Controllable Hydrothermal Synthesis and Photocatalytic Performance of Bi2MoO6 Nano/Microstructures
Abstract
1. Introduction
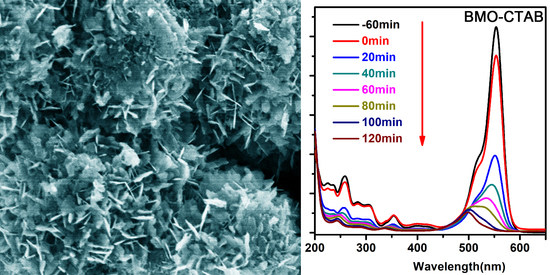
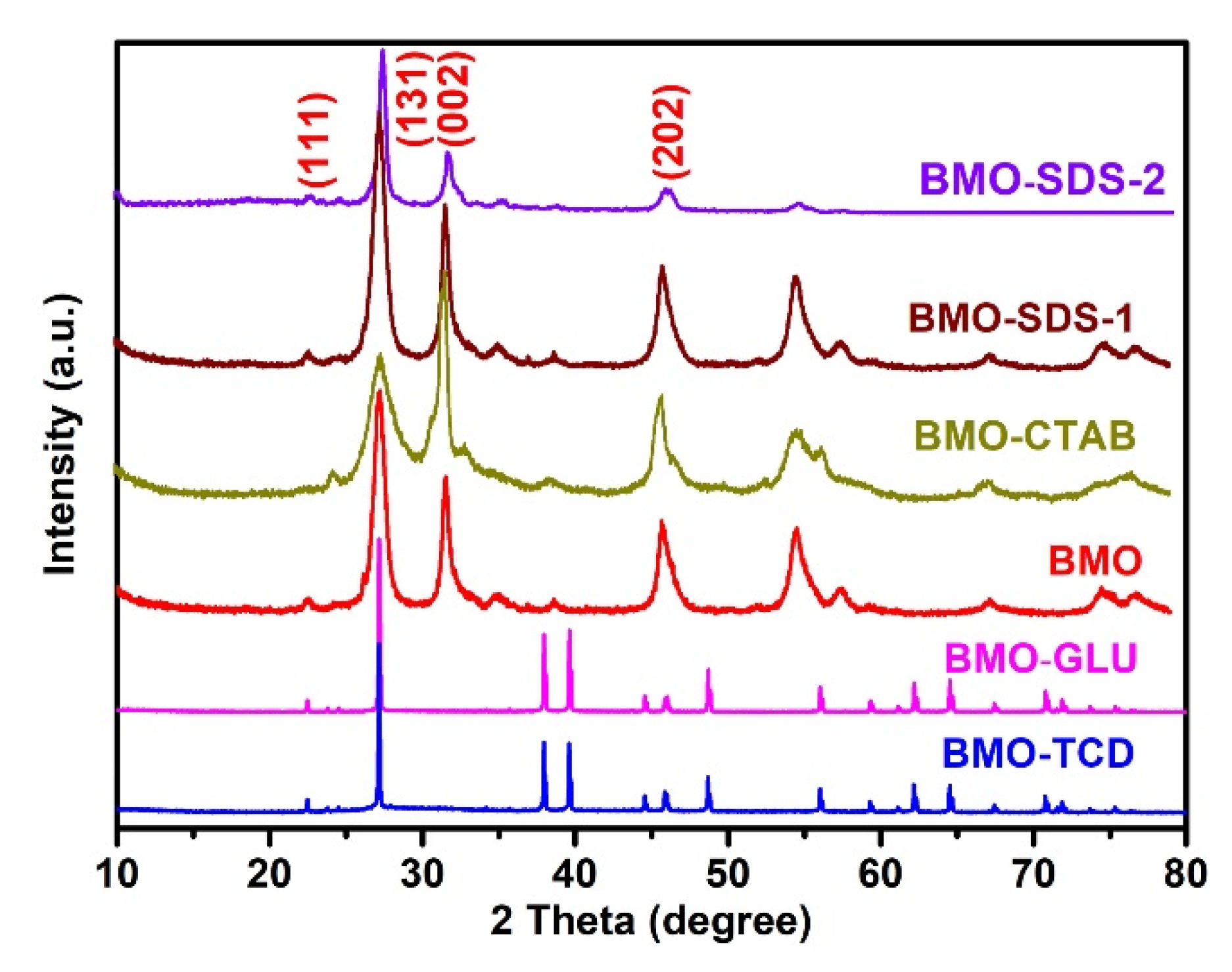
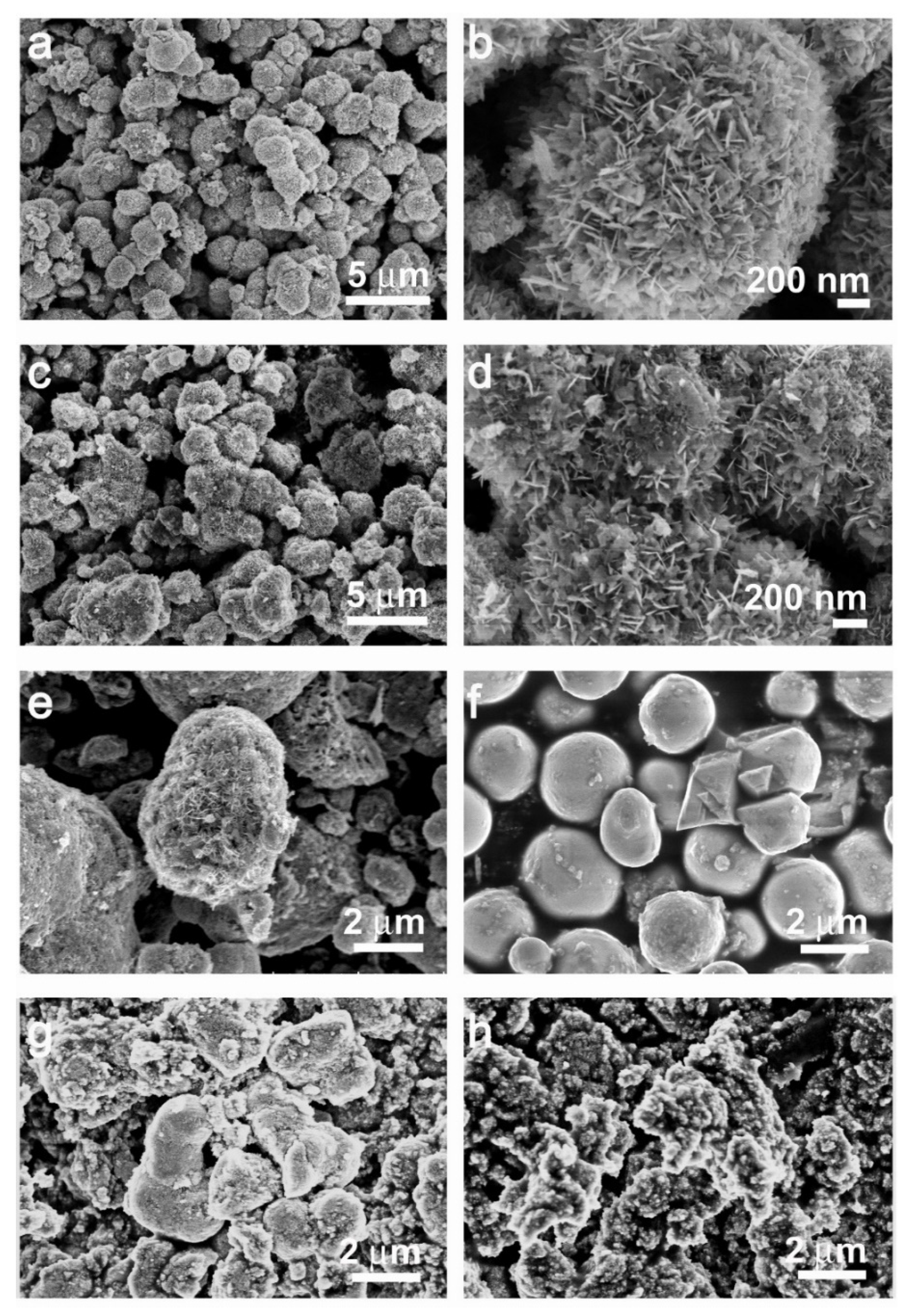
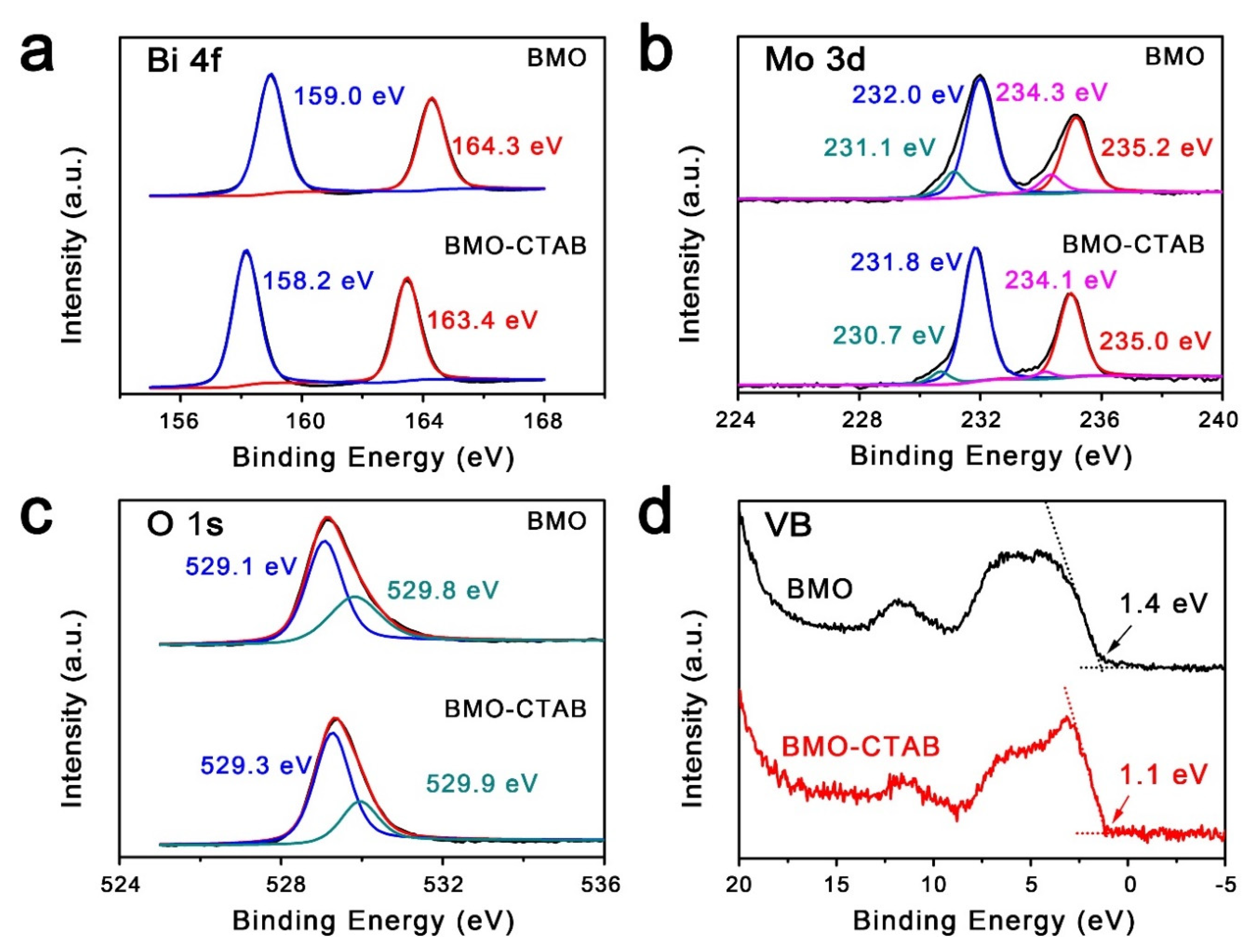
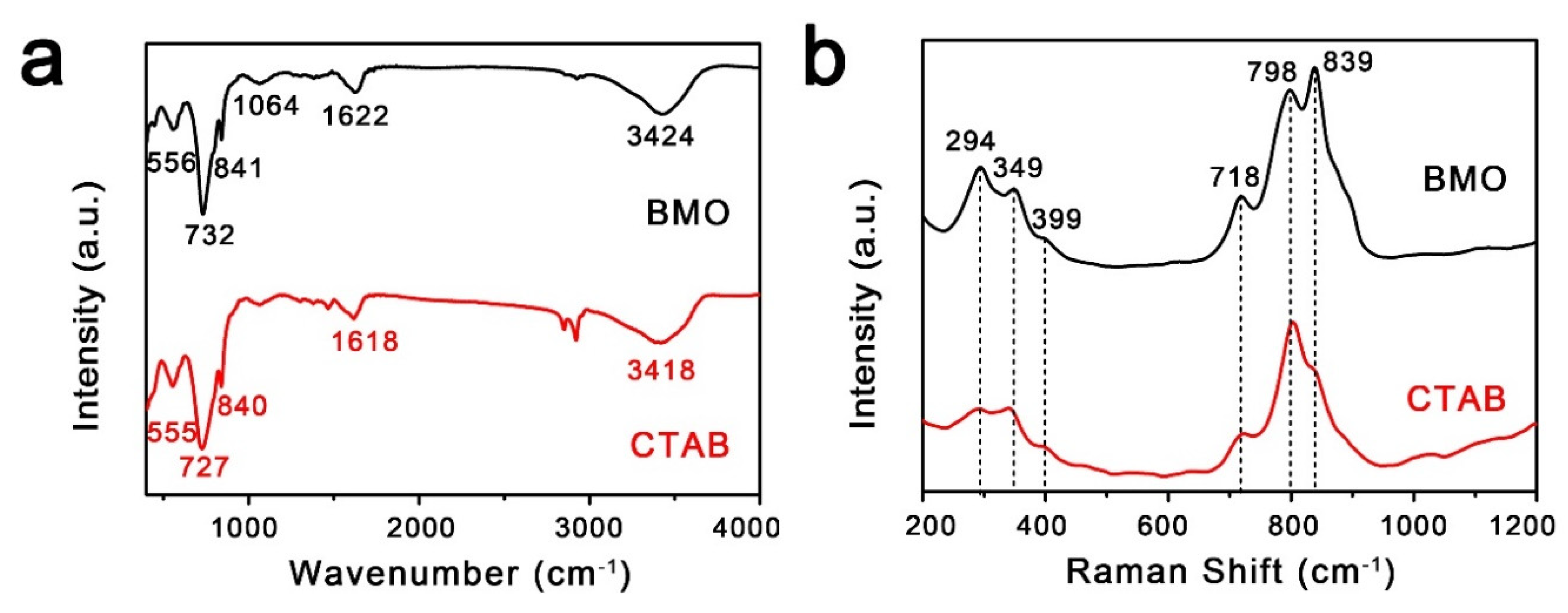
2. Results and Discussion
3. Experimental Section
3.1. Preparation of Different Morphologies of Bi2MoO6
3.2. Characterizations
3.3. Photocatalytic Property Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, X.Y.; Zhang, C.L. Recent advances in structure design for enhancing photocatalysis. J. Mater. Sci. 2019, 54, 8831–8851. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.L.; Lyu, M.Q.; Luo, B.; Wang, S.C.; Liu, G.; Cheng, H.M.; Wang, L.Z. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2019, 31, 1801369. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.J.; Yang, T.; Rui, Z.B.; Ji, H.B. Perovskite-based photocatalysts for organic contaminants removal: Current status and future perspectives. Catal. Today 2019, 327, 47–63. [Google Scholar] [CrossRef]
- Na, J.S.; Gong, B.; Scarel, G.; Parsons, G.N. Surface polarity shielding and hierarchical ZnO nano-architectures produced using sequential hydrothermal crystal synthesis and thin film atomic layer deposition. ACS Nano 2009, 3, 3191–3199. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, J.; Li, C.Z. Hierarchical porous NiCo2O4 nanowires for high-rate supercapacitors. Chem. Commun. 2012, 48, 4465–4467. [Google Scholar] [CrossRef]
- Akbari, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano Struct. Nano Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Hrbek, J. Inverse oxide/metal catalysts: A versatile approach for activity tests and mechanistic studies. Surf. Sci. 2010, 604, 241–244. [Google Scholar] [CrossRef]
