Highly Effective, Regiospecific Hydrogenation of Methoxychalcone by Yarrowia lipolytica Enables Production of Food Sweeteners
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Substrates
3.2. Microorganisms
3.3. Screening
3.4. Gas Chromatography
3.5. Preparative Scale
3.6. TLC and NMR Analysis
3.7. Increasing the Concentrations of Tested Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
References
- Hutteau, F.; Mathlouthi, M.; Portmann, M.O.; Kilcast, D. Physicochemical and psychophysical characteristics of binary mixtures of bulk and intense sweeteners. Food Chem. 1998, 63, 9–16. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am. J. Clin. Nutr. 2006, 84, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Fructose and risk of cardiometabolic disease. Curr. Atheroscler. Rep. 2012, 14, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.S.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars With the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [Green Version]
- Bornet, F.R.J. Undigestible sugars in food products. Am. J. Clin. Nutr. 1994, 59, 763S–769S. [Google Scholar] [CrossRef]
- Hunter, S.R.; Reister, E.J.; Cheon, E.; Mattes, R.D. Low Calorie Sweeteners Differ in Their Physiological Effects in Humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.Y.; Friel, J.K.; MacKay, D.S. The effect of the artificial sweeteners on glucose metabolism in healthy adults: A randomized, double-blinded, crossover clinical trial. Appl. Physiol. Nutr. Metab. 2020, 45, 606–612. [Google Scholar] [CrossRef]
- Ben Shoshan-Galeczki, Y.; Niv, M.Y. Structure-based screening for discovery of sweet compounds. Food Chem. 2020, 315, 126286. [Google Scholar] [CrossRef]
- Gwak, M.J.; Chung, S.J.; Kim, Y.J.; Lim, C.S. Relative sweetness and sensory characteristics of bulk and intense sweeteners. Food Sci. Biotechnol. 2012, 21, 889–894. [Google Scholar] [CrossRef]
- Ibdah, M.; Martens, S.; Gang, D.R. Biosynthetic Pathway and Metabolic Engineering of Plant Dihydrochalcones. J. Agric. Food Chem. 2018, 66, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Microbial transformations of 4′-methylchalcones as an efficient method of obtaining novel alcohol and dihydrochalcone derivatives with antimicrobial activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef] [Green Version]
- Janeczko, T.; Gładkowski, W.; Kostrzewa-Susłow, E. Microbial transformations of chalcones to produce food sweetener derivatives. J. Mol. Catal. B Enzym. 2013, 98, 55–61. [Google Scholar] [CrossRef]
- Eichenberger, M.; Lehka, B.J.; Folly, C.; Fischer, D.; Martens, S.; Simón, E.; Naesby, M. Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab. Eng. 2017, 39, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Inglett, G.E.; Krbechek, L.; Dowling, B.; Wagner, R. Dihydrochalcone Sweeteners–Sensory and Stability Evaluation. J. Food Sci. 1969, 34, 101–104. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef]
- Han, G.E.; Kang, H.T.; Chung, S.; Lim, C.; Linton, J.A.; Lee, J.H.; Kim, W.; Kim, S.H.; Lee, J.H. Novel neohesperidin dihydrochalcone analogue inhibits adipogenic differentiation of human adipose-derived stem cells through the Nrf2 pathway. Int. J. Mol. Sci. 2018, 19, 2215. [Google Scholar] [CrossRef] [Green Version]
- Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review. Molecules 2019, 24, 4468. [Google Scholar] [CrossRef] [Green Version]
