Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids
Abstract
1. Introduction
2. Results and Discussion
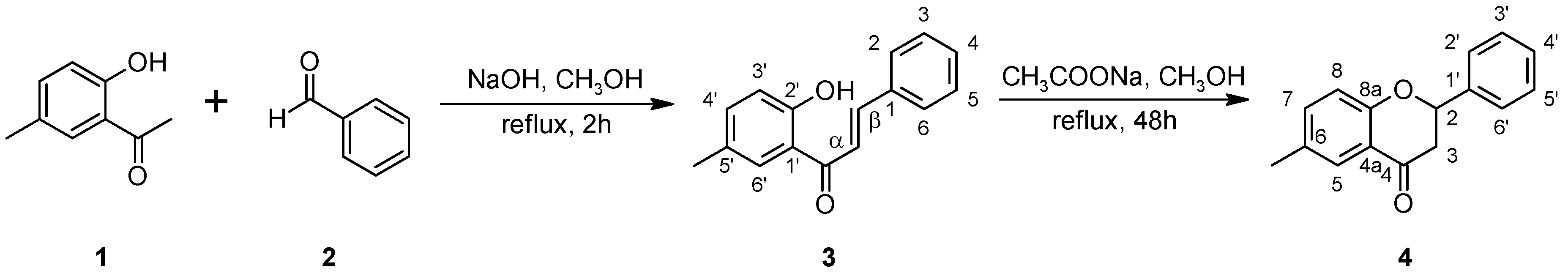
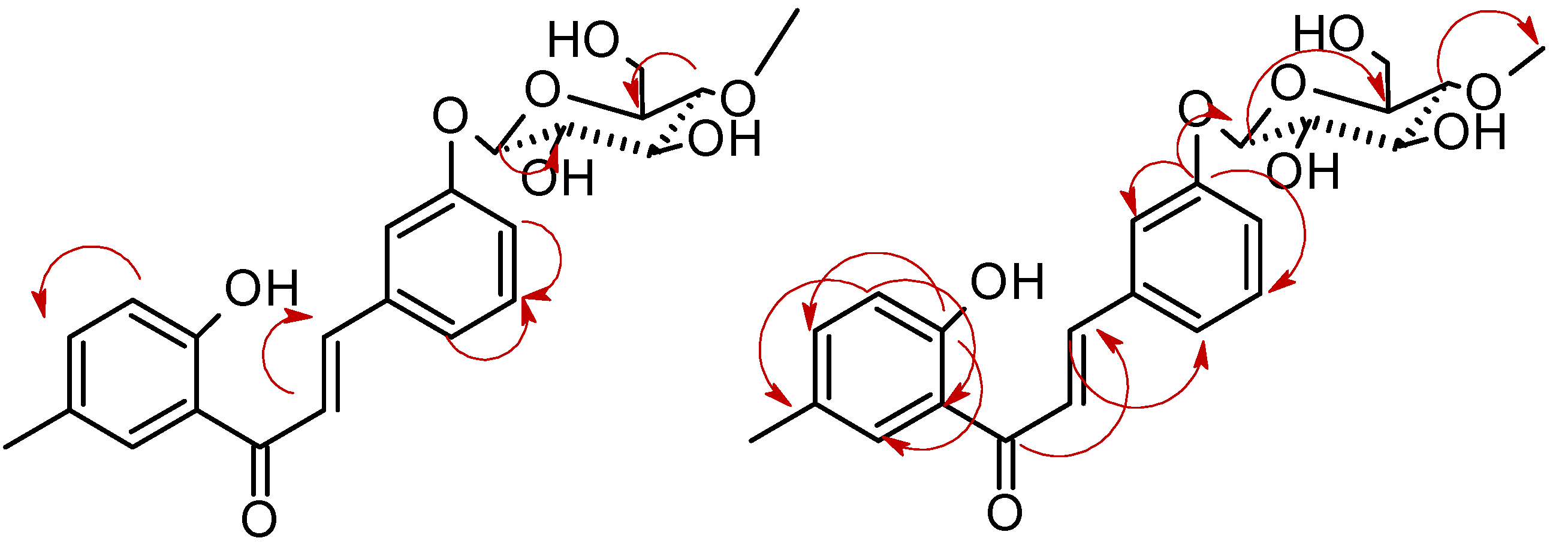
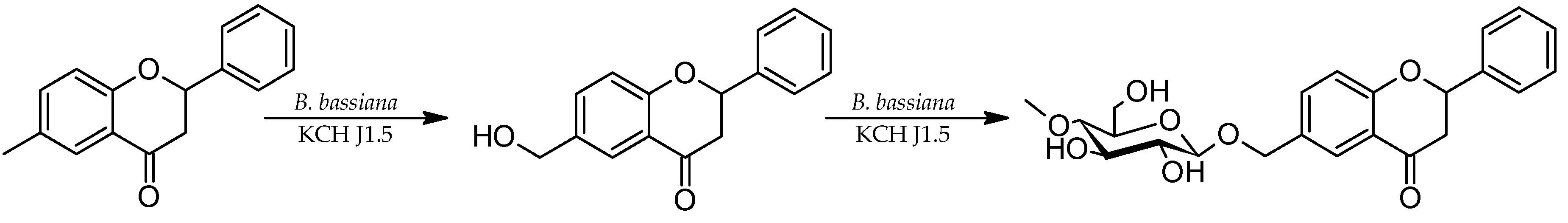
2.1. Biotransformations of 2′-hydroxy-5′-methylchalcone (3)
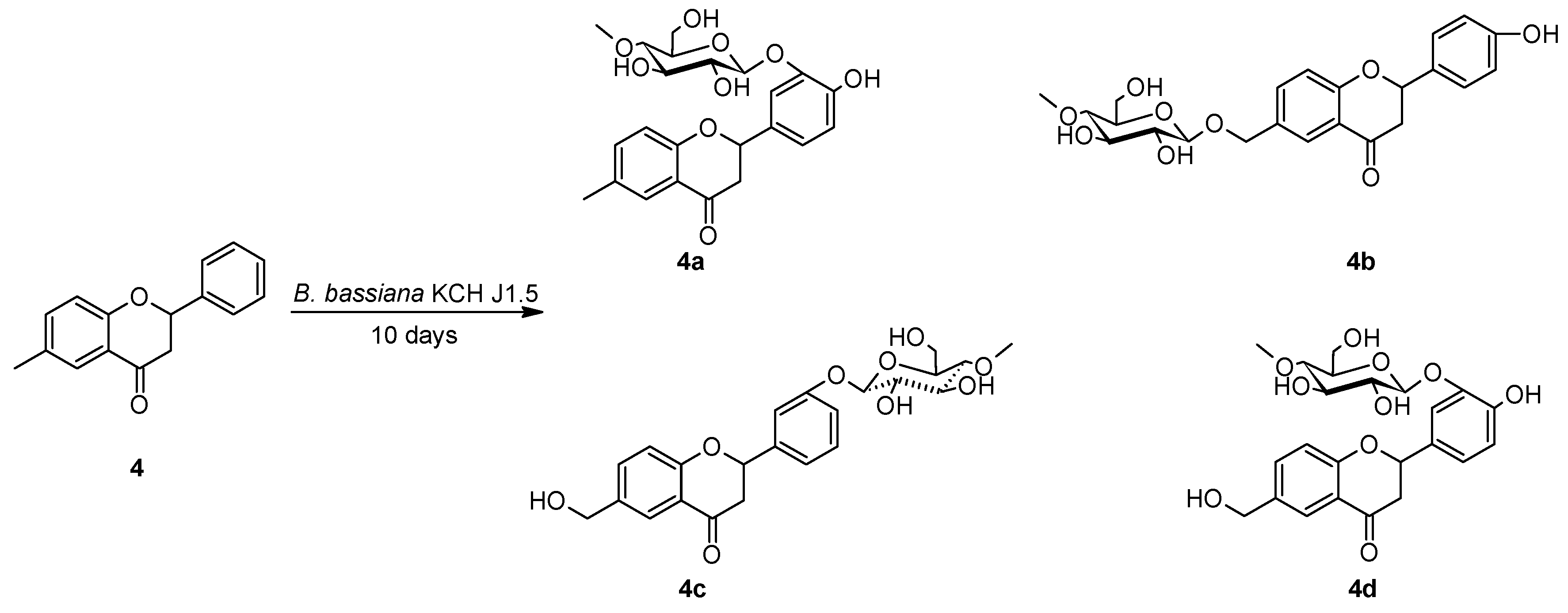
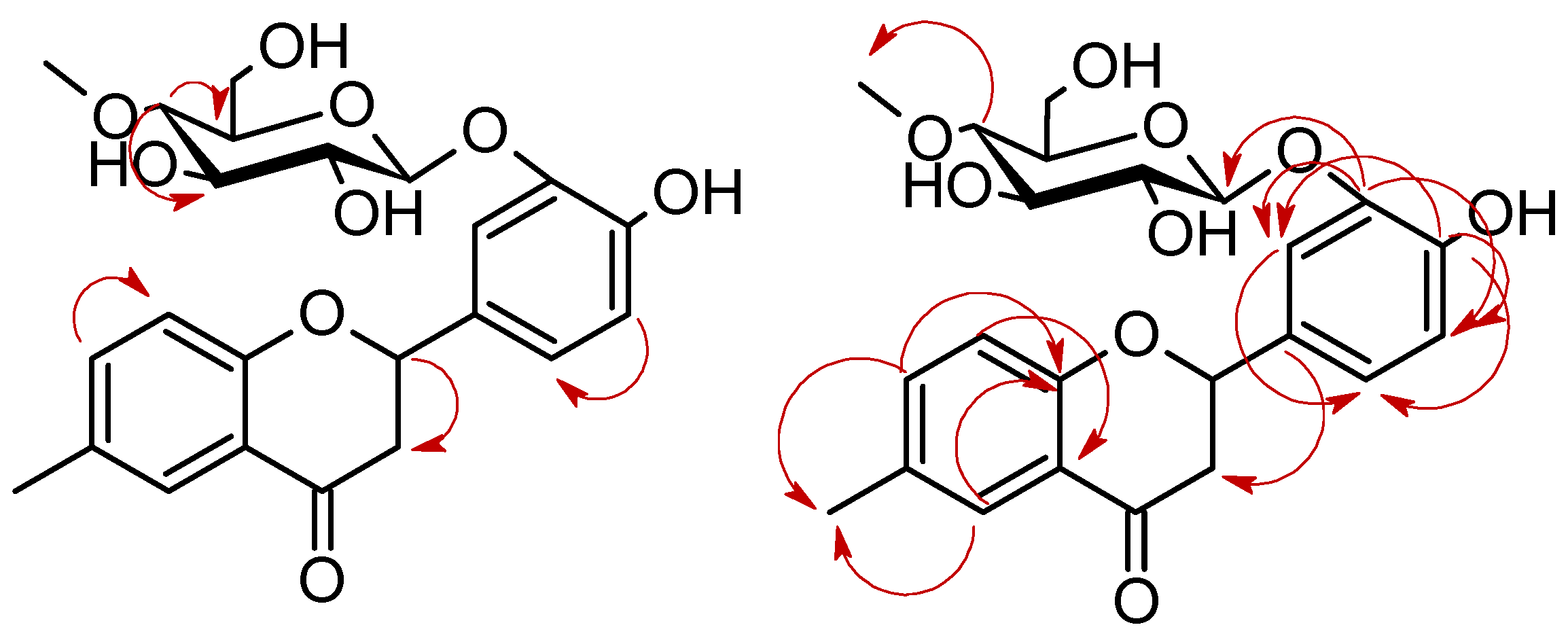
2.2. Biotransformations of 6-methylflavanone (4) in the culture of B. bassiana KCH J1.5
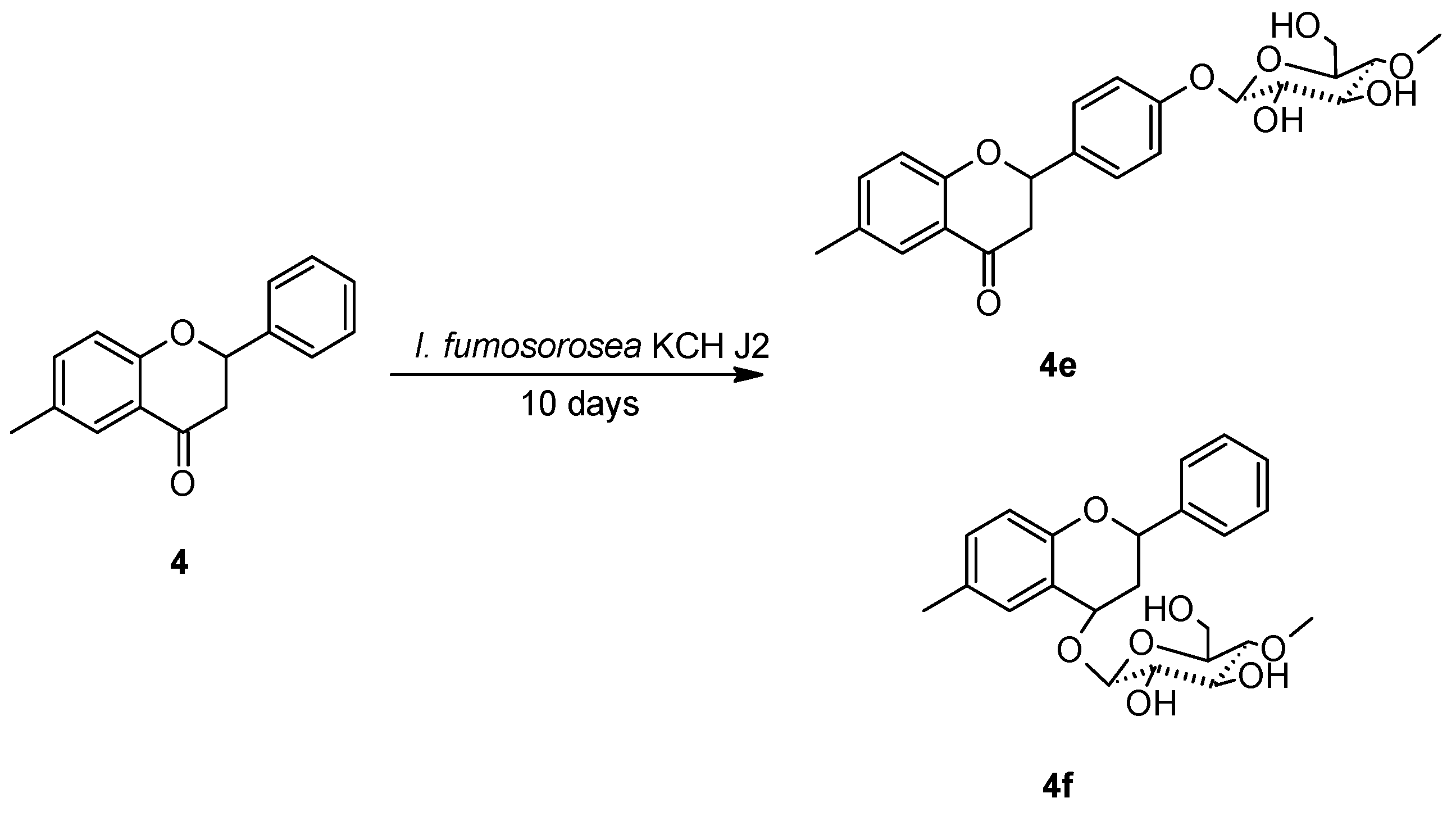
2.3. Biotransformations of 6-methylflavanone (4) in the culture of I. fumosorosea KCH J2
3. Materials and Methods
3.1. Substrates
3.1.1. 2′-Hydroxy-5′-methylchalcone (3)
3.1.2. 6-Methylflavanone (4)
3.2. Microorganisms
3.3. Analysis
3.4. Screening Procedure
3.5. The Semi-Preparative Biotransformations
3.5.1. 2′-Hydroxy-5′-methylchalcone 3-O-β-D-(4″-O-methyl)-glucopyranoside (3a)
3.5.2. 4′-Hydroxy-6-methylflavanone 3′-O-β-D-(4″-O-methyl)-glucopyranoside (4a)
3.5.3. 4′-Hydroxyflavanone 6-methylene-O-β-D-(4″-O-methyl)-glucopyranoside (4b)
3.5.4. 6-Hydroxymethylflavanone 3′-O-β-D-(4″-O-methyl)-glucopyranoside (4c)
3.5.5. 4′-Hydroxy-6-hydroxymethylflavanone 3′-O-β-D-(4″-O-methyl)-glucopyranoside (4d)
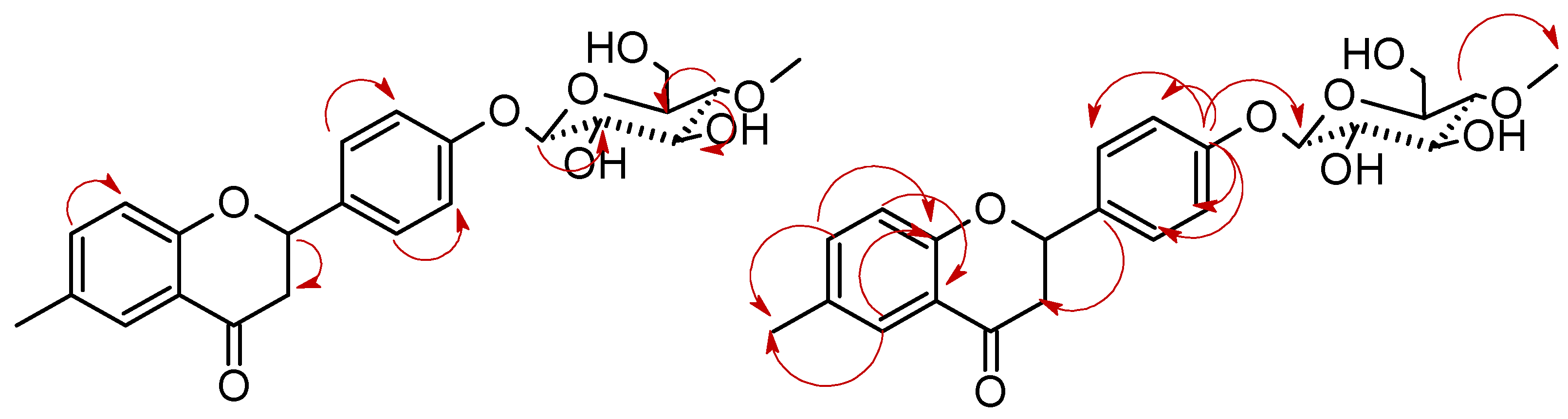
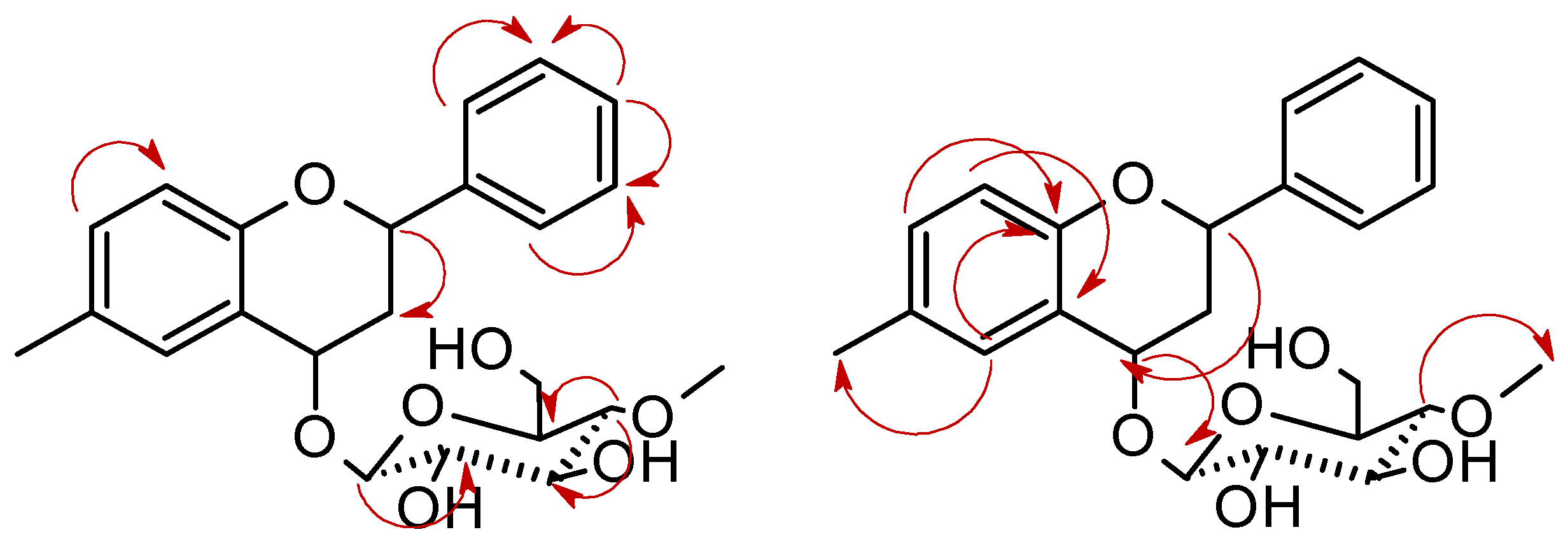
3.5.6. 6-Methylflavanone 4′-O-β-D-(4″-O-methyl)-glucopyranoside (4e)
3.5.7. 2-Phenyl-6-methylchromane 4-O-β-D-(4″-O-methyl)-glucopyranoside (4f)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzym. Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Susłow, E.; Dymarska, M.; Białońska, A.; Janeczko, T. Enantioselective conversion of certain derivatives of 6-hydroxyflavanone. J. Mol. Catal. B Enzym. 2014, 102, 59–65. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef]
- Plaza, M.; Pozzo, T.; Liu, J.; Ara, K.Z.G.; Turner, C.; Nordberg Karlsson, E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, V.H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Grzeszczuk, J.; Urbaniak, M.; Janeczko, T.; Pląskowska, E.; Stępień, Ł.; Kostrzewa-Susłow, E. Glycosylation of 6-methylflavone by the strain Isaria fumosorosea KCH J2. PLoS ONE 2017, 12, e0184885. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of methoxylated flavonoids in the cultures of Isaria fumosorosea KCH J2. Molecules 2018, 23, 2578. [Google Scholar] [CrossRef]