- He, R.G.; Xu, D.F.; Cheng, B.; Yu, J.G.; Ho, W.K. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Bai, J.W.; Li, X.M.; Hao, Z.W.; Liu, L. Enhancement of 3D Bi2MoO6 mesoporous spheres photocatalytic performance by vacancy engineering. J. Colloid Interface Sci. 2020, 560, 510–518. [Google Scholar] [CrossRef]
- Sun, Y.G.; Zhang, Y.Q.; Xu, Y.L.; Yu, X.F.; Ding, D.R.; Liu, X.J. Synthesis and visible-light photocatalytic Properties of ZnO flake-like ensembles. Micro Nano Lett. 2012, 11, 1147–1150. [Google Scholar] [CrossRef]
- Gupta, N.K.; Ghafari, Y.; Kim, S.; Bae, J.; Kim, K.S.; Saifuddin, M. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) Nanoparticles at Neutral pH. Sci. Rep. 2020, 10, 4942. [Google Scholar] [CrossRef] [PubMed]
- Pino, E.; Calderón, C.; Herrera, F.; Cifuentes, G.; Arteaga, G. Photocatalytic Degradation of Aqueous Rhodamine 6G Using Supported TiO2 Catalysts. A Model for the Removal of Organic Contaminants from Aqueous Samples. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Yu, J.G.; Low, J.X.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Comp. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Zhang, L.W.; Xu, T.G.; Zhao, X.; Zhu, Y.F. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Li, S.J.; Chen, J.L.; Hu, S.W.; Wang, H.L.; Jiang, W.; Chen, X.B. Facile construction of novel Bi2WO6/Ta3N5 Z-scheme heterojunction nanofibers for efficient degradation of harmful pharmaceutical pollutants. Chem. Eng. J. 2020, 402, 126165. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, S.W.; Jiang, W.; Zhang, J.L.; Xu, K.B. In situ construction of WO3 nanoparticles decorated Bi2MoO6 microspheres for boosting photocatalytic degradation of refractory pollutants. J. Colloid Interface Sci. 2019, 556, 335–344. [Google Scholar] [CrossRef]