- Sinjman, P.W.; Joubert, E.; Ferreira, D.; Li, X.C.; Ding, Y.; Green, I.R.; Gelderblom, W.C.A. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Silva, V.D.; Stambuk, B.U.; da Graca Nascimento, M. Efficient chemoselective biohydrogenation of 1,3-diaryl-2-propen-1-ones catalyzed by Saccharomyces cerevisiae yeasts in biphasic system. J. Mol. Catal. B Enzym. 2010, 63, 157–163. [Google Scholar] [CrossRef]
- Raimondi, S.; Romano, D.; Amaretti, A.; Molinari, F.; Rossi, M. Enoate reductases from non conventional yeasts: Bioconversion, cloning, and functional expression in Saccharomyces cerevisiae. J. Biotechnol. 2010, 156, 279–285. [Google Scholar] [CrossRef]
- Janeczko, T.; Dymarska, M.; Siepka, M.; Gniłka, R.; Leśniak, A.; Popłoński, J.; Kostrzewa-Susłow, E. Enantioselective reduction of flavanone and oxidation of cis- and trans-flavan-4-ol by selected yeast cultures. J. Mol. Catal. B Enzym. 2014, 109, 47–52. [Google Scholar] [CrossRef]
- Łużny, M.; Krzywda, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Effective Hydrogenation of 3-(2″-furyl)- and 3-(2″-thienyl)-1-(2′-hydroxyphenyl)-prop-2-en-1-one in Selected Yeast Cultures. Molecules 2019, 24, 3185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Shamin, R.V.; Stepanova, N.N.; Morgunov, G.I.; Lunina, J.N.; Allayarov, R.K.; Samoilenko, V.A.; Morgunov, I.G. Fermentation Conditions and Media Optimization for Isocitric Acid Production from Ethanol by Yarrowia lipolytica. Biomed. Res. Int. 2018, 2018, 2543210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, T.; Zannini, E.; Arendt, E.K.; Coffey, A. A review of polyols—Biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Mirończuk, A.M.; Celińska, E.; Białas, W.; Rymowicz, W. Scale-up of the erythritol production technology—Process simulation and techno-economic analysis. J. Clean. Prod. 2020, 257, 120533. [Google Scholar] [CrossRef]
- Janek, T.; Dobrowolski, A.; Biegalska, A.; Mirończuk, A.M. Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb. Cell Fact. 2017, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef]
- Braga, A.; Belo, I. Biotechnological production of γ-decalactone, a peach like aroma, by Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2016, 32, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celińska, E.; Kubiak, P.; Białas, W.; Dziadas, M.; Grajek, W. Yarrowia lipolytica: The novel and promising 2-phenylethanol producer. J. Ind. Microbiol. Biotechnol. 2013, 40, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowska, A.; Bajzert, J.; Babij, K.; Szołtysik, M.; Stefaniak, T.; Willak-Janc, E.; Chrzanowska, J. Reduced IgE and IgG antigenic response to milk proteins hydrolysates obtained with the use of non-commercial serine protease from Yarrowia lipolytica. Food Chem. 2020, 302, 125350. [Google Scholar] [CrossRef]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage lipid and polysaccharide metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Borkowska, M.; Białas, W.; Korpys, P.; Nicaud, J.M. Robust signal peptides for protein secretion in Yarrowia lipolytica: Identification and characterization of novel secretory tags. Appl. Microbiol. Biotechnol. 2018, 102, 5221–5233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juszczyk, P.; Rymowicz, W.; Kita, A.; Rywińska, A. Biomass production by Yarrowia lipolytica yeast using waste derived from the production of ethyl esters of polyunsaturated fatty acids of flaxseed oil. Ind. Crops Prod. 2019, 138, 111590. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hapeta, P.; Rakicka, M.; Dulermo, R.; Gamboa-Meléndez, H.; Cruz-Le Coq, A.-M.; Nicaud, J.-M.; Lazar, Z. Transforming sugars into fat—Lipid biosynthesis using different sugars in Yarrowia lipolytica. Yeast 2017, 34, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, A.; Drzymała, K.; Rzechonek, D.A.; Mituła, P.; Mirończuk, A.M. Lipid production from waste materials in seawater-based medium by the yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.K.; Alper, H.S. Yarrowia lipolytica: More than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 2019, 103, 9251–9262. [Google Scholar] [CrossRef]
