MOF Embedded and Cu Doped CeO2 Nanostructures as Efficient Catalyst for Adipic Acid Production: Green Catalysis
Abstract
1. Introduction
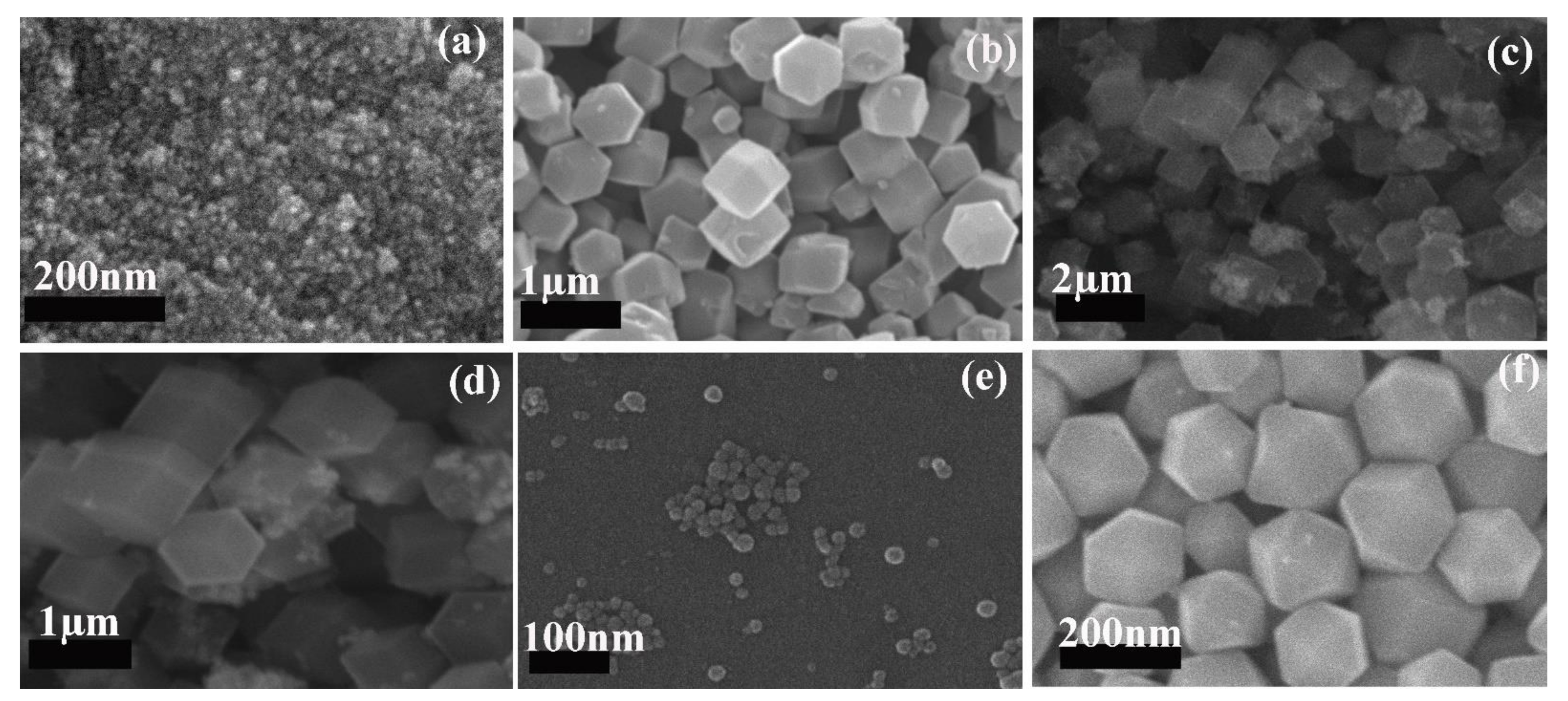
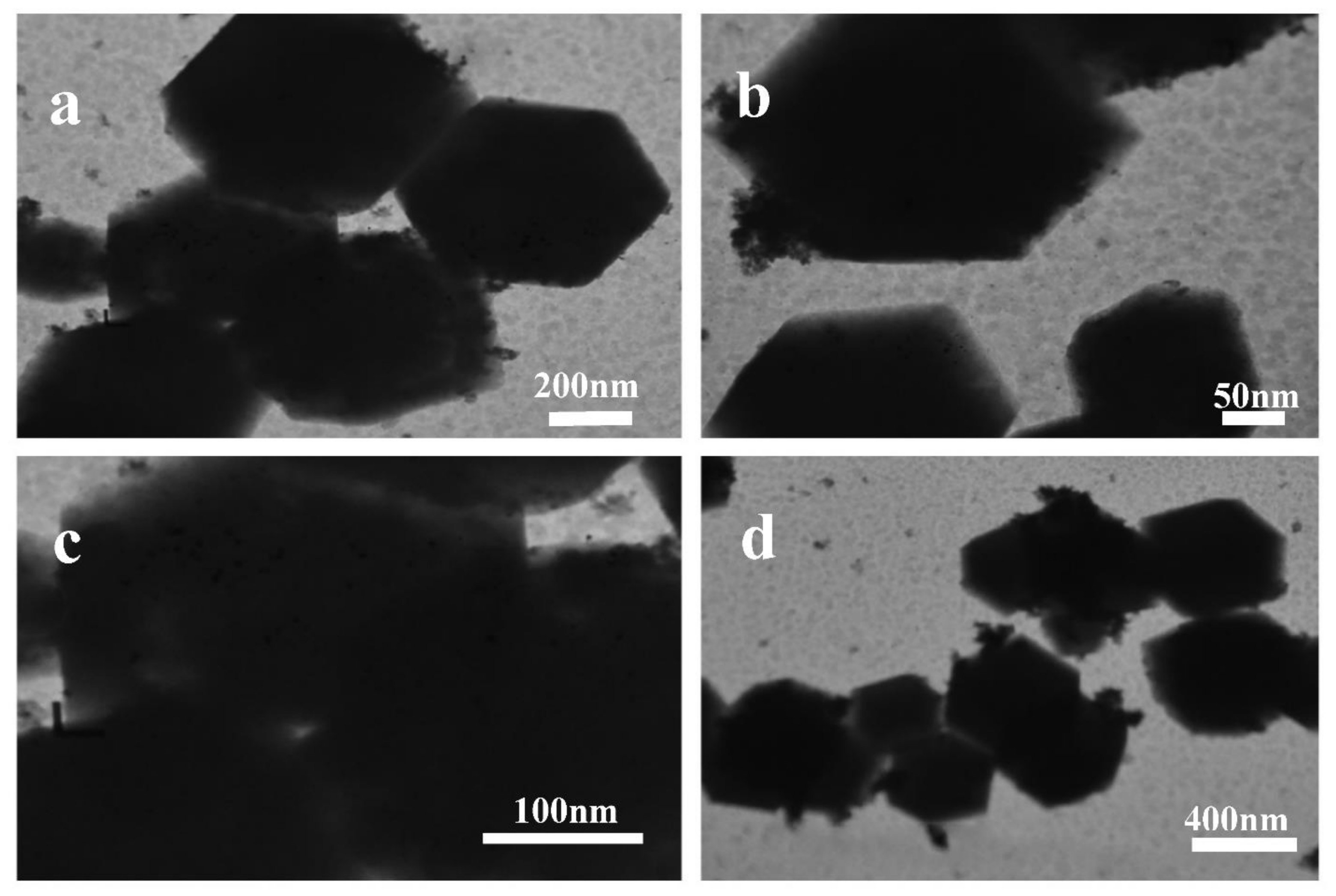
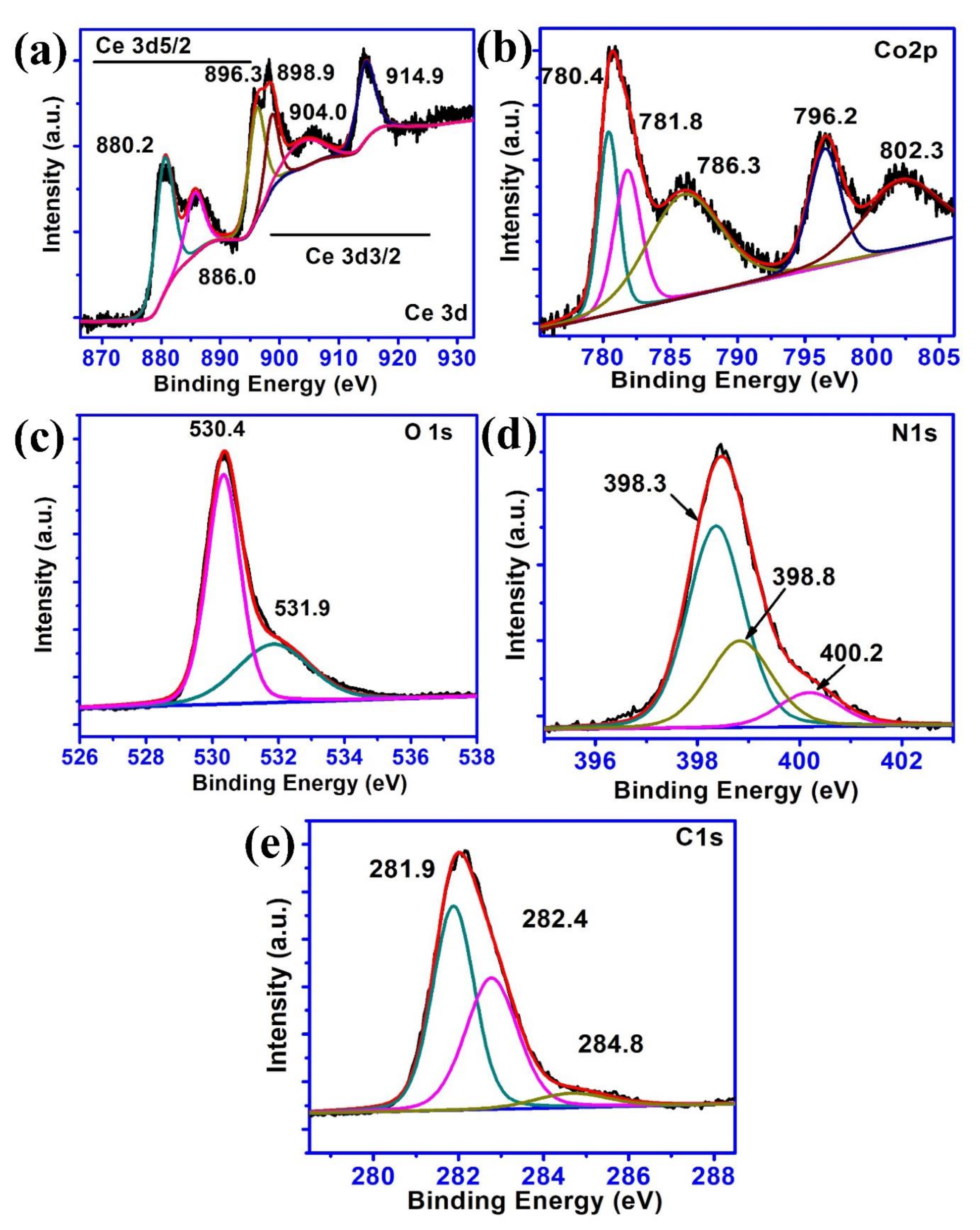
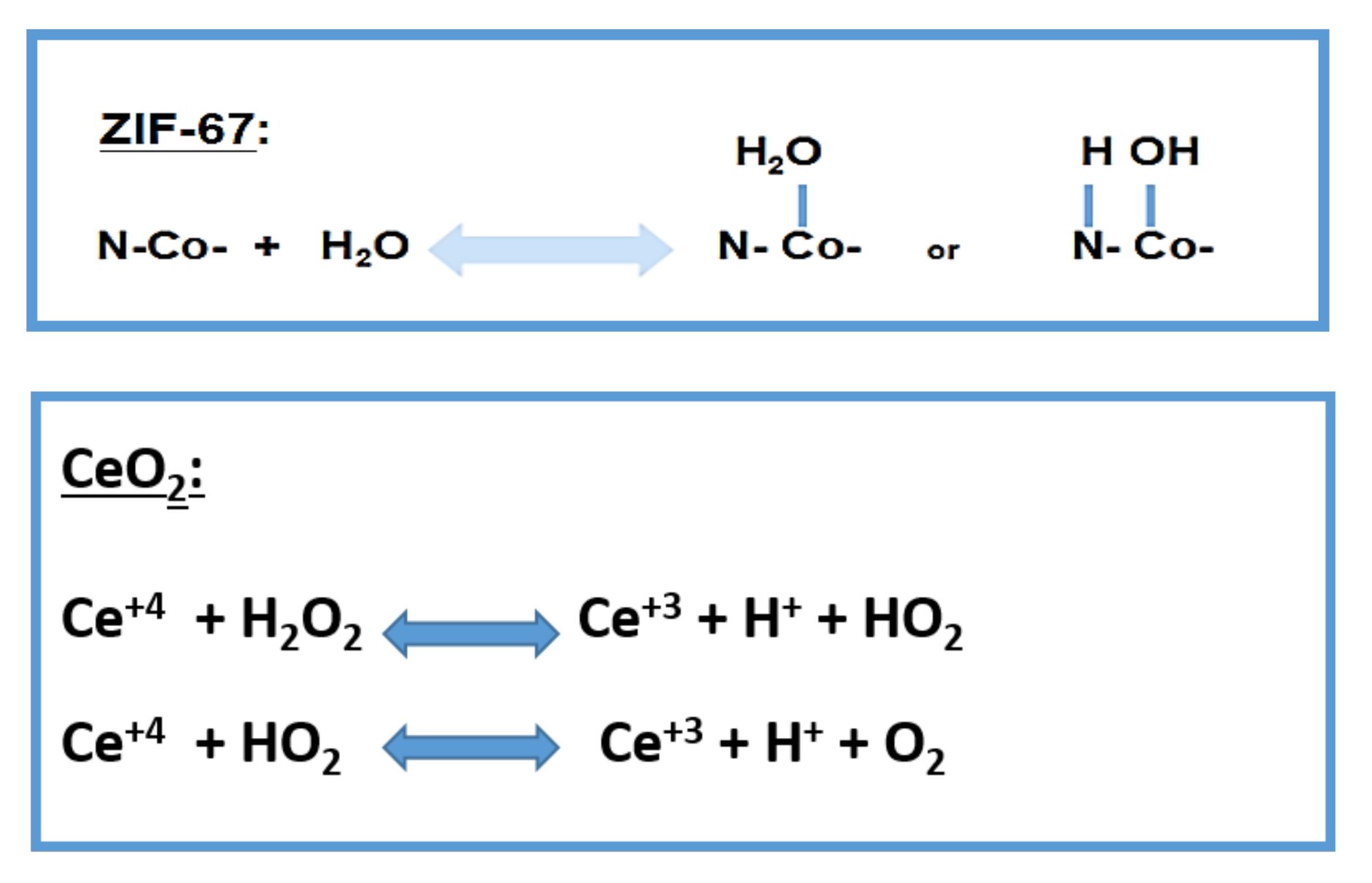
2. Results and Discussion
Catalytic Activity
3. Methods and Materials
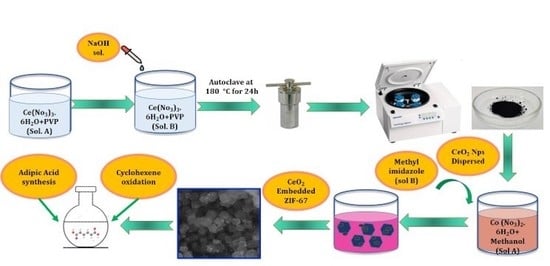
3.1. Synthesis of CeO2 Nanoparticles
3.2. Synthesis of ZIF-67 and ZIF-67/CeO2
3.3. Synthesis of Cu Doped CeO2 Nanospheres (10% vs. 90%)
3.4. Oxidation of Cyclohexene with H2O2
3.5. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Zhao, Q.; Yu, T.; Du, X.; Hao, X. Highly dispersed Ag nanoparticles embedded on the surface of CeO2/CF nanowires derived from three-dimensional structured Cu foam for toluene catalytic oxidation. J. Mol. Catal. 2020, 486, 110879. [Google Scholar] [CrossRef]
- Nyk, M.; Kumar, R.; Ohulchansky, T.Y.; Bergey, E.J.; Prasad, P.N. High contrast in vitro and in vivo photoluminescence bioimaging using near infrared to near infrared up-conversion in Tm3+ and Yb3+ doped fluoride nanophosphors. Nano Lett. 2008, 8, 3834–3838. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L.; Zhou, J.; Li, F.; Cao, T.; Huang, C. Multimodal-Luminescence Core–Shell Nanocomposites for Targeted Imaging of Tumor Cells. Chem. Eur. J. 2009, 15, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Weidenkaff, A.; Ebbinghaus, S.G.; Lippert, T. Ln1-x A x CoO3 (Ln = Er, La; A = Ca, Sr)/Carbon Nanotube Composite Materials Applied for Rechargeable Zn/Air Batteries. Chem. Mater. 2002, 14, 1797–1805. [Google Scholar] [CrossRef]
- Rajan, A.R.; Vilas, V.; Rajan, A.; John, A.; Philip, D. Synthesis of nanostructured CeO2 by chemical and biogenic methods: Optical properties and bioactivity. Ceram. Int. 2020, 46, 14048–14055. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, K.; Li, J.; Gao, J.; Zhao, H.; Yu, R.; Tang, R. Multi-shelled CeO2 hollow microspheres as superior photocatalysts for water oxidation. Nanoscale 2014, 6, 4072–4077. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Y.; Huang, B.; Li, X.; Guo, L.; Zheng, A.; Luque, R.; Sun, Y. Photoactive CeO2/SBA-15 functionalized materials as efficient systems for mono-dehydration of sugar alcohols. Molecular Catalysis. J. Mol. Catal. 2020, 487, 110844. [Google Scholar] [CrossRef]
- You, Y.; Shi, C.; Chang, H.; Guo, L.; Xu, L.; Li, J. The promoting effects of amorphous CePO4 species on phosphorus-doped CeO2/TiO2 catalysts for selective catalytic reduction of NOx by NH3. J. Mol. Catal. 2018, 453, 47–54. [Google Scholar] [CrossRef]