- Li, S.J.; Xue, B.; Chen, J.L.; Liu, Y.P.; Zhang, J.L.; Wang, H.W.; Liu, J.S. Constructing a plasmonic p-n heterojunction photocatalyst of 3D Ag/Ag6Si2O7/Bi2MoO6 for efficiently removing broad-spectrum antibiotics. Sep. Purif. Technol. 2020, 254, 117579. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, S.W.; Jiang, W.; Zhou, Y.T.; Liu, J.S.; Wang, Z.H. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef]
- Ma, Y.; Jia, Y.L.; Wang, L.N.; Yang, M.; Bi, Y.P.; Qi, Y.X. Hierarchical nanosheetbased Bi2MoO6 nanotubes with remarkably improved electrochemical performance. J. Power Sour. 2016, 331, 481–486. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, C.; Mu, J.; Huang, X.; Zhang, Z.; Guo, Z.; Zhang, P.; Liu, Y. Hierarchical heterostructures of Bi2MoO6 on carbon nanofibers: Controllable solvothermal fabrication and enhanced visible photocatalytic properties. J. Mater. Chem. 2012, 22, 577–584. [Google Scholar] [CrossRef]
- Wu, M.H.; Wang, Y.X.; Xu, Y.; Ming, J.; Zhou, M.; Xu, R.; Fu, Q.; Lei, Y. Self-supported Bi2MoO6 nanowall for photoelectrochemical water splitting. ACS Appl. Mater. Inter. 2017, 9, 23647–23653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.G.; Wu, C.C.; Cui, Z.; Rao, P.H. Enhancing photocatalytic activity of Bi2MoO6 via surface co-doping with Ni2+ and Ti4+ ions. J. Phys. Chem. Solid. 2019, 129, 209–216. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Wang, H. Hydrothermal synthesis and photocatalytic properties of bismuth molybdate materials. Mater. Chem. Phys. 2009, 116, 134–142. [Google Scholar] [CrossRef]