- Dou, F.; Wang, Z.; Li, G.; Dun, B. Microbial transformation of flavonoids by Isaria fumosorosea ACCC 37814. Molecules 2019, 24, 1028. [Google Scholar] [CrossRef]
- Hyung Ko, J.; Gyu Kim, B.; Joong-Hoon, A. Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol. Lett. 2006, 258, 263–268. [Google Scholar] [CrossRef]
- Gurung, R.B.; Kim, E.H.; Oh, T.J.; Sohng, J.K. Enzymatic synthesis of apigenin glucosides by glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13. Mol. Cells 2013, 36, 355–361. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Wang, C.; Wang, X.; Xu, Y.; Yu, H.; Wu, P.; Li, S.; Han, L.; Gunatilaka, A.A.L.; et al. Methylglucosylation of aromatic amino and phenolic moieties of drug-like biosynthons by combinatorial biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4980–E4989. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Bai, J.; Yue, Q.; Zhang, M.; Li, J.; Wang, C.; Xu, Y. Methylglucosylation of phenolic compounds by fungal glycosyltransferase-methyltransferase functional modules. J. Agric. Food Chem. 2019, 67, 8573–8580. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Dymarska, M.; Janeczko, T. Microbial transformations of 3-methoxyflavone by strains of Aspergillus niger. Pol. J. Microbiol. 2014, 63, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.L.; Lu, Y.H.; Wei, D.Z. Flavonoids from Cleistocalyx operculatus. Phytochemistry 2004, 65, 445–447. [Google Scholar] [CrossRef]
- Ye, C.L.; Liu, Y.; Wei, D.Z. Antioxidant and anticancer activity of 3′-formyl-4′, 6′-dihydroxy-2′-methoxy-5′-methylchalcone and (2S)-8-formyl-5-hydroxy-7-methoxy-6-methylflavanone. J. Pharm. Pharm. 2007, 59, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Tung, B.T.; Nguyen, P.H.; Thuong, P.T.; Yoo, S.S.; Kim, E.H.; Kim, S.K.; Oh, W.K. C-methylated flavonoids from Cleistocalyx operculatus and their inhibitory effects on novel influenza A (H1N1) neuraminidase. J. Nat. Prod. 2010, 73, 1636–1642. [Google Scholar] [CrossRef]
- Chen, W.Q.; Song, Z.J.; Xu, H.H. A new antifungal and cytotoxic C-methylated flavone glycoside from Picea neoveitchii. Bioorg. Med. Chem. Lett. 2012, 22, 5819–5822. [Google Scholar] [CrossRef]
