterocyclic carbene ligands. Adv. Synth. Catal. 2007, 349, 1692–1700. [Google Scholar] [CrossRef]
- Endo, K.; Herbert, M.B.; Grubbs, R.H. Investigations into ruthenium metathesis catalysts with six-membered chelating NHC ligands: Relationship between catalyst structure and stereoselectivity. Organometallics 2013, 32, 5128–5135. [Google Scholar] [CrossRef]
- Lehman, S.E.; Wagener, K.B. Synthesis of ruthenium olefin metathesis catalysts with linear alkyl carbene complexes. Organometallics 2005, 24, 1477–1482. [Google Scholar] [CrossRef]
- Samojłowicz, C.; Bieniek, M.; Pazio, A.; Makal, A.; Woźniak, K.; Poater, A.; Cavallo, L.; Wójcik, J.; Zdanowski, K.; Grela, K. The doping effect of fluorinated aromatic solvents on the rate of ruthenium-catalysed olefin metathesis. Chemistry 2011, 17, 12981–12993. [Google Scholar] [CrossRef]
- Clavier, H.; Urbina-Blanco, C.A.; Nolan, S.P. Indenylidene ruthenium complex bearing a sterically demanding NHC ligand: An efficient catalyst for olefin metathesis at room temperature. Organometallics 2009, 28, 2848–2854. [Google Scholar] [CrossRef]
- Yu, B.; Hamad, F.B.; Sels, B.; Van Hecke, K.; Verpoort, F. Ruthenium indenylidene complexes bearing N-alkyl/N-mesityl-substituted N-heterocyclic carbene ligands. Dalton Trans. 2015, 44, 11835–11842. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, Y.; He, X.; Hamad, F.B.; Ding, F.; Van Hecke, K.; Yusubov, M.S.; Verpoort, F. SIMes/PCy3 mixed ligand-coordinated alkyl group-tagged ruthenium indenylidene complexes: Synthesis, characterization and metathesis activity. Appl. Organomet. Chem. 2018, 32, e4548. [Google Scholar] [CrossRef]
- Vehlow, K.; Maechling, S.; Blechert, S. Ruthenium metathesis catalysts with saturated unsymmetrical N-heterocyclic carbene ligands. Organometallics 2006, 25, 25–28. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Wyrębek, P.; Trzybiński, D.; Woźniak, K.; Grela, K. In a quest for selectivity paired with activity: A ruthenium olefin metathesis catalyst bearing an unsymmetrical phenanthrene-based N-heterocyclic carbene. Chemistry 2020, 26, 3782–3794. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, T.K.; Bieniek, M.; Skowerski, K.; Grela, K. A new tool in the toolbox: Electron-withdrawing group activated ruthenium catalysts for olefin metathesis. Synlett 2013, 24, 903–919. [Google Scholar] [CrossRef]
- Schmid, T.E.; Dumas, A.; Colombel-Rouen, S.; Crévisy, C.; Baslé, O.; Mauduit, M. From environmentally friendly reusable ionic-tagged ruthenium-based complexes to industrially relevant homogeneous catalysts: Toward a sustainable olefin metathesis. Synlett 2017, 28, 773–798. [Google Scholar]
- Smoleń, M.; Marczyk, A.; Kośnik, W.; Trzaskowski, B.; Kajetanowicz, A.; Grela, K. Ruthenium-catalysed olefin metathesis in environmentally friendly solvents: 2-methyltetrahydrofuran revisited. Eur. J. Org. Chem. 2019, 2019, 640–646. [Google Scholar] [CrossRef]
- Du Toit, J.I.; van der Gryp, P.; Loock, M.M.; Tole, T.T.; Marx, S.; Jordaan, J.H.L.; Vosloo, H.C.M. Industrial viability of homogeneous olefin metathesis: Beneficiation of linear alpha olefins with the diphenyl-substituted pyridinyl alcoholato ruthenium carbene precatalyst. Catal. Today 2016, 275, 191–200. [Google Scholar] [CrossRef]
- Du Toit, J.I.; Jordaan, M.; Huijsmans, C.A.A.; Jordaan, J.H.L.; Van Sittert, C.G.C.E.; Vosloo, H.C.M. Improved metathesis lifetime: Chelating pyridinyl-alcoholato ligands in the second generation Grubbs precatalyst. Molecules 2014, 19, 5522–5537. [Google Scholar] [CrossRef]
- Interestingly, the amount of isomerization products did not seem to decrease with the decrease in the ruthenium concentration. This results seems interesting in the light of recent publication of Fogg [See: Ref. [56]].