- Shi, Y.; Feng, S.; Cao, C. Hydrothermal synthesis and characterization of Bi2MoO6 and Bi2WO6. Mater. Lett. 2000, 44, 215–218. [Google Scholar] [CrossRef]
- Zheng, Y.; Duan, F.; Wu, J.; Liu, L.; Chen, M.; Xie, Y. Enhanced photocatalytic activity of bismuth molybdates with the preferentially exposed {010} surface under visible light irradiation. J. Mol. Catal. A Chem. 2009, 303, 9–14. [Google Scholar] [CrossRef]
- Yang, Z.X.; Shen, M.; Dai, K.; Zhang, X.H.; Chen, H. Controllable synthesis of Bi2MoO6 nanosheets and their facet-dependent visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2018, 430, 505–514. [Google Scholar] [CrossRef]
- Ghasemian, M.B.; Mayyas, M.; Idrus-Saidi, S.A.; Jamal, M.A.; Yang, J.; Mofarah, S.S.; Adabifiroozjaei, E.; Tang, J.B.; Syed, N.; O’Mullane, A.P.; et al. Self-Limiting Galvanic Growth of MnO2 Monolayers on a Liquid Metal—Applied to Photocatalysis. Adv. Funct. Mater. 2019, 29, 1901649. [Google Scholar] [CrossRef]
- Idrus-Saidi, S.A.; Tang, J.B.; Ghasemian, M.B.; Yang, J.; Han, J.L.; Syed, N.; Daeneke, T.; Abbasi, R.; Koshy, P.; O’Mullane, A.P.; et al. Liquid metal core–shell structures functionalised via mechanical agitation: The example of Field’s metal. J. Mater. Chem. A 2019, 7, 17876–17887. [Google Scholar] [CrossRef]
- Cao, D.D.; Wang, Q.Y.; Wu, Y.; Zhu, S.X.; Jia, Y.; Wang, R.L. Solvothermal synthesis and enhanced photocatalytic hydrogen production of Bi/Bi2MoO6 co-sensitized TiO2 nanotube arrays. Sep. Purif. Technol. 2020, 250, 117132. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A.; Ranganathan, S.; Vembuli, T.; Kumaravel, S.; Erusappan, E. Fabrication of novel Bi2MoO6/N-rGO catalyst for the efficient photocatalytic degradation of harmful dyes. Mater. Res. Bull. 2020, 125, 110782. [Google Scholar] [CrossRef]