- Nobakht, M.; Grkovic, T.; Trueman, S.J.; Wallace, H.M.; Katouli, M.; Quinn, R.J.; Brooks, P.R. Chemical constituents of kino extract from Corymbia torelliana. Molecules 2014, 19, 17862–17871. [Google Scholar] [CrossRef]
- Nobakht, M.; Trueman, S.J.; Wallace, H.M.; Brooks, P.R.; Streeter, K.J.; Katouli, M. Antibacterial properties of flavonoids from kino of the eucalypt tree, Corymbia torelliana. Plants 2017, 6, 39. [Google Scholar] [CrossRef]
- Salmazzo, G.R.; Verdan, M.H.; Silva, F.; Cicarelli, R.M.; da Silva Mota, J.; Salvador, M.J.; de Carvalho, J.E.; Cardoso, C.A.L. Chemical composition and antiproliferative, antioxidant and trypanocidal activities of the fruits from Campomanesia xanthocarpa (Mart.) O. Berg (Myrtaceae). Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Hall, B.J.; Chebib, M.; Hanrahan, J.R.; Johnston, G.A.R. 6-Methylflavanone, a more efficacious positive allosteric modulator of γ-aminobutyric acid (GABA) action at human recombinant α2β2γ2L than at α1β2γ2L and α1β2 GABAA receptors expressed in Xenopus oocytes. Eur. J. Pharm. 2006, 512, 97–104. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Jensen, A.B.; Meyling, N.V.; Eilenberg, J. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 2014, 59, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Valero-Jiménez, C.A.; Wiegers, H.; Zwaan, B.J.; Koenraadt, C.J.M.; van Kan, J.A.L. Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol. 2016, 133, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Cito, A.; Barzanti, G.P.; Strangi, A.; Francardi, V.; Zanfini, A.; Dreassi, E. Cuticle-degrading proteases and toxins as virulence markers of Beauveria bassiana (Balsamo) Vuillemin. J. Basic Microbiol. 2016, 56, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St. Leger, R.J.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Chen, J.; Lai, Y.; Wang, L.; Zhai, S.; Zou, G.; Zhou, Z.; Cui, C.; Wang, S. CRISPR/Cas9-mediated efficient genome editing via blastospore-based transformation in entomopathogenic fungus Beauveria bassiana. Sci. Rep. 2017, 7, 45763. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, E.; Urbaniak, M.; Hoc, N.; Grzeszczuk, J.; Dymarska, M.; Stępień, Ł.; Pląskowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Cascade biotransformation of dehydroepiandrosterone (DHEA) by Beauveria species. Sci. Rep. 2018, 8, 13449. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): Biology, ecology and use in biological control. Biocontrol Sci. Technol. 2008, 18, 865–901. [Google Scholar] [CrossRef]
- Weng, Q.; Zhang, X.; Chen, W.; Hu, Q. Secondary metabolites and the risks of Isaria fumosorosea and Isaria farinosa. Molecules 2019, 24, 664. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, I.S. Microbial metabolism of the prenylated chalcone xanthohumol. J. Nat. Prod. 2006, 69, 1522–1524. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and antiproliferative activity of glycosides obtained by biotransformation of xanthohumol. Bioorg. Med. Chem. Lett. 2013, 23, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Bartmańska, A.; Tronina, T. Glycosylation of xanthohumol by fungi. Z. Nat. C J. Biosci. 2008, 63, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Hofer, B. Recent developments in the enzymatic O-glycosylation of flavonoids. Appl. Microbiol. Biotechnol. 2016, 100, 4269–4281. [Google Scholar] [CrossRef] [PubMed]
- Júnior, G.M.V.; Sousa, C.M.D.M.; Cavalheiro, A.J.; Lago, J.H.G.; Chaves, M.H. Phenolic derivatives from fruits of Dipteryx lacunifera Ducke and evaluation of their antiradical activities. Helv. Chim. Acta 2008, 91, 2159–2167. [Google Scholar] [CrossRef]
- Moon, B.H.; Lee, Y.; Ahn, J.H.; Lim, Y. Complete assignment of 1H and 13C NMR data of dihydroxyflavone derivatives. Magn. Reson. Chem. 2006, 44, 99–101. [Google Scholar] [CrossRef]
- Park, Y.; Moon, B.H.; Lee, Y.; Yoon, Y.; Ahn, J.H.; Lim, Y. 1H and 13C-NMR data of hydroxyflavone derivatives. Magn. Reson. Chem. 2007, 45, 488–495. [Google Scholar] [CrossRef]
- Cuca-Suárez, L.; Monache, F. 6-C-formyl and 6-C-hydroxymethyl flavonoides from Petiveria Alliacea. Phytochemistry 1992, 31, 2481–2482. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Huszcza, E. Microbial glycosylation of flavonoids. Pol. J. Microbiol. 2016, 65, 137–151. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Regioselective O-glycosylation of flavonoids by fungi Beauveria bassiana, Absidia coerulea and Absidia glauca. Bioorg. Chem. 2019, 93, 102750. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Tavares, H.R.; Barros, A.I.N.R.A.; Cavaleiro, J.A.S. NMR and structural and conformational features of 2′-hydroxychalcones and flavones. Spectrosc. Lett. 1997, 30, 1655–1667. [Google Scholar] [CrossRef]
- Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. Chem. Biol. Drug Des. 2012, 80, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, T.; Gładkowski, W.; Kostrzewa-Susłow, E. Microbial transformations of chalcones to produce food sweetener derivatives. J. Mol. Catal. B Enzym. 2013, 98, 55–61. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Kostrzewa-Susłow, E.; Janeczko, T.; Środa-Pomianek, K.; Poła, A.; Uryga, A.; Michalak, K. Cyclization of flavokawain B reduces its activity against human colon cancer cells. Hum. Exp. Toxicol. 2020, 39, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Murti, Y.; Mishra, P. Synthesis and evaluation of flavanones as anticancer agents. Indian J. Pharm. Sci. 2014, 76, 163–166. [Google Scholar]
- Gładkowski, W.; Siepka, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Popłoński, J.; Mazur, M.; Żarowska, B.; Łaba, W.; Maciejewska, G.; Wawrzeńczyk, C. Synthesis and antimicrobial activity of methoxy-substituted γ-Oxa-ε -lactones derived from flavanones. Molecules 2019, 24, 4151. [Google Scholar] [CrossRef]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Xue, X.; Zhang, F.; Xu, Q.; Liang, X. Structural characterization of iridoid glucosides by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1941–1954. [Google Scholar] [CrossRef]

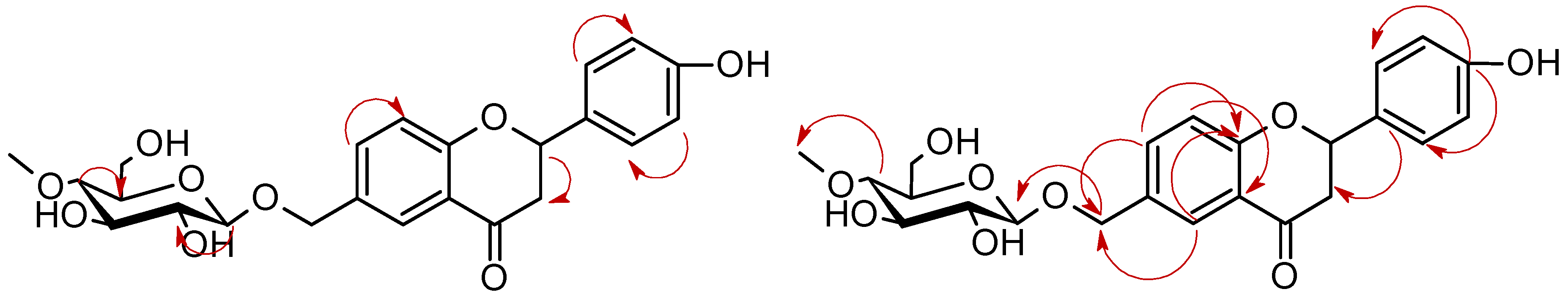
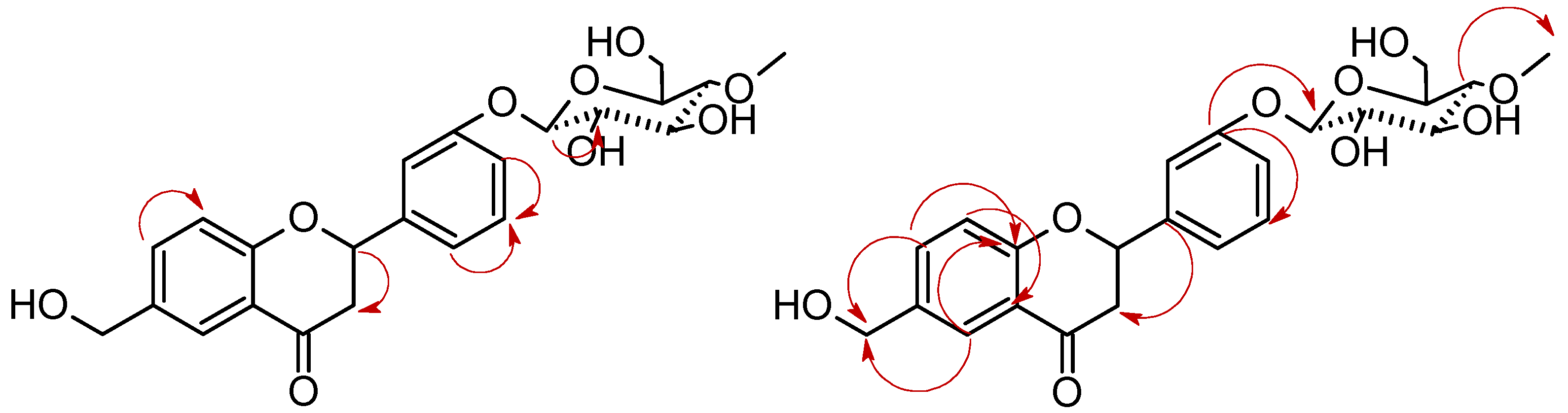
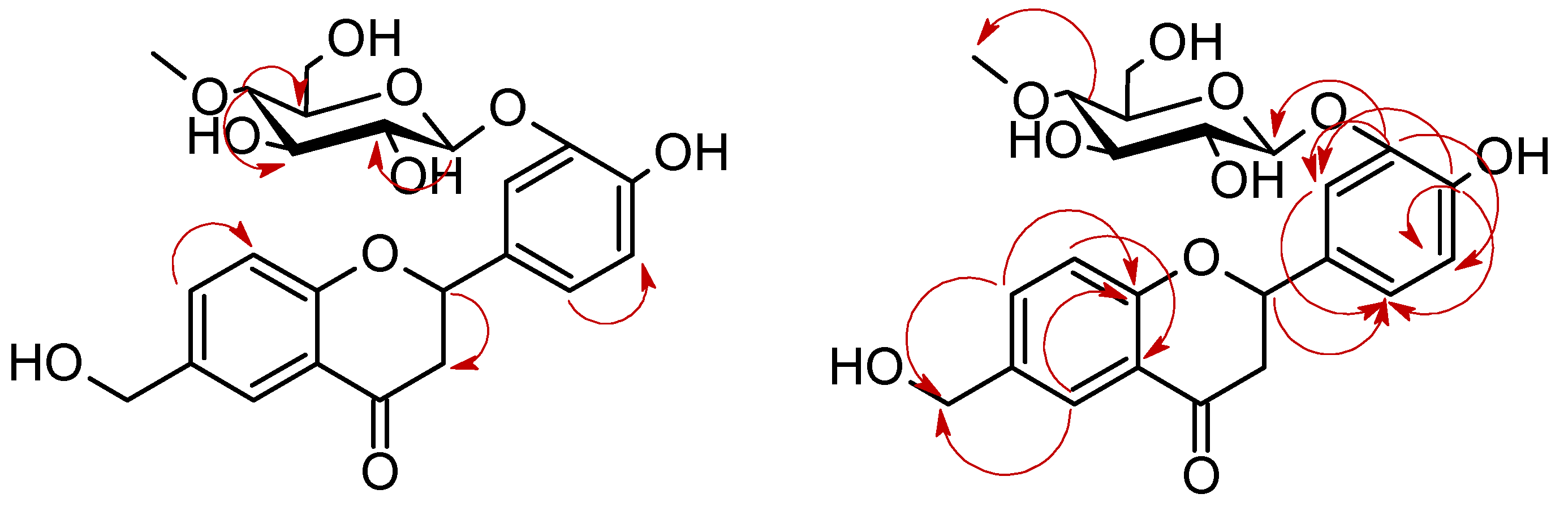
| Proton | Compound | |
|---|---|---|
| 3 | 3a | |
| H-2 | 7.68 (m) | 7.61 (m) |
| H-3 | 7.45 (dd) J = 4.8, 1.8 | - |
| H-4 | 7.45 (dd) J = 4.8, 1.8 | 7.16 (ddd) J = 8.1, 2.3, 0.6 |
| H-5 | 7.45 (dd) J = 4.8, 1.8 | 7.40 (dd) J = 13.0, 5.0 |
| H-6 | 7.68 (m) | 7.47 (d) J = 7.7 |
| H- α | 7.68 (m) | 8.03 (d) J α, β= 15.5 |
| H- β | 7.92 (d) J β, α = 15.5 | 7.87 (d) J β, α = 15.5 |
| H-2′ | - | - |