- Carrettin, S.; Concepción, P.; Corma, A.; López Nieto, J.M.L.; Puntes, V.F. Nanocrystalline CeO2 increases the activity of Au for CO oxidation by two orders of magnitude. Angew. Chem. Int. Ed. 2004, 43, 2538–2540. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zheng, H.P.; Liu, H.C.; Yan, C.H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.; Parker, S.C.; Watson, G.W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595, 223–232. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X.; Chen, Z.; Huang, Z.; Jing, G. Oxygen vacancy defect engineering in Mn-doped CeO2 nanostructures for nitrogen oxides emission abatement. J. Mol. Catal. 2019, 47, 110512. [Google Scholar] [CrossRef]
- Li, Z.; Han, F.; Li, C.; Jiao, X.; Chen, D. Hollow CeO2 dodecahedrons: One-step template synthesis and enhanced catalytic performance. RSC Adv. 2016, 6, 60975–60982. [Google Scholar] [CrossRef]
- Feng, Z.; Ren, Q.; Peng, R.; Mo, S.; Zhang, M.; Fu, M.; Chen, L.; Ye, D. Effect of CeO2 morphologies on toluene catalytic combustion. Catal. Today 2019, 332, 177–182. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Alhumaimess, M.; Aldosari, O.; Alshammari, H.; Kamel, M.M.; Betiha, M.A.; Hassan, H.M.A. Ionic liquid green synthesis of CeO2 nanorods and nano-cubes: Investigation of the shape dependent on catalytic performance. J. Mol. Liq. 2019, 279, 649–656. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, D.; Shi, Z.; Zhang, X. Thickness-dependent morphology, microstructure, adsorption and surface free energy of sputtered CeO2 films. Ceram. Int. 2020, 46, 13925–13931. [Google Scholar] [CrossRef]
- An, T.; Deng, X.; Liu, S.; Wang, S.; Ju, J.; Dou, C. Growth and roughness dependent wetting properties of CeO2 films prepared by glancing angle deposition. Ceram. Int. 2018, 44, 9742–9745. [Google Scholar] [CrossRef]
- Zanon, A.; Chaemchuen, S.; Mousavi, B.; Verpoot, F. 1 Zn-doped ZIF-67 as catalyst for the CO2 fixation into cyclic carbonates. J. CO2 Util. 2017, 20, 282–291. [Google Scholar] [CrossRef]
- Yang, H.; He, X.W.; Wang, F.; Kang, Y.; Zhang, J. Doping copper into ZIF-67 for enhancing gas uptake capacity and visible-light-driven photocatalytic degradation of organic dye. J. Mater. Chem. 2012, 22, 21849–21851. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhai, B.; Liang, Y.; Li, Y. Hybrid photocatalysts using semiconductor/MOF/graphene oxide for superior photodegradation of organic pollutants under visible light. Mater. Sci. Semicond. Process. 2020, 107, 104838. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Zhang, Y.; Wang, Z.; Feng, J.; Song, S.; Zhang, H. CeO2 nanowires self-inserted into porous Co3O4 frameworks as high-performance “noble metal free” hetero-catalysts. Chem. Sci. 2016, 7, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, H.; Luque, R.; Li, Y. Metal−organic framework encapsulated Pd nanoparticles: Towards advanced heterogeneous catalysts. Chem. Sci. 2014, 5, 3708–3714. [Google Scholar] [CrossRef]