- Nascimento, D.L.; Fogg, D.E. Origin of the breakthrough productivity of ruthenium–cyclic alkyl amino carbene catalysts in olefin metathesis. J. Am. Chem. Soc. 2019, 141, 19236–19240. [Google Scholar] [CrossRef]
- Bailey, G.A.; Foscato, M.; Higman, C.S.; Day, C.S.; Jensen, V.R.; Fogg, D.E. Bimolecular coupling as a vector for decomposition of fast-initiating olefin metathesis catalysts. J. Am. Chem. Soc. 2018, 140, 6931–6944. [Google Scholar] [CrossRef]
- Bidange, J.; Dubois, J.-L.; Couturier, J.-L.; Fischmeister, C.; Bruneau, C. Ruthenium catalyzed ethenolysis of renewable oleonitrile. Eur. J. Lipid Sci. Technol. 2014, 116, 1583–1589. [Google Scholar] [CrossRef]
- Alexander, K.A.; Paulhus, E.A.; Lazarus, G.M.L.; Leadbeater, N.E. Exploring the reactivity of a ruthenium complex in the metathesis of biorenewable feedstocks to generate value-added chemicals. J. Organomet. Chem. 2016, 812, 74–80. [Google Scholar] [CrossRef]
- Bidange, J.; Fischmeister, C.; Bruneau, C. Ethenolysis: A green catalytic tool to cleave carbon–carbon double bonds. Chemistry 2016, 22, 12226–12244. [Google Scholar] [CrossRef]
- Marx, V.M.; Sullivan, A.H.; Melaimi, M.; Virgil, S.C.; Keitz, B.K.; Weinberger, D.S.; Bertrand, G.; Grubbs, R.H. Cyclic alkyl amino carbene (CAAC) ruthenium complexes as remarkably active catalysts for ethenolysis. Angew. Chem. Int. Ed. 2015, 54, 1919–1923. [Google Scholar] [CrossRef]
- Gawin, R.; Kozakiewicz, A.; Guńka, P.A.; Dąbrowski, P.; Skowerski, K. Bis(cyclic alkyl amino carbene) ruthenium complexes: A versatile, highly efficient tool for olefin metathesis. Angew. Chem. Int. Ed. 2017, 56, 981–986. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Chwalba, M.; Gawin, A.; Tracz, A.; Grela, K. Non-glovebox ethenolysis of ethyl oleate and fame at larger scale utilizing a cyclic (alkyl)(amino)carbene ruthenium catalyst. Eur. J. Lipid Sci. Technol. 2020, 122, 1900263. [Google Scholar] [CrossRef]