- Khazaee, Z.; Khavar, A.H.C.; Mahjoub, A.R.; Motaee, A.; Srivastava, V.; Sillanpää, M. Template-confined growth of X-Bi2MoO6 (X: F, Cl, Br, I) nanoplates with open surfaces for photocatalytic oxidation; experimental and DFT insights of the halogen doping. Solar Energy 2020, 196, 567–581. [Google Scholar] [CrossRef]
- Suebsom, P.; Phuruangrat, A.; Suwanboon, S.; Thongtem, S.; Thongtem, T. Enhanced visible-light-driven photocatalytic activity of heterostructure Ag/Bi2MoO6 nanocomposites synthesized by photoreduction method. Inorg. Chem. Commun. 2020, 119, 108120. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Q.Y.; Zhang, H.; Wu, Y.; Jia, Y.; Jin, R.C.; Gao, S.M. CTAB-assisted solvothermal construction of hierarchical Bi2MoO6/Bi5O7Br with improved photocatalytic performances. Sep. Purif. Technol. 2020, 242, 116775. [Google Scholar] [CrossRef]
- Li, S.J.; Shen, X.F.; Liu, J.S.; Zhang, L.S. Synthesis of Ta3N5/Bi2MoO6 core-shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Li, N.; Gao, H.; Wang, X.; Zhao, S.J.; Lv, D.; Yang, G.Q.; Gao, X.Y.; Fan, H.K.; Gao, Y.Q.; Ge, L. Novel indirect Z-scheme g-C3N4/Bi2MoO6/Bi hollow microsphere heterojunctions with SPR-promoted visible absorption and highly enhanced photocatalytic performance. Chin. J. Chem. 2020, 41, 426–434. [Google Scholar] [CrossRef]
- Sun, Y.G.; Zou, R.J.; Tian, Q.W.; Wu, J.H.; Chen, Z.G.; Hu, J.Q. Hydrothermal synthesis, growth mechanism, and properties of three-dimensional micro/nanoscaled hierarchical architecture films of hemimorphite zinc silicate. CrystEngComm 2011, 13, 2273–2280. [Google Scholar] [CrossRef]