- Peng, W.; Zheng, G.; Wang, Y.; Cao, S.; Ji, Z.; Huan, Y.; Zou, M.; Yan, X. Zn doped ZIF67-derived porous carbon framework as efficient bifunctional electrocatalyst for water splitting. Int. J. Hydrog. Energy 2019, 44, 19782–19791. [Google Scholar] [CrossRef]
- Tuan, D.D.; Lin, K.Y.A. Ruthenium supported on ZIF-67 as an enhanced catalyst for hydrogen generation from hydrolysis of sodium borohydride. Chem. Eng. J. 2018, 351, 48–55. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, X.; Wei, H.; Li, B.; Jin, B.; Li, L. A large-scale continuous-flow process for the production of adipic acid via catalytic oxidation of cyclohexene with H2O2. Green Chem. 2012, 14, 2868–2875. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef]
- Adili, L.; Roufegarinejad, L.; Tabibiazar, M.; Hamishehkar, H.; Alizadeh, A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: Its application in cake and beef burger. LWT Food Sci. Technol. 2020, 126, 109277. [Google Scholar] [CrossRef]
- Vibha, K.; Negi, S. Enzymatic plasticising of lignin and styrene with adipic acid to synthesize a biopolymer with high antioxidant and thermostability. Polym. Degrad. Stab. 2020, 174, 109081. [Google Scholar] [CrossRef]
- Acid, A. World Market Outlook and Forecast up to 2027; Merchant Research and Consulting Ltd.: Birmingham, UK, 2018. [Google Scholar]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43. [Google Scholar] [CrossRef] [PubMed]
- ALINI, S.; BABINI, P. The Industrial Oxidation of KA Oil to Adipic Acid. In Handbook of Advanced Methods and Processes in Oxidation Catalysis: From Laboratory to Industry; World Scientific: Paris, France, 2014; pp. 320–333. [Google Scholar]
- Mazzi, A.; Paul, S.; Cavani, F.; Wojciesak, R. Cyclohexane Oxidation to Adipic Acid Under Green Conditions: A Scalable and Sustainable Process. Chemcatchem 2018, 10, 3680–3682. [Google Scholar] [CrossRef]
- Shang, M. The Direct Synthesis of Adipic Acid from Cyclohexene and Hydrogen Peroxide by a Continuous Micro-Flow Process. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2016. [Google Scholar]
- Lesage, G.; Penate, I.Q.; Cognet, P.; Poux, M. Green process for adipic acid synthesis: Oxidation by hydrogen peroxide in water micromelusions using Benzalkonium Chloride C12-14 surfactant. Int. J. Chem. React. Eng. 2012, 10, 1–18. [Google Scholar] [CrossRef][Green Version]