| H-3′ | 6.94 (d) J = 8.4 | 6.89 (d) J = 8.4 |
| H-4′ | 7.32 (dd) J = 8.4, 2.1 | 7.40 (dd) J = 13.0, 5.0 |
| H-5′ | - | - |
| H-6′ | 7.68 (m) | 8.12 (d) J = 1.3 |
| H-1″ | - | 5.03 (d) J = 7.8 |
| H-2″ | - | 3.52 (ddd) J = 15.1, 9.1, 5.1 |
| H-3″ | - | 3.66 (m) |
| H-4″ | - | 3.19 (m) |
| H-5″ | - | 3.61 (ddd) J = 9.7, 5.8, 2.2 |
| H-6″ | - | 3,92 (ddd) J = 11.8, 5.6, 2.1 3.73 (dt) J = 12.1, 6.2 |
| C4″-OCH3 | - | 3.57 (s) |
| C2′-OH | 12.63 (s) | 12.67 (s) |
| C5′-CH3 | 2.36 (s) | 2.35 (s) |
| Carbon | Compound | |
|---|---|---|
| 3 | 3a | |
| C-1 | 134.8 | 137.1 |
| C-2 | 128.8 | 116.7 |
| C-3 | 129.2 | 159.2 |
| C-4 | 131.0 | 120.2 |
| C-5 | 129.2 | 130.8 |
| C-6 | 128.8 | 124.3 |
| C- α | 120.4 | 122.0 |
| C- β | 145.4 | 145.6 |
| C-1′ | 119.8 | 120.5 |
| C-2′ | 161.7 | 162.5 |
| C-3′ | 118.5 | 118.7 |
| C-4′ | 137.7 | 138.5 |
| C-5′ | 128.1 | 129.3 |
| C-6′ | 129.5 | 131.1 |
| C-1″ | - | 101.7 |
| C-2″ | - | 75.0 |
| C-3″ | - | 78.1 |
| C-4″ | - | 80.5 |
| C-5″ | - | 77.2 |
| C-6″ | - | 62.3 |
| C4″-OCH3 | - | 60.6 |
| C = O | 193.8 | 194.9 |
| C5′-CH3 | 20.8 | 20.4 |
| Proton | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 4 | 4a | 4b | 4c | 4d | 4e | 4f | |
| H-2 | 5.59 (dd) J2,3ax = 13.0, J2,3eq = 2.8 | 5.46 (dd) J2,3ax = 12.8, J2,3eq = 2.9 | 5.52 (dd) J2,3ax = 13.0, J2,3eq = 2.8 | 5.61 (dd) J2,3ax = 12.5, J2,3eq = 3.0 | 5.48 (dd) J2,3ax = 12.8, J2,3eq = 2.9 | 5.53 (dd) J2,3ax = 12.9, J2,3eq = 2.9 | 5.36 (dd) J = 12.1, J = 1.9 |
| H-3ax | 3.11 (dd) J3ax,2 = 13.0, J3ax,3eq = 16.7 | 3.11 (dd) J3ax,2 = 12.8, J3ax,3eq = 16.7 | 3.16 (dt) J = 8.3, J3ax,3eq = 16.8 | 3.13 (dd) J3ax,2 = 12.5, J3ax,3eq = 16.8 | 3.15 (dd) J3ax,2 = 12.9, J3ax,3eq = 16.8 | 3.12 (dd) J3ax,2 = 12.9, J3ax,3eq = 16.7 | 2.43 (dt) J = 14.1, J = 2.2 |
| H-3eq | 2.85 (d) J3eq,2 =2.9 | 2.78 (dd) J3eq,2 = 2.9, J3eq,3ax = 16.8 | 2.79 (dd) J3eq,2 = 2.9, J3eq,3ax = 16.8 | 2.90 (d) J = 3.2 | 2.80 (dd) J3eq,2 = 2.9, J3eq,3ax = 16.7 | 2.79 (dd) J3eq,2 = 2.9, J3eq,3ax = 16.8 | 2.07 (ddd) J = 6.0, J = 3.6, J = 1.2 |
| H-4 | - | - | - | - | - | - | 4.92 (t) J = 2.8 |
| H-5 | 7.63 (s) | 7.61 (d) J5,7 = 2.3 | 7.83 (d) J5,7 =2.2 | 7.82 (d) J5,7 =2.1 | 7.82 (d) J5,7 = 2.3 | 7.62 (d) J5,7 = 1.5 | 7.22 (d) J5,7 = 1.9 |