- Li, W.F.; Sun, Y.G.; Xu, J.L. Controllable hydrothermal synthesis and properties of ZnO hierarchical micro/nanostructures. Nano-Micro Lett. 2012, 4, 98–102. [Google Scholar] [CrossRef]
- Sun, M.Z.; Guo, P.Y.; Wang, M.; Ren, F.Y. The effect of calcination temperature on the photocatalytic performance of Bi2MoO6 for the degradation of phenol under visible light. Opt. Int. J. Light Electron Opt. 2019, 199, 163319. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Guo, P.; Sun, M.; Zhang, Y.; Tong, Z.; You, M.; Lv, C. Hydrothermal synthesis of B-doped Bi2MoO6 and its high photocatalytic performance for the degradation of rhodamine B. J. Phys. Chem. Solid. 2018, 113, 86–93. [Google Scholar] [CrossRef]
- Rangel, R.; Cedeno, V.J.; Espino, J.; Bartolo-Perez, P.; Rodriguez-Gattorno, G.; Alvarado-Gil, J.J. Comparing the Efficiency of N-Doped TiO2 and N-Doped Bi2MoO6 Photo Catalysts for MB and Lignin Photodegradation. Catalysts 2018, 8, 668. [Google Scholar] [CrossRef]
- Ji, T.; Cui, Z.; Zhang, W.L.; Cao, Y.J.; Zhang, Y.F.; He, S.A.; Xu, M.D.; Sun, Y.G.; Zou, R.J.; Hu, J.Q. UV and visible light synergetic photodegradation using rutile TiO2 nanorod arrays based on a p–n Junction. Dalton Trans. 2017, 46, 4296. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.; Farajnezhad, M.; Tadjarodi, A. 3D hierarchical flower-like nanostructure of Bi2MoO6: Mechanochemical synthesis, the effect of synthesis parameters and photocatalytic activity. Mater. Res. Bull. 2017, 87, 92. [Google Scholar] [CrossRef]
- Shen, H.D.; Xue, W.W.; Fu, F.; Sun, J.F.; Zhen, Y.Z.; Wang, D.J.; Shao, B.; Tang, J.W. Efficient Degradation of Phenol and 4-Nitrophenol by Surface Oxygen Vacancies and Plasmonic Silver Co-Modified Bi2MoO6 Photocatalysts. Chem. Eur. J. 2018, 24, 18463–18478. [Google Scholar] [CrossRef] [PubMed]