- Deng, Y.; Ma, Z.; Wang, K.; Chen, J. Clean synthesis of adipic acid by direct oxidation of cyclohexene with H2O2 over peroxytungstate–organic complex catalysts. Green Chem. 1999, 1, 275–276. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Trautwein, G.; Garcia-Garcia, A. Influence of peroxometallic intermediaries present on polyoxometalates nanoparticles surface on the adipic acid synthesis. J. Mol. Catal. A Chem. 2014, 394, 211–216. [Google Scholar] [CrossRef]
- Raj, K.; Partow, S.; Correia, K.; Khusnutdinova, A.N.; Yahunin, A.F.; Mahadevan, R. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metab. Eng. Commun. 2018, 6, 28–32. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd–Lewis acid catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R.; Jhonson, B.F.G.; O’Connell, T.J.; Sanakar, G.; Khimyak, T. Bimetallic nanocatalysts for the conversion of muconic acid to adipic acid. Chem. Commun. 2003, 1126–1127. [Google Scholar] [CrossRef]
- Zhao, R.; Ji, D.; Lv, G.; Qian, G.; Yan, L.; Wang, X.; Sou, J. A highly efficient oxidation of cyclohexane over Au/ZSM-5 molecular sieve catalyst with oxygen as oxidant. Chem. Commun. 2004, 35, 904–905. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lin, K.J.; Prasad, M.R.; Fu, S.J.; Chang, S.Y.; Shyu, S.G.; Sheu, S.H.; Chen, C.H.; Chuang, C.H.; Lin, M.T. Synthesis of a reusable oxotungsten-containing SBA-15 mesoporous catalyst for the organic solvent-free conversion of cyclohexene to adipic acid. Catal. Commun. 2007, 8, 1060–1064. [Google Scholar] [CrossRef]
- Saedi, Z.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Muhammadpoor-Baltork, I. MIL-101 metal–organic framework: A highly efficient heterogeneous catalyst for oxidative cleavage of alkenes with H2O2. Catal. Commun. 2012, 17, 18–22. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Kholdeeva, O.A.; Jhung, S.H.; Chang, J.S. Titanium and cerium-containing mesoporous silicate materials as catalysts for oxidative cleavage of cyclohexene with H2O2: A comparative study of catalytic activity and stability. Appl. Catal. A 2008, 345, 195–200. [Google Scholar] [CrossRef]
- Kehoe, A.B.; Scanlon, D.O.; Watson, W. Role of lattice distortions in the oxygen storage capacity of divalently doped CeO2. Chem. Mater. 2011, 23, 4464–4468. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Sun, Y.; Zhou, K. Synthesis of one-dimensional Ce1 − xYxO2 − x/2 (0 ≤ x ≤ 1) solid solutions and their catalytic properties: The role of oxygen vacancies. J. Phys. Chem. C 2010, 114, 8926–8932. [Google Scholar] [CrossRef]
- Li, T.; Xiang, G.; Zhuang, J.; Wang, X. Enhanced catalytic performance of assembled ceria necklace nanowires by Ni doping. Chem. Commun. 2011, 47, 6060–6062. [Google Scholar] [CrossRef]
