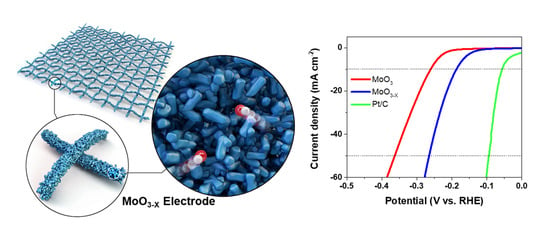
Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity
Abstract
1. Introduction
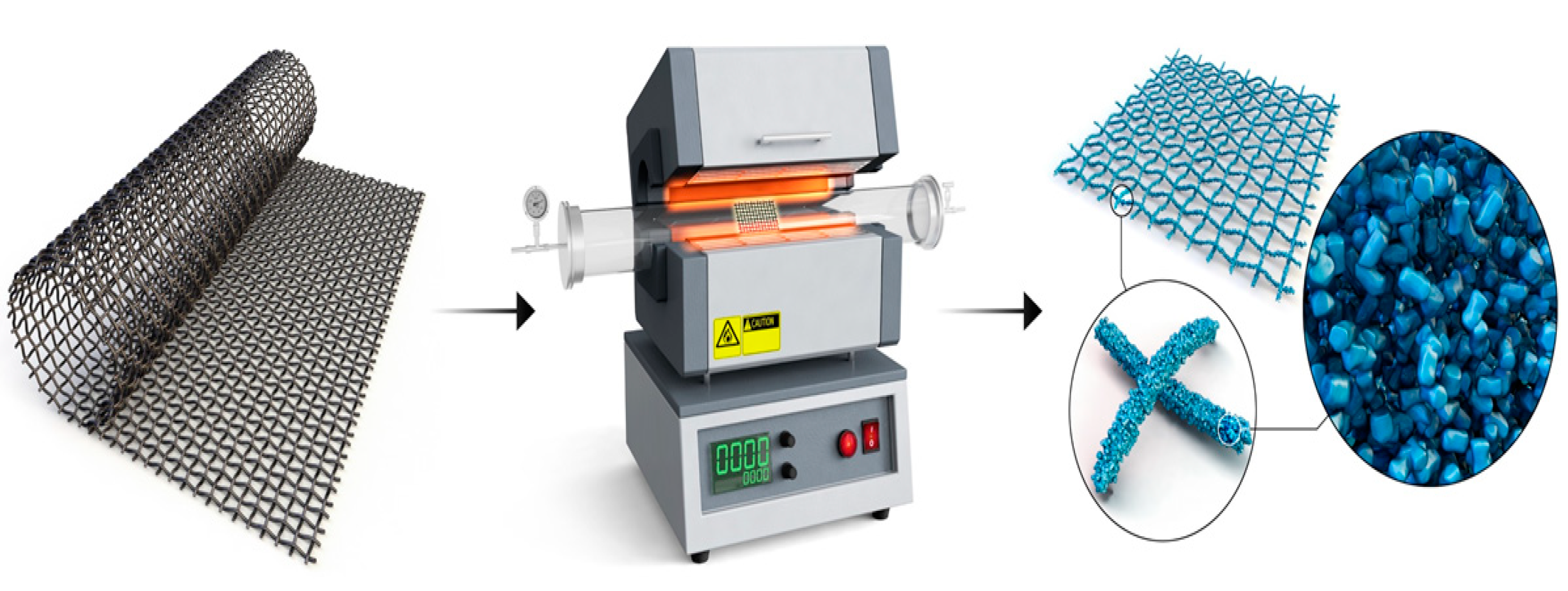
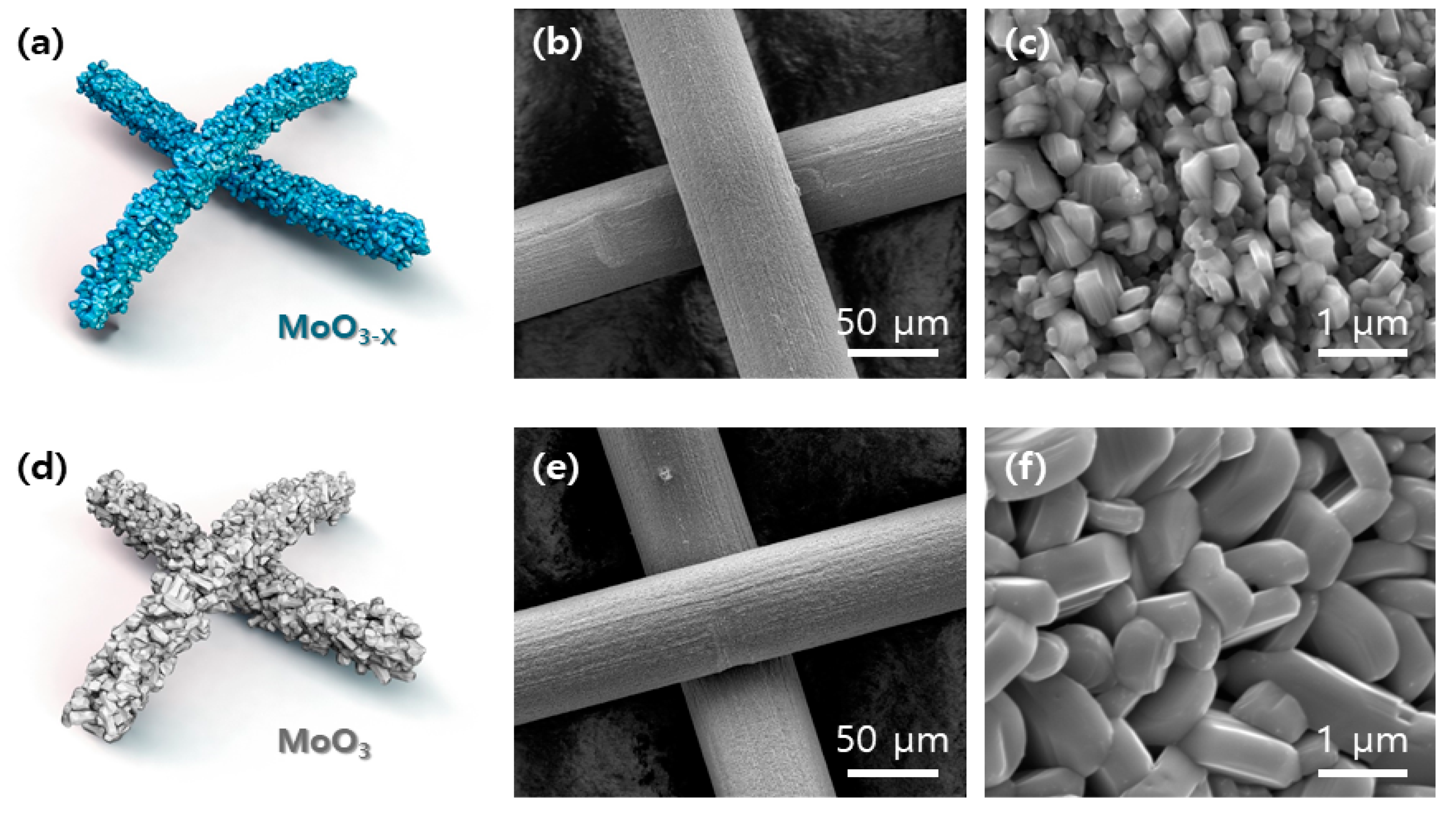
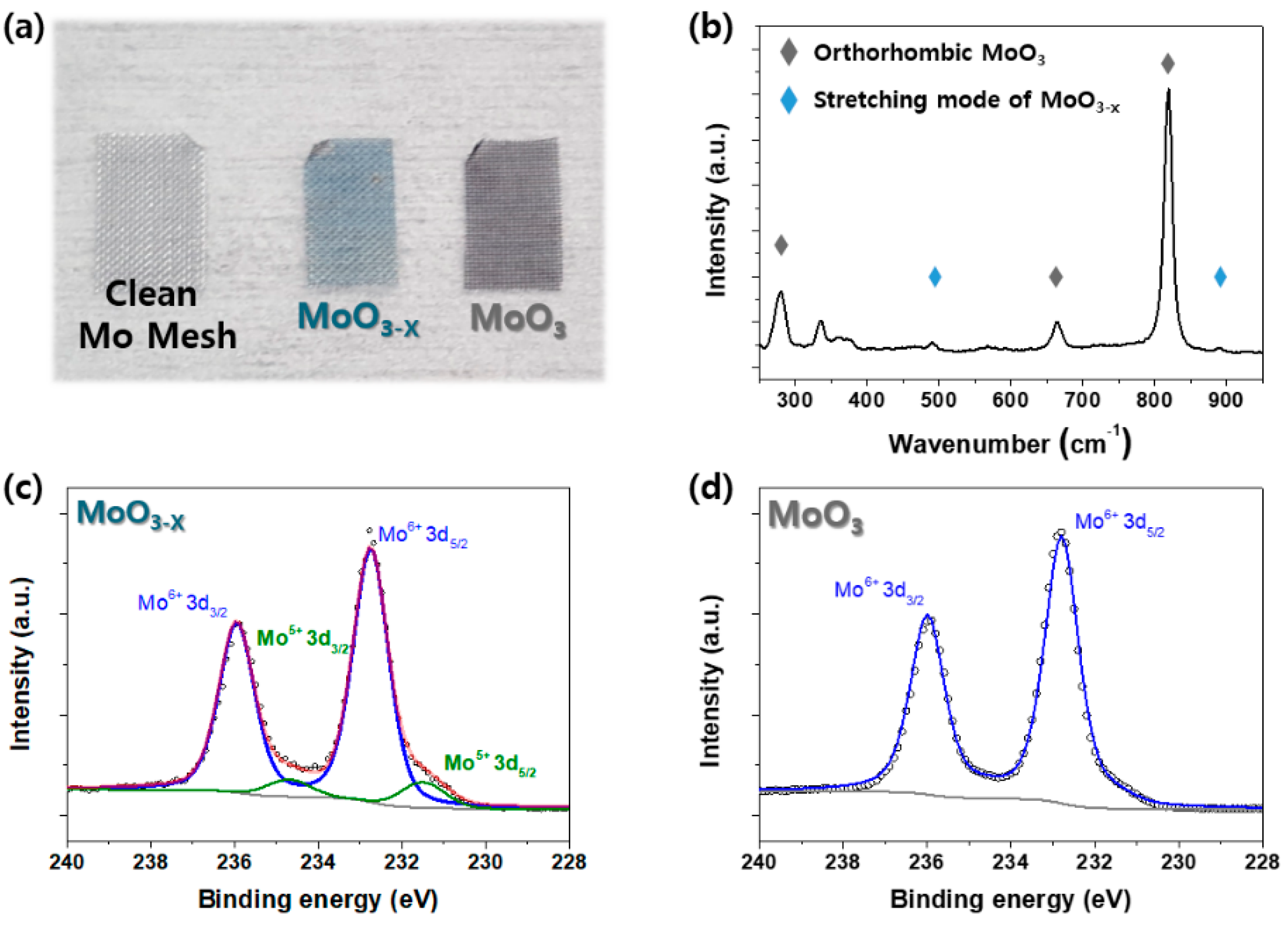
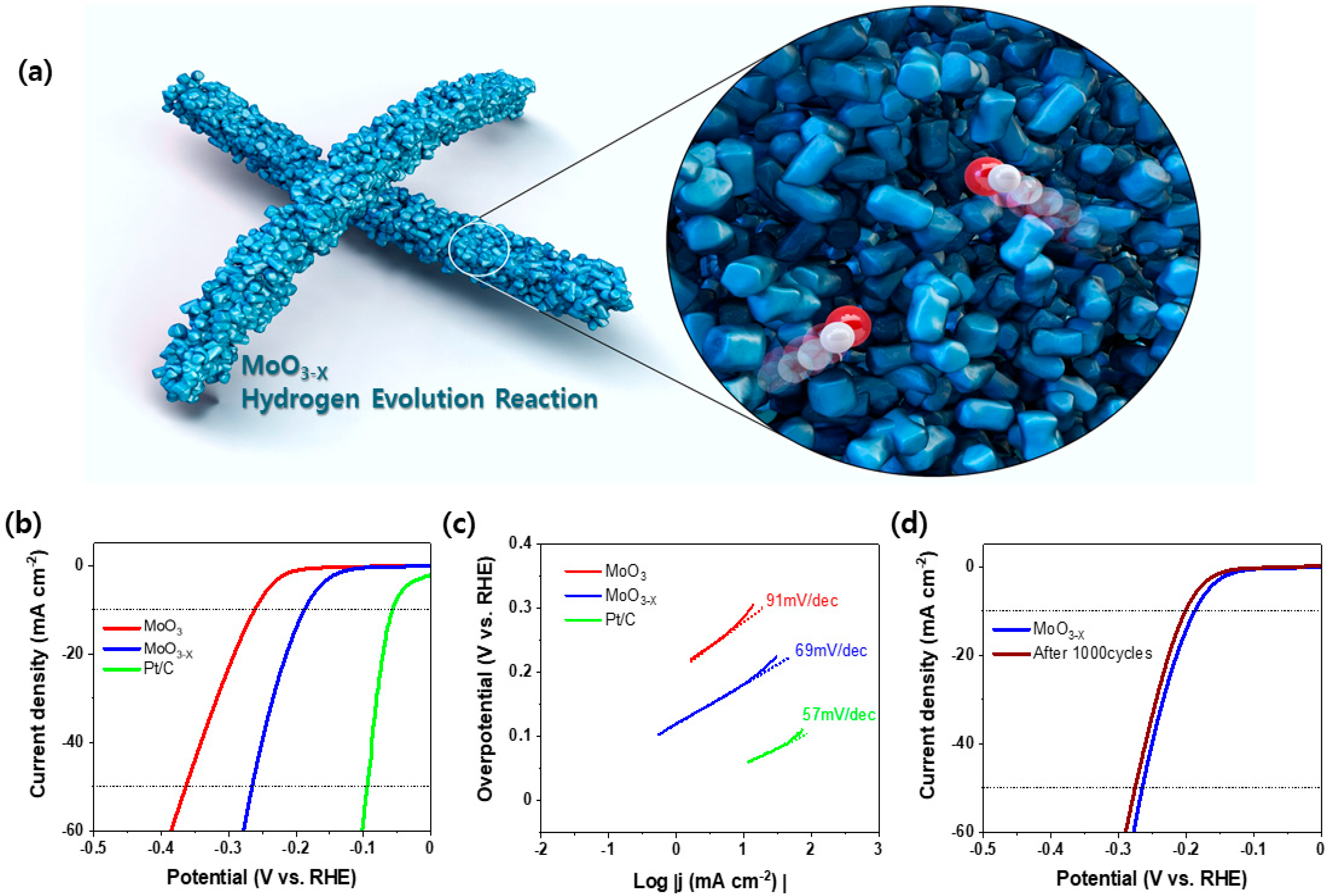
2. Results
3. Materials and Methods
3.1. Preparations of Materials
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2014, 414, 332–337. [Google Scholar] [CrossRef]
- Schlapbach, L. Hydrogen-fuelled vehicles. Nature 2009, 406, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. A realizable renewable energy future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. advancing the electrochemistry of the hydrogen evolution reaction through combining experiment and theory. Angew. Chem. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Liu, D.; Zou, Y.; Wang, S. Water-plasma-enabled exfoliation of ultrathin layered double hydroxide nanosheets with multivacancies for water oxidation. Adv. Mater. 2017, 29, 1701546. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.-C.; Fang, S.; Yang, H. Ca2Mn2O5 as oxygen-deficient perovskite electrocatalyst for oxygen evolution reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Wu, M.; Ke, S.; Chen, W.; Zhang, S.; Zhu, M.; Zhang, Y.; Foo, M.L.; Tang, L. Optimization of the facet structure of cobalt oxide catalysts for enhanced hydrogen evolution reaction. Catal. Sci. Technol. 2020, 10, 1040–1047. [Google Scholar] [CrossRef]