- Phuruangrat, A.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Synthesis and characterization of visible light-driven W-doped Bi2MoO6 hotocatalyst and its photocatalytic activities. Mater. Lett. 2017, 194, 114–117. [Google Scholar] [CrossRef]
- Li, H.D.; Li, W.J.; Gu, S.N.; Wang, F.Z.; Zhou, H.L.; Liu, X.T.; Ren, C.J. Enhancement of photocatalytic activity in Tb/Eu co-doped Bi2MoO6: The synergistic effect of Tb-Eu redox cycles. RSC Adv. 2016, 6, 48089–48098. [Google Scholar] [CrossRef]



| Surfactant | SDS-1 | BMO (None) | TCD | SDS-2 | CTAB | GLU |
|---|---|---|---|---|---|---|
| Surface area (m2/g) | 45.120 | 48.959 | 72.191 | 45.213 | 41.170 | 4.892 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, T.; Ha, E.; Wu, M.; Hu, X.; Wang, J.; Sun, Y.; Li, S.; Hu, J. Controllable Hydrothermal Synthesis and Photocatalytic Performance of Bi2MoO6 Nano/Microstructures. Catalysts 2020, 10, 1161. https://doi.org/10.3390/catal10101161
Ji T, Ha E, Wu M, Hu X, Wang J, Sun Y, Li S, Hu J. Controllable Hydrothermal Synthesis and Photocatalytic Performance of Bi2MoO6 Nano/Microstructures. Catalysts. 2020; 10(10):1161. https://doi.org/10.3390/catal10101161
Chicago/Turabian StyleJi, Tao, Enna Ha, Mingzhou Wu, Xin Hu, Jie Wang, Yangang Sun, Shijie Li, and Junqing Hu. 2020. "Controllable Hydrothermal Synthesis and Photocatalytic Performance of Bi2MoO6 Nano/Microstructures" Catalysts 10, no. 10: 1161. https://doi.org/10.3390/catal10101161
APA StyleJi, T., Ha, E., Wu, M., Hu, X., Wang, J., Sun, Y., Li, S., & Hu, J. (2020). Controllable Hydrothermal Synthesis and Photocatalytic Performance of Bi2MoO6 Nano/Microstructures. Catalysts, 10(10), 1161. https://doi.org/10.3390/catal10101161