- Engl, P.S.; Santiago, C.B.; Gordon, C.P.; Liao, W.-C.; Fedorov, A.; Copéret, C.; Sigman, M.S.; Togni, A. Exploiting and understanding the selectivity of Ru-N-heterocyclic carbene metathesis catalysts for the ethenolysis of cyclic olefins to α,ω-dienes. J. Am. Chem. Soc. 2017, 139, 13117–13125. [Google Scholar] [CrossRef]
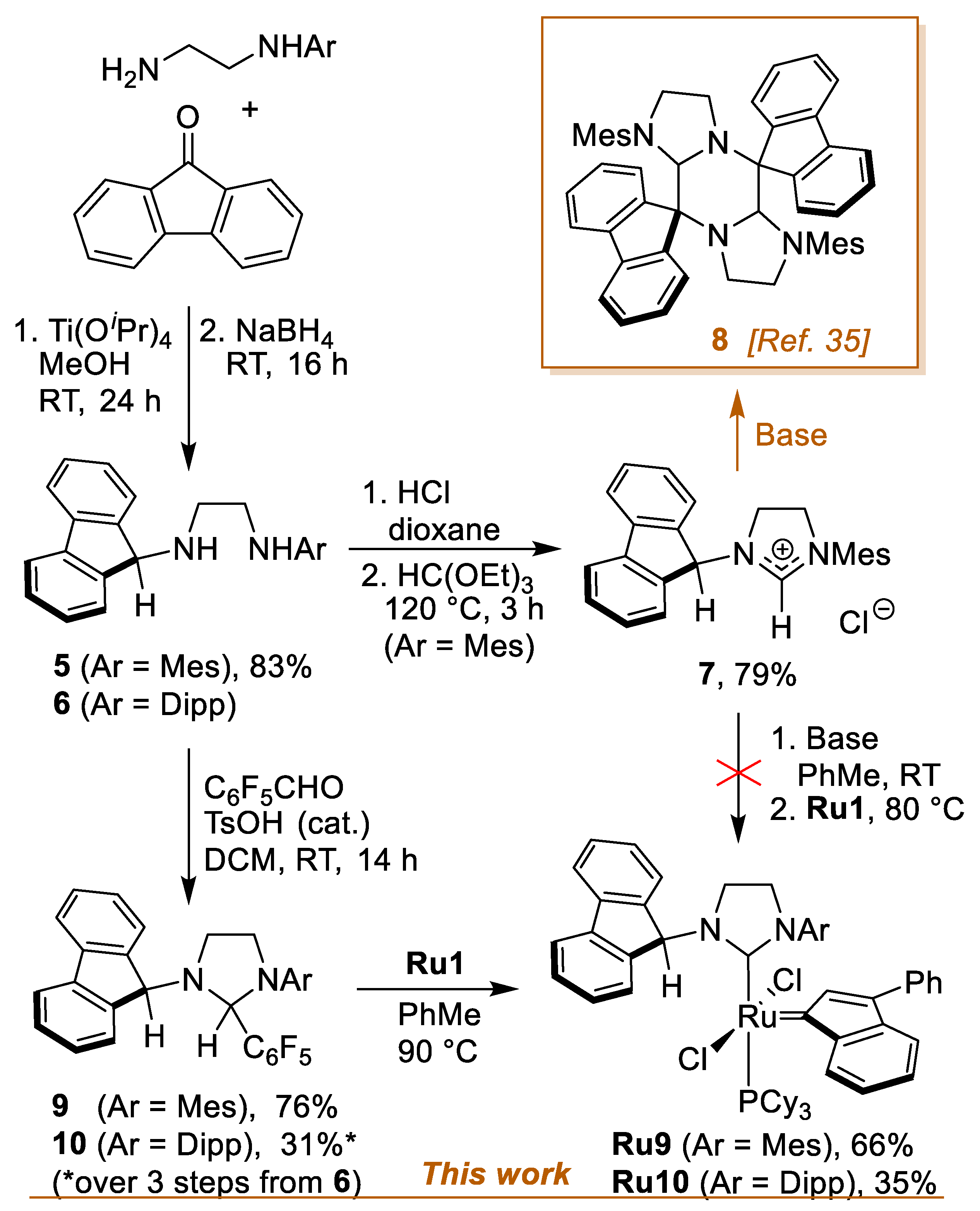
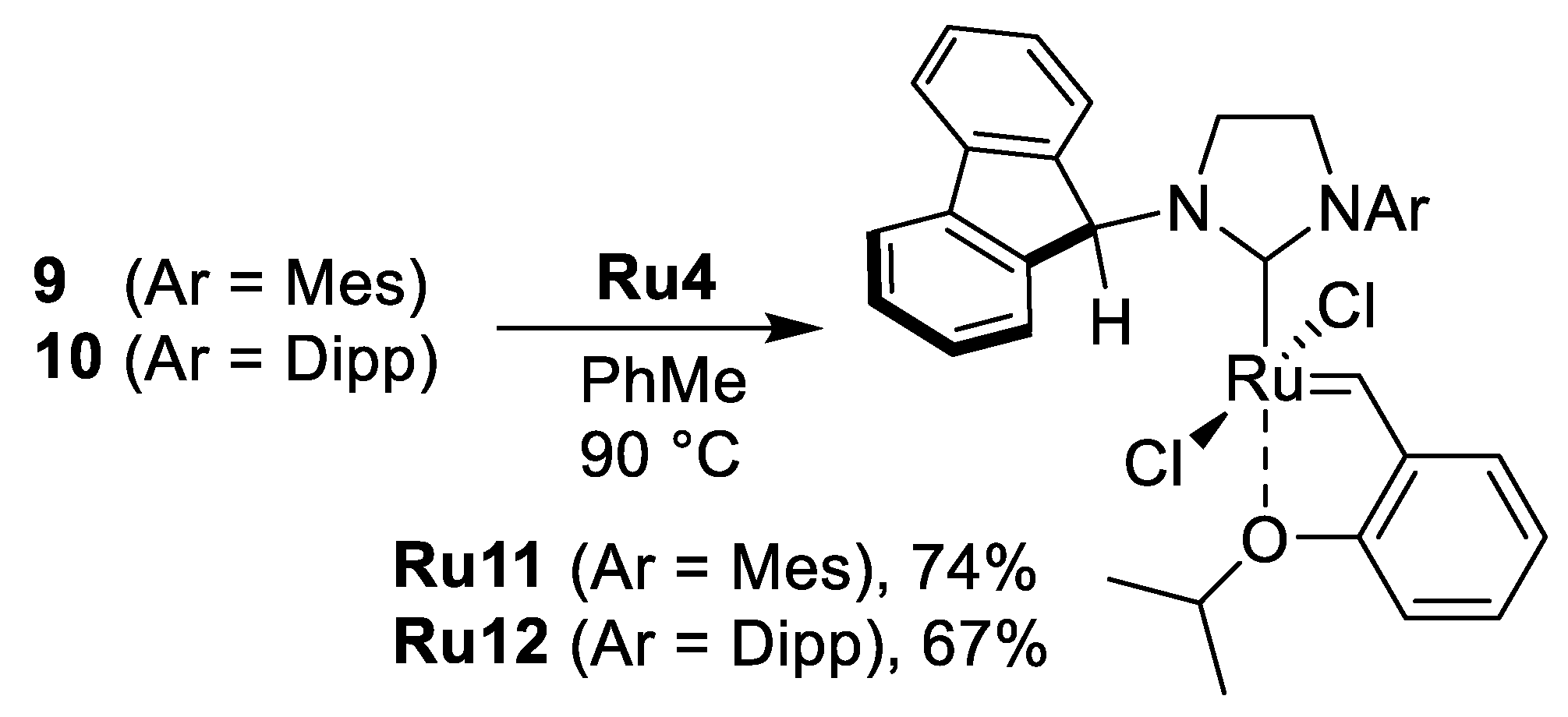
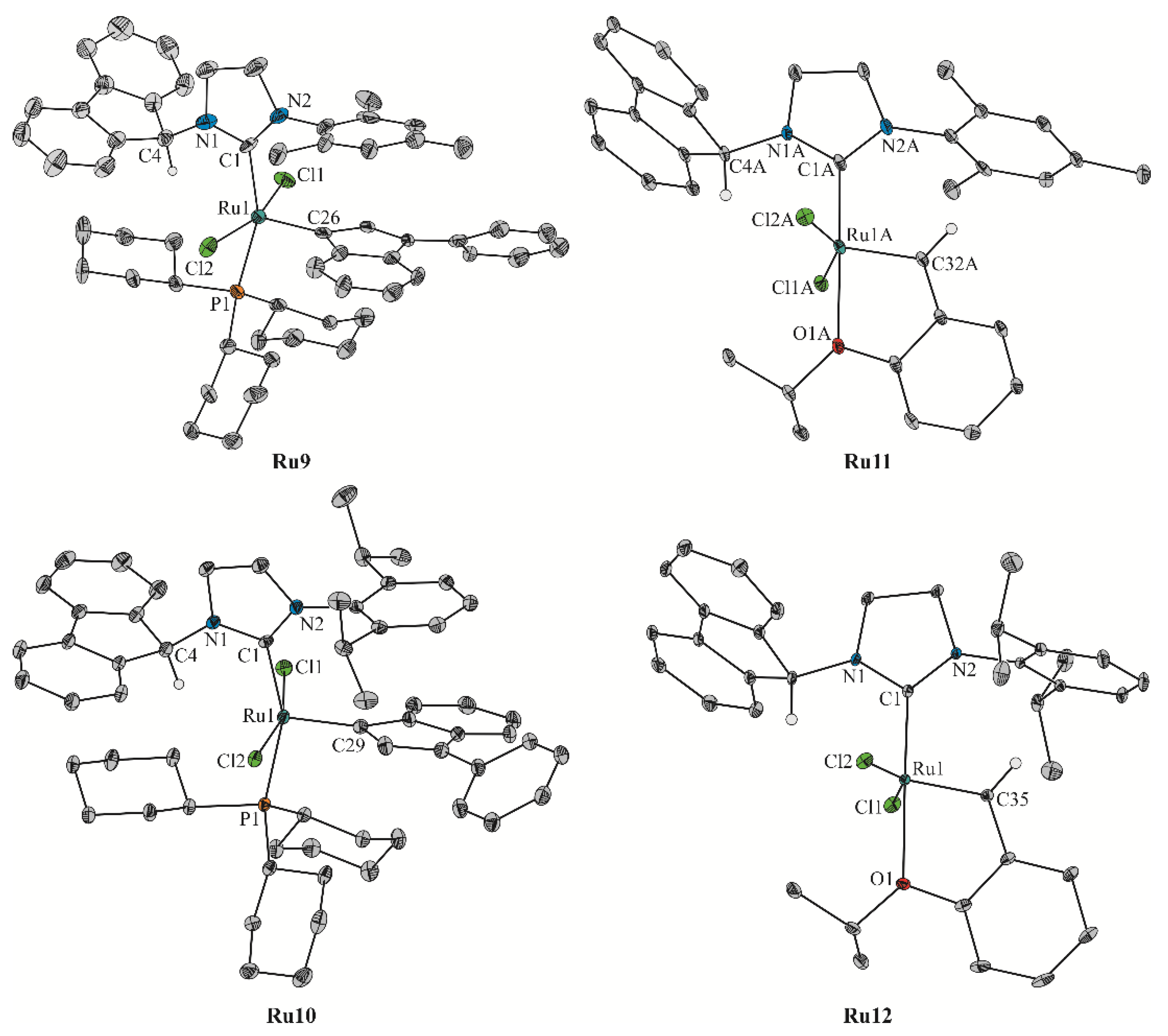
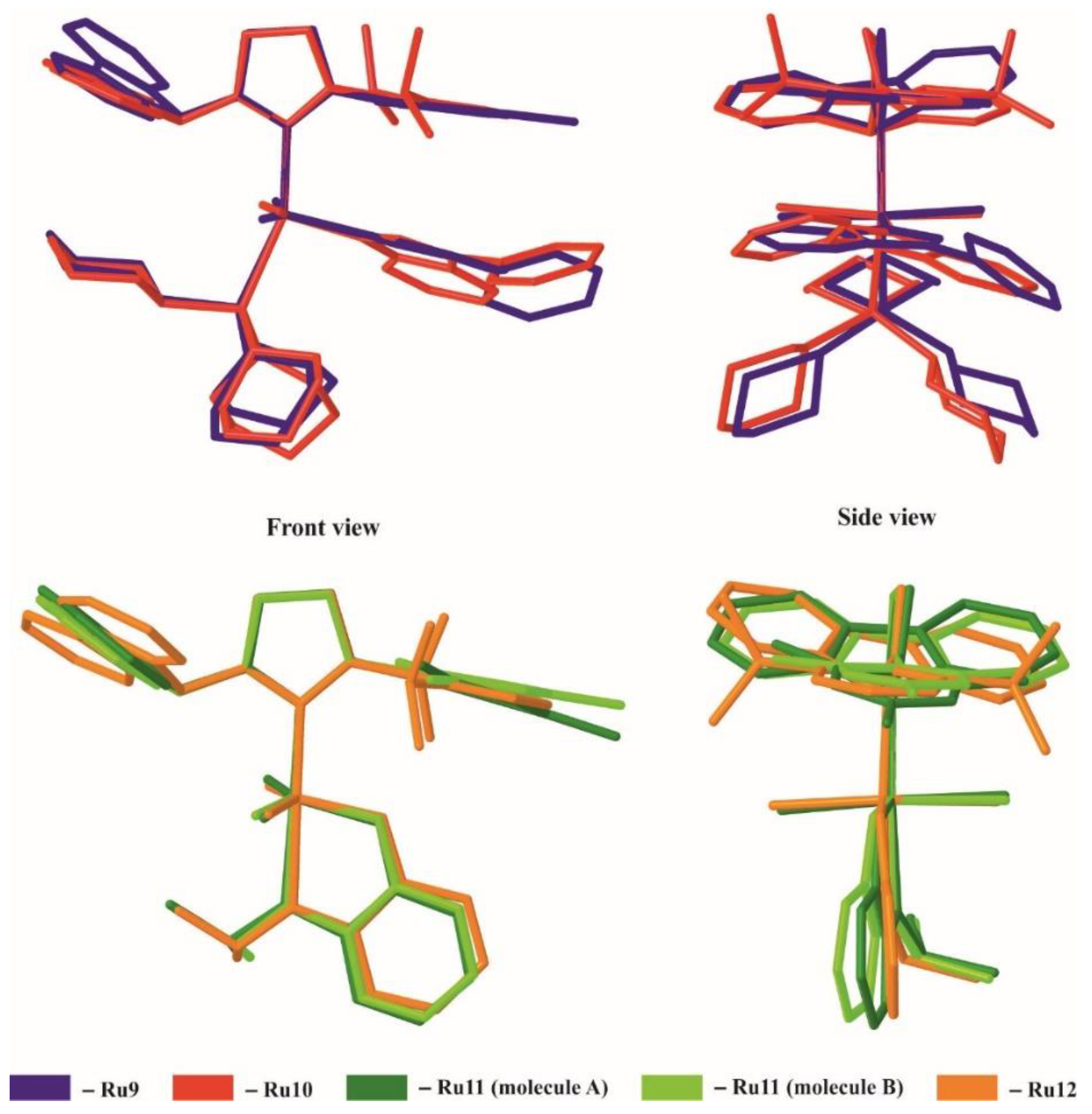
| Geometrical Parameters (Å, °) | Ru9 | Ru10 | Ru11 (Molecule A/Molecule B) | Ru12 |
|---|---|---|---|---|
| Bond lengths (Å) | ||||
| C1–N1 | 1.324(12) | 1.345(6) | 1.337(8)/1.364(9) | 1.349(3) |
| C1–N2 | 1.333(13) | 1.343(6) | 1.372(8)/1.361(8) | 1.352(3) |
| Ru–C | 2.076(9) | 2.082(4) | 1.974(7)/1.958(7) | 1.975(2) |
| Ru–Cl1 | 2.378(2) | 2.397(2) | 2.325(2)/2.320(2) | 2.317(2) |
| Ru–Cl2 | 2.384(2) | 2.412(2) | 2.331(2)/2.336(2) | 2.329(2) |
| Ru–O | − | − | 2.274(5)/2.277(5) | 2.294(2) |
| Ru–P | 2.440(2) | 2.423(2) | − | − |