- Qin, J.; Lu, J.; Cao, M.; Hu, C. Synthesis of porous CuO–CeO2 nanospheres with an enhanced low-temperature CO oxidation activity. Nanoscale 2010, 2, 2739–2743. [Google Scholar] [CrossRef]
- Lin, M.; Fu, Z.Y.; Tan, H.R.; Tan, J.P.Y.; Ng, S.C.; Teo, E. Hydrothermal synthesis of CeO2 nanocrystals: Ostwald ripening or oriented attachment? Cryst. Growth Des. 2012, 12, 3296–3303. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Qin, Z.; Sun, H.; Jiao, X.; Chen, D. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale 2013, 5, 11770–11775. [Google Scholar] [CrossRef]
- Yang, F.; Wei, J.; Liu, W.; Guo, J.; Yang, Y. Copper doped ceria nanospheres: Surface defects promoted catalytic activity and a versatile approach. J. Mater. Chem. A 2014, 2, 5662–5667. [Google Scholar] [CrossRef]
- Wu, H.; Qian, X.; Zhu, H.; Ma, S.; Zhu, G.; Long, Y. Controlled synthesis of highly stable zeolitic imidazolate framework-67 dodecahedra and their use towards the templated formation of a hollow Co3O4 catalyst for CO oxidation. RSC Adv. 2016, 6, 6915–6920. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.P.; Yang, M.; Huo, L.H.; Zhao, H.; Grenier, J.C. Facile synthesis and excellent electrochemical performance of reduced graphene oxide–Co3O4 yolk-shell nanocages as a catalyst for oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 13534–13542. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Yu, J. Combustion synthesis and characterization of nanocrystalline CeO2-based powders via ethylene glycol–nitrate process. Mater. Lett. 2006, 60, 57–62. [Google Scholar] [CrossRef]
- Kumar, K.S.; Jaya, N.V. Synthesis and Characterization of Pure and Sn-Doped CeO2 Nanoparticles. Asian J. Chem. 2013, 25, 6095–6098. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 nanoparticles/nitrogen-doped carbon composites with extraordinary catalytic activity for oxygen evolution reaction (OER). Nanomicro Lett. 2018, 10, 15. [Google Scholar] [CrossRef]
- Xu, Q.; Lei, W.; Li, X.; Qi, X.; Yu, J.; Liu, G.; Wang, J.; Zhang, P. Efficient removal of formaldehyde by nanosized gold on well-defined CeO2 nanorods at room temperature. Environ. Sci. Technol. 2014, 48, 9702–9708. [Google Scholar] [CrossRef]
- Briggs, D. Practical Surface Analysis Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; John Wiley: New York, NY, USA, 1990; Volume 1, p. 657. [Google Scholar]