| H-6 | - | - | - | - | - | - | - |
| H-7 | 7.40 (dt) J = 10.9, J = 4.7 | 7.39 (m) | 7.61 (dd) J7,8 = 8.5, J7,5 = 2.2 | 7.58 (dd) J7,8 = 8.5, J7,5 = 2.3 | 7.56 (dd) J7,8 = 8.5, J7,5 = 2.1 | 7.39 (m) | 7.08 (dd) J7,8 = 8.3, J7,5 = 1.9 |
| H-8 | 6.98 (d) J8,7 = 8.4 | 6.96 (d) J8,7 = 8.4 | 7.02 (d) J8,7 = 8.6 | 7.07 (dd) J8,7 = 8.3, J = 1.7 | 7.02 (d) J8,7 = 8.5 | 6.95 (d) J8,7 = 8.4 | 6.80 (d) J8,7 = 8.3 |
| H-2′ | 7.58 (d) J2′,3′ = 7.6 | 7.39 (m) | 7.41 (m) | 7.29 (m) | 7.41 (dd) J = 4.5, J = 2.1 | 7.49 (m) | 7.50 (d) J = 7.2 |
| H-3′ | 7.45 (t) J = 7.5 | - | 6.90 (m) | - | - | 7.12 (m) | 7.40 (t) J = 7.6 |
| H-4′ | 7.40 (dt) J = 10.9, J = 4.7 | - | - | 7.07 (dd) J = 8.3, J = 1.7 | - | - | 7.33 (m) |
| H-5′ | 7.45 (t) J = 7.5 | 6.91 (d) J = 8.2 | 6.90 (m) | 7.35 (t) J = 8.0 | 6.92 (d) J = 8.2 | 7.12 (m) | 7.40 (t) J = 7.6 |
| H-6′ | 7.58 (d) J = 7.6 | 7.16 (dt) J = 2.0, J = 8.2 | 7.41 (m) | 7.21 (d) J = 7.7 | 7.17 (dd) J6′,5′ = 8.3, J = 1.9 | 7.49 (m) | 7.50 (d) J = 7.2 |
| H-1″ | - | 4.79 (d) J = 7.9 | 4.36 (d) J = 7.8 | 4.98 (d) J = 7.8 | 4.82 (d) J = 7.9 | 4.97 (d) J = 7.8 | 4.49 (d) J = 7.8 |
| H-2″ | - | 3.50 (t) J = 9.4 | 3.26 (m) | 3.48 (m) | 3.50 (t) J = 8.2 | 3.48 (ddd) J = 9.8, J = 5.0, J = 2.0 | 3.30 (m) |
| H-3″ | - | 3.63 (m) | 3.50 (m) | 3.62 (ddd) J = 9.1, J = 7.6, J = 4.2 | 3.63 (d) J = 6.6 | 3.63 (t) J = 9.0 | 3.52 (m) |
| H-4″ | - | 3.22 (m) | 3.10 (m) | 3.21 (m) | 3.21 (m) | 3.21 (dd) J = 9.6, J = 9.1 | 3.12 (dd) J = 9.6, J = 9.0 |
| H-5″ | - | 3.45 (ddd) J = 9.8, J = 4.9, J = 1.9 | 3.26 (m) | 3.48 (m) | 3.46 (m) | 3.48 (ddd) J = 9.8, J = 5.0, J = 2.0 | 3.30 (m) |
| H-6″ | - | 3.85 (dt) J = 8.5, J = 4.4 3.69 (m) | 3.82 (d) J = 10.0 3.66 (m) | 3.84 (dd) J = 9.3, J = 5.2 3.68 (m) | 3.85 (d) J = 10.6 3.68 (m) | 3.83 (d) J = 12.2 3.69 (m) | 3.89 (m) 3.71 (dt) J = 11.4, J = 5.8 |
| -CH2- | - | - | 4.86 (dd) J = 11.8 J = 2.6 4.61 (dt) J = 11.7 J =2.7 | 4.61 (d) J = 5.2 | 4.61 (m) | - | - |
| C6-CH3 | 2.32 (s) | 2.31 (s) | - | - | - | 2,31 (s) | 2.27 (s) |
| C4″-OCH3 | - | 3.55 (s) | 3.53 (s) | 3.56 (s) | 3.55 (s) | 3.56 (s) | 3.54 (s) |
| Carbon | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 4 | 4a | 4b | 4c | 4d | 4e | 4f | |