| Ru=C | 1.922(11) | 1.872(5) | 1.850(7)/1.819(7) | 1.828(2) |
| Angles (°) | ||||
| Cl–Ru–Cl | 162.80(10) | 163.55(4) | 151.01(6)/149.64(6) | 151.33(2) |
| N–C–N | 110.0(8) | 107.8(4) | 106.5(6)/105.9(6) | 106.85(18) |
| C–Ru–O | − | − | 176.5(2)/178.8(2) | 176.42(7) |
| C–Ru–P | 156.3(3) | 157.63(13) | − | − |
| A | 81.5(5) | 81.42(16) | 84.49(19)/92.1(2) | 97.44(8) |
| B | 93.0(5) | 100.75(19) | 90.4(2)/89.3(3) | 86.18(9) |
| C | 8.5(3) | 14.11(14) | − | − |
| D | − | − | 19.8(2)/18.8(3) | 11.45(10) |
| τ5 | 0.11 | 0.10 | 0.42/0.49 | 0.42 |
| Entry | Substrate | Product | Catalyst | Conv. (%) |
|---|---|---|---|---|
| 1 a | 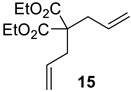 | 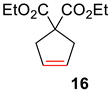 | Ru2 | 100 |
| Ru9 | 93 | |||
| Ru10 | 92 | |||
| Ru5 | 100 | |||
| Ru11 | 98 | |||
| Ru12 | 98 | |||
| 2 | 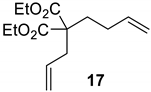 | 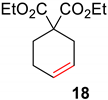 | Ru2 | 100 |
| Ru9 | 99 | |||
| Ru10 | 96 | |||
| Ru5 | 100 | |||
| Ru11 | 87 | |||
| Ru12 | 96 | |||