- Kim, M.H.; Choo, K.H. Low-temperature continuous wet oxidation of trichloroethylene over CoOx/TiO2 catalysts. Catal. Commun. 2007, 8, 462–466. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Han, L.; Liu, W.; Zhang, C.; Zhang, X.; Wang, S.; Yang, Y. Mesoporous-shelled CeO2 hollow nanospheres synthesized by a one-pot hydrothermal route and their catalytic performance. Cryst. Eng. Comm. 2013, 15, 7769–7775. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Martínez, L.; Roman, E.; De Segovia, J.L.; Poupard, S.; Creus, J.; Pedraza, F. Surface study of cerium oxide based coatings obtained by cathodic electrodeposition on zinc. Appl. Surf. Sci. 2011, 257, 6202–6207. [Google Scholar] [CrossRef]
- Qian, J.; Chen, F.; Zhao, X.; Chen, Z. China rose petal as biotemplate to produce two-dimensional ceria nanosheets. J. Nanopart. Res. 2011, 13, 7149–7158. [Google Scholar] [CrossRef]
- Park, K.S.; Jin, S.; Kang, K.H.; Lee, J.; Song, I.; Lee, B.S.; Kim, S.; Sohn, J.; Pak, C.; Kim, G.; et al. Characterization of Zeolitic Imidazolate Framework-Derived Polyhedral Carbonaceous Material and Its Application to Electrocatalyst for Oxygen Reduction Reaction. Int. J. Electrochem. Sci. 2016, 11, 9295–9306. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Liu, F.; Majeed, M.K.; Feng, Z.; Cui, Y.; Yang, J.; Qian, Y. Metal-organic framework-derived Co0. 85Se nanoparticles in N-doped carbon as a high-rate and long-lifespan anode material for potassium ion batteries. Mater. Today Energy 2018, 10, 241–248. [Google Scholar]
- Kuruppathparambil, R.R.; Jose, T.; Babu, R.; Hwang, G.Y.; Kathalikkatail, A.C.; Kim, D.W.; Park, D.W. A room temperature synthesizable and environmental friendly heterogeneous ZIF-67 catalyst for the solvent less and co-catalyst free synthesis of cyclic carbonates. Appl. Catal. B 2016, 182, 562–569. [Google Scholar] [CrossRef]
- Lee, H.; Ko, J.H.; Kwon, W.H.; Park, Y.K. Conversion of acetic acid from the catalytic pyrolysis of xylan over CeO2. J. Nanosci. Nanotechnol. 2016, 16, 4480–4482. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Weng, D. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, M.; Noyori, R. A “green” route to adipic acid: Direct oxidation of cyclohexenes with 30 percent hydrogen peroxide. Science 1998, 281, 1646–1647. [Google Scholar] [CrossRef]
- Wang, L.; Wan, C.; Fu, Y.; Chen, H.; Liu, X.; Li, M. Study on the effects of adipic acid on properties of dicyandiamide-cured electrically conductive adhesive and the interaction mechanism. J. Electron. Mater. 2014, 43, 132–136. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Hashemi, M.M. One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nano-rods in a Brønsted acidic ionic liquid. RSC Adv. 2015, 5, 31298–31302. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Hashemi, M.M. Simple and green oxidation of cyclohexene to adipic acid with an efficient and durable silica-functionalized ammonium tungstate catalyst. Catal. Commun. 2014, 43, 169–172. [Google Scholar] [CrossRef]