| C-2 | 80.3 | 80.0 | 80.4 | 80.0 | 80.1 | 80.0 | 73.8 |
| C-3 | 45.1 | 44.9 | 44.8 | 44.9 | 44.7 | 44.9 | 37.5 |
| C-4 | 191.9 | 192.3 | 192.3 | 191.9 | 192.3 | 192.2 | 70.2 |
| C-4a | 121.6 | 121.6 | 121.5 | 121.5 | 121.5 | 121.5 | 120.5 |
| C-5 | 127.0 | 126.9 | 126.7 | 125.2 | 125.2 | 126.9 | 132.7 |
| C-6 | 131.6 | 131.5 | 132.3 | 136.7 | 136.5 | 131.5 | 129.6 |
| C-7 | 137.7 | 137.7 | 136.7 | 135.6 | 135.5 | 137.8 | 131.5 |
| C-8 | 118.8 | 118.8 | 118.8 | 117.1 | 118.8 | 118.7 | 117.5 |
| C-8a | 160.5 | 160.6 | 162.0 | 161.4 | 161.5 | 160.5 | 154.1 |
| C-1′ | 140.5 | 131.8 | 131.0 | 141.9 | 131.6 | 133.9 | 142.7 |
| C-2′ | 127.3 | 117.9 | 129.0 | 115.4 | 117.7 | 128.7 | 127.1 |
| C-3′ | 129.5 | 146.1 | 116,2 | 158.9 | 146.1 | 117.3 | 129.2 |
| C-4′ | 129.3 | 149.0 | 158.7 | 118.8 | 149.0 | 158.8 | 128.6 |
| C-5′ | 129.5 | 116.8 | 116.2 | 130.5 | 116.8 | 117.3 | 129.2 |
| C-6′ | 127.3 | 123.3 | 129.0 | 120.8 | 123.3 | 128.7 | 127.1 |
| C-1″ | - | 104.2 | 103.0 | 101.5 | 104.0 | 101.6 | 101.4 |
| C-2″ | - | 75.0 | 75.2 | 75.0 | 74.9 | 74.9 | 75.3 |
| C-3″ | - | 77.5 | 78.1 | 78.1 | 77.5 | 77.9 | 78.2 |
| C-4″ | - | 80.1 | 80,5 | 80.1 | 80.2 | 80.1 | 80.6 |
| C-5″ | - | 77.3 | 76.9 | 77.0 | 77.2 | 77.0 | 76.9 |
| C-6″ | - | 62.0 | 62.4 | 62.1 | 62.0 | 62.0 | 62.6 |
| C6-CH2-OH | - | - | 70.8 | 63.9 | 63.9 | - | - |
| C-4″-OCH3 | - | 60.6 | 60.5 | 60.5 | 60.6 | 60.5 | 60.5 |
| C6-CH3 | 20.4 | 20.4 | - | - | - | 20.4 | 20.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids. Catalysts 2020, 10, 1148. https://doi.org/10.3390/catal10101148
Krawczyk-Łebek A, Dymarska M, Janeczko T, Kostrzewa-Susłow E. Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids. Catalysts. 2020; 10(10):1148. https://doi.org/10.3390/catal10101148
Chicago/Turabian StyleKrawczyk-Łebek, Agnieszka, Monika Dymarska, Tomasz Janeczko, and Edyta Kostrzewa-Susłow. 2020. "Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids" Catalysts 10, no. 10: 1148. https://doi.org/10.3390/catal10101148
APA StyleKrawczyk-Łebek, A., Dymarska, M., Janeczko, T., & Kostrzewa-Susłow, E. (2020). Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids. Catalysts, 10(10), 1148. https://doi.org/10.3390/catal10101148