| 3 | 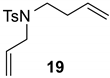 | 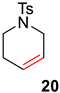 | Ru2 | 100 |
| Ru9 | 87 | |||
| Ru10 | 98 | |||
| Ru5 | 98 | |||
| Ru11 | 92 | |||
| Ru12 | 100 | |||
| 4 | 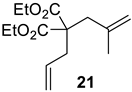 | 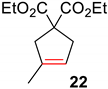 | Ru2 | 100 |
| Ru9 | 100 | |||
| Ru10 | 75 | |||
| Ru5 | 100 | |||
| Ru11 | 100 | |||
| Ru12 | 98 | |||
| 5 | 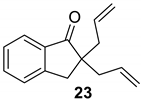 | 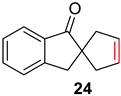 | Ru2 | 99 |
| Ru9 | 80 | |||
| Ru10 | 100 | |||
| Ru5 | 98 | |||
| Ru11 | 86 | |||
| Ru12 | 86 | |||
| 6 |  | 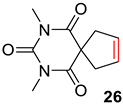 | Ru2 | 100 |
| Ru9 | 67 | |||
| Ru10 | 84 | |||
| Ru5 | 100 | |||
| Ru11 | 62 | |||
| Ru12 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jolly, P.I.; Marczyk, A.; Małecki, P.; Trzybiński, D.; Woźniak, K.; Kajetanowicz, A.; Grela, K. Specialized Olefin Metathesis Catalysts Featuring Unsymmetrical N-Heterocyclic Carbene Ligands Bearing N-(Fluoren-9-yl) Arm. Catalysts 2020, 10, 599. https://doi.org/10.3390/catal10060599
Jolly PI, Marczyk A, Małecki P, Trzybiński D, Woźniak K, Kajetanowicz A, Grela K. Specialized Olefin Metathesis Catalysts Featuring Unsymmetrical N-Heterocyclic Carbene Ligands Bearing N-(Fluoren-9-yl) Arm. Catalysts. 2020; 10(6):599. https://doi.org/10.3390/catal10060599
Chicago/Turabian StyleJolly, Phillip I., Anna Marczyk, Paweł Małecki, Damian Trzybiński, Krzysztof Woźniak, Anna Kajetanowicz, and Karol Grela. 2020. "Specialized Olefin Metathesis Catalysts Featuring Unsymmetrical N-Heterocyclic Carbene Ligands Bearing N-(Fluoren-9-yl) Arm" Catalysts 10, no. 6: 599. https://doi.org/10.3390/catal10060599
APA StyleJolly, P. I., Marczyk, A., Małecki, P., Trzybiński, D., Woźniak, K., Kajetanowicz, A., & Grela, K. (2020). Specialized Olefin Metathesis Catalysts Featuring Unsymmetrical N-Heterocyclic Carbene Ligands Bearing N-(Fluoren-9-yl) Arm. Catalysts, 10(6), 599. https://doi.org/10.3390/catal10060599