- Agúndez, J.; Ares, C.; Márquez-Álvarez, C.; Pariente, J.P. Catalytic oxidation of cyclohexene by supported gold nanoclusters synthesized in a two-liquid phases system containing eucalyptus essential oil. Mol. Catal. 2020, 488, 110922. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoinead, A.A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal−organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, B.; Chaemcheun, S.; Moosavi, B.; Luo, Z.; Gholampur, N.; Verpoort, F. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates. New J. Chem. 2016, 40, 5170–5176. [Google Scholar] [CrossRef]
- Dai, J.; Guo, Y.; Xu, L.; Zhuang, G.; Zheng, Y.; Sun, D.; Huang, J.; Li, Q. Bovine serum albumin templated porous CeO2 to support Au catalyst for benzene oxidation. Mol. Catal. 2020, 486, 110849. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Li, J.; Zhen, J.; Zhang, H. Clean synthesis of Cu2O@ CeO2 core@ shell nanocubes with highly active interface. NPG Asia Mater. 2015, 7, e158. [Google Scholar] [CrossRef]
- Cai, W.; Chen, F.; Shen, X.; Chen, L.; Zhang, J. Enhanced catalytic degradation of AO7 in the CeO2–H2O2 system with Fe3+ doping. Appl. Catal. B 2010, 101, 160–168. [Google Scholar] [CrossRef]
- Ji, P.; Wang, L.; Chen, F.; Zhang, J. Ce3+-centric organic pollutant elimination by CeO2 in the presence of H2O2. ChemCatChem 2010, 2, 1552–1554. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.M.; Hwang, G.Y.; Jeong, G.S.; Kim, M.; Kim, D.W.; Park, D.W. A solid solution zeolitic imidazolate framework as a room temperature efficient catalyst for the chemical fixation of CO2. Green Chem. 2016, 18, 6349–6356. [Google Scholar] [CrossRef]
- Jose, T.; Hwang, Y.; Kim, D.W.; Kim, M.; Park, D.W. Functionalized zeolitic imidazolate framework F-ZIF-90 as efficient catalyst for the cycloaddition of carbon dioxide to allyl glycidyl ether. Catal. Today 2015, 245, 61–67. [Google Scholar] [CrossRef]
- Fang, W.; Chen, J.; Zhou, X.; Chen, J.; Ye, Z.; Li, Y. Zeolitic Imidazolate Framework-67 Derived CeO2@ Co3O4 Core-shell Microspheres with Enhanced Catalytic Activity towards Toluene Oxidation. Ind. Eng. Chem. Res. 2020, 59, 10328–10337. [Google Scholar] [CrossRef]
- Kan, J.; Deng, L.; Li, B.; Huang, Q.; Zhu, S.; Shen, S.; Chen, Y. Performance of co-doped Mn-Ce catalysts supported on cordierite for low concentration chlorobenzene oxidation. Appl. Catal. A 2017, 530, 21–29. [Google Scholar] [CrossRef]
- Rao, B.G.; Sudarsanam, P.; Nallappareddy, P.R.G.; Reddy, M.Y.; Rao, T.V.; Reddy, B.M. Selective allylic oxidation of cyclohexene over a novel nanostructured CeO2–Sm2O3/SiO2 catalyst. Res. Chem. Intermed. 2018, 44, 6151–6168. [Google Scholar] [CrossRef]