- Lovell, E.; Lu, X.; Zhang, Q.; Scott, J.; Amal, R. From passivation to activation–tunable nickel/nickel oxide for hydrogen evolution electrocatalysis. Chem. Commun. 2020, 56, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Verma, M.; Sohn, Y.; Deshpande, P.A.; Pradhan, D. Highly active tungsten oxide nanoplate electrocatalysts for the hydrogen evolution reaction in acidic and near neutral electrolytes. ACS Omega 2017, 2, 7039–7047. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, C.; Zhang, Y.; Zheng, Q.; Zhang, S.; Mu, X.; Chen, C.; Ma, J.; Mu, S. Vacancy-coordinated hydrogen evolution reaction on MoO3−x anchored atomically dispersed MoRu pairs. J. Mater. Chem. A 2019, 7, 14466–14472. [Google Scholar] [CrossRef]
- Kim, H.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, X.; Cheng, X.; Xu, Y.-M.; Gao, S.; Zhao, H.; Zhou, X.; Huo, L.-H. Oxygen-vacancy-enriched porous α-MoO3 nanosheets for trimethylamine sensing. ACS Appl. Nano Mater. 2019, 2, 8016–8026. [Google Scholar] [CrossRef]
- Yu, M.; Shao, H.; Wang, G.; Yang, F.; Liang, C.; Rozier, P.; Wang, C.-Z.; Lu, X.; Simon, P.; Feng, X. Interlayer gap widened α-phase molybdenum trioxide as high-rate anodes for dual-ion-intercalation energy storage devices. Nat. Commun. 2020, 11, 1348. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Yazdani, S.; Kashfi-Sadabad, R.; Huan, T.D.; Morales-Acosta, M.D.; Pettes, M.T. Polyelectrolyte-assisted oxygen vacancies: A new route to defect engineering in molybdenum oxide. Langmuir 2018, 34, 6296–6306. [Google Scholar] [CrossRef]
- Shi, X.-R.; Wang, J.; Hermann, K. Theoretical cluster studies on the catalytic sulfidation of MoO3. J. Phys. Chem. C 2010, 114, 6791–6801. [Google Scholar] [CrossRef]
- Borgschulte, A.; Sambalova, O.; Delmelle, R.; Jenatsch, S.; Hany, R.; Nüesch, F. Hydrogen reduction of molybdenum oxide at room temperature. Sci. Rep. 2017, 7, 40761. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Zhao, S.-X.; Yu, L.; Zheng, X.-X.; Wang, Y.-F.; Yu, L.-Q.; Nan, C.-W.; Cao, G. Oxygen vacancy-enriched MoO3−x nanobelts for asymmetric supercapacitors with excellent room/low temperature performance. J. Mater. Chem. A 2019, 7, 13205–13214. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J.; et al. Mesoporous MoO3-x material as an efficient electrocatalyst for hydrogen evolution reactions. Adv. Energy Mater. 2016, 6, 1600528. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Self-supported earth-abundant nanoarrays as efficient and robust electrocatalysts for energy-related reactions. ACS Catal. 2018, 8, 6707–6732. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.-P.; Yuan, Z.-Y. Three-dimensional electrocatalysts for sustainable water splitting reactions. Eur. J. Inorg. Chem. 2016, 2016, 1916–1923. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148. [Google Scholar] [CrossRef]
- Kashfi-Sadabad, R.; Yazdani, S.; Huan, T.D.; Cai, Z.; Pettes, M.T. Role of oxygen vacancy defects in the electrocatalytic activity of substoichiometric molybdenum oxide. J. Phys. Chem. C 2018, 122, 18212–18222. [Google Scholar] [CrossRef]
- Oleksak, R.P.; Kapoor, M.; Perea, D.; Holcomb, G.R.; Doğan, Ö.N. The role of metal vacancies during high-temperature oxidation of alloys. NPJ Mater. Degrad. 2018, 2, 25. [Google Scholar] [CrossRef]
- Yamashita, T.; Yokoyama, H. Molybdenum anode: A novel electrode for enhanced power generation in microbial fuel cells, identified via extensive screening of metal electrodes. Biotechnol. Biofuels 2018, 11, 39. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, C.; Wang, S.; Hou, Y. 3D arrays of molybdenum sulphide nanosheets on Mo meshes: Efficient electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2015, 174, 653–659. [Google Scholar] [CrossRef]
- Manthiram, K.; Alivisatos, A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef]
- Dieterle, M.; Weinberg, G.; Mestl, G. Raman spectroscopy of molybdenum oxides. Phys. Chem. Chem. Phys. 2002, 4, 812–821. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2Nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.S.; Haque, F.; Mohiuddin, M.; Carey, B.J.; Syed, N.; Zavabeti, A.; Zhang, B.; Khan, H.; Berean, K.J.; Ou, J.Z.; et al. Highly active two dimensional α-MoO3−x for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24223–24231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Lee, Y.-W.; Hong, J.; Sohn, J.I. Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity. Catalysts 2020, 10, 1180. https://doi.org/10.3390/catal10101180
Jo S, Lee Y-W, Hong J, Sohn JI. Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity. Catalysts. 2020; 10(10):1180. https://doi.org/10.3390/catal10101180
Chicago/Turabian StyleJo, Seunghwan, Young-Woo Lee, John Hong, and Jung Inn Sohn. 2020. "Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity" Catalysts 10, no. 10: 1180. https://doi.org/10.3390/catal10101180
APA StyleJo, S., Lee, Y.-W., Hong, J., & Sohn, J. I. (2020). Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity. Catalysts, 10(10), 1180. https://doi.org/10.3390/catal10101180