- Hakat, Y.; Kotbagi, T.V.; Bakker, M.G. Silver supported on hierarchically porous SiO2 and Co3O4 monoliths: Efficient heterogeneous catalyst for oxidation of cyclohexene. J. Mol. Catal. A Chem. 2016, 411, 61–71. [Google Scholar] [CrossRef]
- Acharyya, S.S.; Ghosh, S.; Bal, R. Nanoclusters of Cu (II) supported on nanocrystalline W (VI) oxide: A potential catalyst for single-step conversion of cyclohexane to adipic acid. Green Chem. 2015, 17, 3490–3499. [Google Scholar] [CrossRef]
- Soares, J.C.S.; Gonçalves, A.H.A.; Zotin FM, Z.; Araújo, L.R.R.; Gaspar, A.B. Cyclohexene to adipic acid synthesis using heterogeneous polyoxometalate catalysts. Mol. Catal. 2018, 458, 223–229. [Google Scholar] [CrossRef]
- Lesage, G.; Peñate, I.Q.; Franceschi, S.; Perez, E.; Garrigues, J.C.; Poux, M.; Cognet, P. Sustainable process for adipic acid production from cyclohexene in microemulsion. Catal. Today 2019, 346, 40–45. [Google Scholar] [CrossRef]
- Moreno, I.; Navascues, N.; Irusta, S.; Santamaria, J. Electrospun Au/CeO2 nanofibers: A highly accessible low-pressure drop catalyst for preferential CO oxidation. J. Catal. 2015, 329, 479–489. [Google Scholar] [CrossRef]







| Catalysts | Yield (%) a | Reaction Conditions | Reactant Conversion (%) b | Product Selectivity (%) c | Reference |
|---|---|---|---|---|---|
| MIL-101 | 90 | 70 °C, 8 h | >99 | Not reported | [45] |
| WO4/silica gel | 84 | 87 °C, 15 h | 100 | 99 | [74] |
| Ag2WO4 | 85 | 75 °C, 18 h | 99 | 98 | [73] |
| Cu/WO3 | 66 | 70 °C, 12 h | 75 | 88 | [88] |
| K3PW12O40 | 77 | 75 °C, 24 h | 100 | Not reported | [89] |
| Na2WO4 | 80 | 85 °C, 8 h | >99 | Not reported | [90] |
| ZIF-67/CeO2 | 72 | 90 °C, 12 h | 100% | >99% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, S.; Pervaiz, E.; Yang, M.; Rabi, O. MOF Embedded and Cu Doped CeO2 Nanostructures as Efficient Catalyst for Adipic Acid Production: Green Catalysis. Catalysts 2021, 11, 304. https://doi.org/10.3390/catal11030304
Bibi S, Pervaiz E, Yang M, Rabi O. MOF Embedded and Cu Doped CeO2 Nanostructures as Efficient Catalyst for Adipic Acid Production: Green Catalysis. Catalysts. 2021; 11(3):304. https://doi.org/10.3390/catal11030304
Chicago/Turabian StyleBibi, Shabahat, Erum Pervaiz, Minghui Yang, and Osama Rabi. 2021. "MOF Embedded and Cu Doped CeO2 Nanostructures as Efficient Catalyst for Adipic Acid Production: Green Catalysis" Catalysts 11, no. 3: 304. https://doi.org/10.3390/catal11030304
APA StyleBibi, S., Pervaiz, E., Yang, M., & Rabi, O. (2021). MOF Embedded and Cu Doped CeO2 Nanostructures as Efficient Catalyst for Adipic Acid Production: Green Catalysis. Catalysts, 11(3), 304. https://doi.org/10.3390/catal11030304