- Bilal, M.; Xu, S.; Iqbal, H.M.N.; Cheng, H. Yarrowia lipolytica as an emerging biotechnological chassis for functional sugars biosynthesis. Crit. Rev. Food Sci. Nutr. 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.A.; Alper, H.S. Synthetic Biology Expands the Industrial Potential of Yarrowia lipolytica. Trends Biotechnol. 2018, 36, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.H.; Ledesma-Amaro, R.; Martinez, J.L. Recombinant β-Carotene Production by Yarrowia lipolytica—Assessing the Potential of Micro-Scale Fermentation Analysis in Cell Factory Design and Bioreaction Optimization. Front. Bioeng. Biotechnol. 2020, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Paula, B.R.S.; Zampieri, D.; Rodrigues, J.A.R.; Moran, P.J.S. Bioreduction of α-Acetoxymethyl Enones: Proposal for an SN2′ Mechanism Catalyzed by Enereductase. Adv. Synth. Catal. 2016, 358, 3555–3571. [Google Scholar] [CrossRef]
- Stuermer, R.; Hauer, B.; Hall, M.; Faber, K. Asymmetric bioreduction of activated C=C bonds using enoate reductases from the old yellow enzyme family. Curr. Opin. Chem. Biol. 2007, 11, 203–213. [Google Scholar] [CrossRef]
- Żyszka-Haberecht, B.; Poliwoda, A.; Lipok, J. Structural constraints in cyanobacteria-mediated whole-cell biotransformation of methoxylated and methylated derivatives of 2′-hydroxychalcone. J. Biotechnol. 2019, 293, 36–46. [Google Scholar] [CrossRef]
- Xue, Z.; Sharpe, P.L.; Hong, S.P.; Yadav, N.S.; Xie, D.; Short, D.R.; Damude, H.G.; Rupert, R.A.; Seip, J.E.; Wang, J.; et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat. Biotechnol. 2013, 31, 734–740. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Zhang, H.; Zhang, X.; Apaliya, M.T.; Zheng, X.; Mahunu, G.K. Effect of Yarrowia lipolytica on postharvest decay of grapes caused by Talaromyces rugulosus and the protein expression profile of T. rugulosus. Postharvest Biol. Technol. 2017, 126, 15–22. [Google Scholar] [CrossRef]
- Selvam, D.G.A.; Thatheyus, A.J. Microbial degradation of petroleum hydrocarbons: An overview. Microb. Action Hydrocarb. 2019, 3, 485–503. [Google Scholar]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; Van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Dymarska, M.; Guzik, U.; Wojcieszyńska, D.; Janeczko, T. Stenotrophomonas maltophilia: A Gram-Negative Bacterium Useful for Transformations of Flavanone and Chalcone. Molecules 2017, 22, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stompor, M.; Kałużny, M.; Żarowska, B. Biotechnological methods for chalcone reduction using whole cells of Lactobacillus, Rhodococcus and Rhodotorula strains as a way to produce new derivatives. Appl. Microbiol. Biotechnol. 2016, 100, 8371–8384. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Ahn, S.; Hwang, D.; Yoon, H.; Jo, G.; Kim, Y.H.; Kim, S.H.; Koh, D.; Lim, Y. 1H and 13C NMR spectral assignments of 2′-hydroxychalcones. Magn. Reson. Chem. 2013, 51, 364–370. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Kostrzewa-Susłow, E.; Janeczko, T.; Środa-Pomianek, K.; Poła, A.; Uryga, A.; Michalak, K. Cyclization of flavokawain B reduces its activity against human colon cancer cells. Hum. Exp. Toxicol. 2020, 39, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Henklewska, M.; Hernández Suárez, B.; Łużny, M.; Kozłowska, E.; Obmińska-Mrukowicz, B.; Janeczko, T. Chalcone Methoxy Derivatives Exhibit Antiproliferative and Proapoptotic Activity on Canine Lymphoma and Leukemia Cells. Molecules 2020, 25, 4362. [Google Scholar] [CrossRef]
- Janeczko, T.; Kostrzewa-Susłow, E. Enantioselective reduction of propiophenone formed from 3-chloropropiophenone and stereoinversion of the resulting alcohols in selected yeast cultures. Tetrahedron Asymmetry 2014, 25, 1264–1269. [Google Scholar] [CrossRef]
- Łużny, M.; Tronina, T.; Kozłowska, E.; Dymarska, M.; Popłoński, J.; Łyczko, J.; Kostrzewa-Susłow, E.; Janeczko, T. Biotransformation of Methoxyflavones by Selected Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2020, 21, 6121. [Google Scholar] [CrossRef]
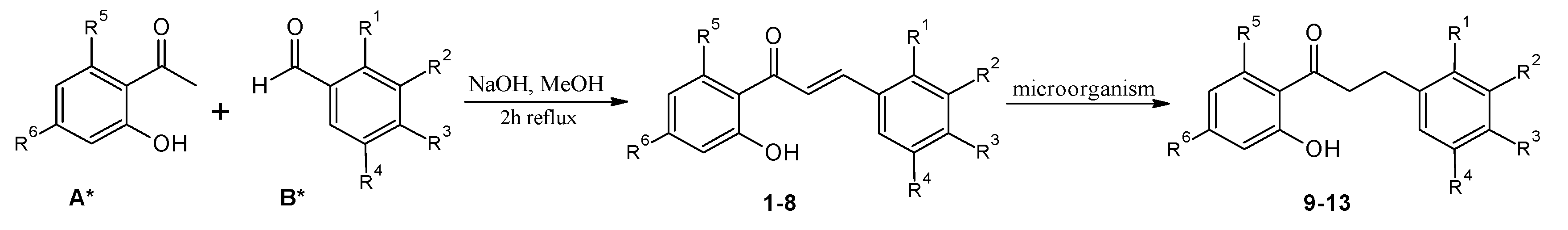



| Chalcones | Dihydrochalcones | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|---|
| 1 | 9 | -OCH3 | -H | -H | -H | -H | -H |
| 2 | 10 | -H | -OCH3 | -H | -H | -H | -H |
| 3 | 11 | -H | -H | -OCH3 | -H | -H | -H |
| 4 | 12 | -OCH3 | -H | -H | -OCH3 | -H | -H |
| 5 | 13 | -H | -OCH3 | -OCH3 | -OCH3 | -H | -H |
| 6 | - | -H | -H | -H | -H | -OCH3 | -OCH3 |
| 7 | - | -H | -H | -OCH3 | -H | -OCH3 | -OCH3 |
| 8 | - | -H | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 |
| Substrate | 2′-hydroxy-2″-methoxychalcone (1) | 2′-hydroxy-3″-methoxychalcone (2) | 2′-hydroxy-4″-methoxychalcone (3) | 2′-hydroxy-2″,5″-dimethoxychalcone (4) | 2′-hydroxy-3″,4″,5″-trimethoxychalcone (5) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time [days] | 1 | 3 | 7 | 1 | 3 | 7 | 1 | 3 | 7 | 1 | 3 | 7 | 1 | 3 | 7 |
| Rhodotorula rubra KCh 4 | 2 | 3 | 4 | 7 | 8 | 10 | 5 | 5 | 8 | 4 | 5 | 7 | 0 | 0 | 2 |
| Yarrowia lipolytica KCh 71 | 98 | 99 | 99 | 96 | 97 | 98 | 88 | 95 | 96 | 99 | 99 | 99 | 7 | 14 | 20 |
| Rhodotorula marina KCh 77 | 3 | 3 | 4 | 8 | 8 | 10 | 10 | 14 | 17 | 2 | 5 | 8 | 0 | 3 | 5 |
| Rhodotorula rubra KCh 82 | 3 | 3 | 3 | 25 | 30 | 43 | 6 | 8 | 13 | 2 | 4 | 6 | 0 | 4 | 6 |
| Candida viswanathii KCh 120 | 98 | 99 | 99 | 70 | 95 | 95 | 98 | 98 | 98 | 0 | 39 | 58 | 6 | 10 | 19 |
| Rhodotorula glutinis KCh 242 | 2 | 3 | 14 | 95 | 95 | 95 | 43 | 75 | 94 | 3 | 6 | 6 | 4 | 4 | 6 |
| Saccharomyces cerevisiae KCh 464 | 10 | 14 | 34 | 6 | 12 | 24 | 74 | 86 | 97 | 3 | 4 | 5 | 3 | 4 | 9 |
| Candida parapsilosis KCh 909 | 44 | 68 | 83 | 33 | 54 | 73 | 29 | 75 | 84 | 12 | 17 | 48 | 4 | 6 | 7 |
| Substrate | Concentration [g/L] | Biotransformation Time | ||||
|---|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 12 h | 24 h | ||
| 1 | 0.1 | 87 ± 1.2 | 96 ± 3.5 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.6 |
| 0.5 | 41 ± 7.1 | 92 ± 3.0 | 98 ± 0.0 | 100 ± 0.0 | 100 ± 0.6 | |
| 1 | 26 ± 4.9 | 57 ± 6.7 | 69 ± 7.6 | 83 ± 7.2 | 94 ± 0.0 | |
| 2 | 15 ± 1.5 | 32 ± 2.1 | 36 ± 1.7 | 43 ± 4.2 | 70 ± 4.5 | |
| 5 | 6 ± 1.0 | 14 ± 2.0 | 16 ± 2.3 | 19 ± 2.5 | 49 ± 0.6 | |
| 2 | 0.1 | 66 ± 8.1 | 91 ± 5.0 | 91 ± 8.1 | 93 ± 1.0 | 95 ± 1.5 |
| 0.5 | 46 ± 5.8 | 77 ± 4.4 | 85 ± 3.2 | 90 ± 1.0 | 93 ± 1.0 | |
| 1 | 27 ± 2.0 | 41 ± 4.7 | 49 ± 4.7 | 60 ± 4.9 | 64 ± 3.5 | |
| 2 | 14 ± 1.5 | 25 ± 1.2 | 30 ± 2.6 | 31 ± 1.4 | 37 ± 3.2 | |
| 5 | 5 ± 0.6 | 8 ± 0.0 | 9 ± 0.6 | 10 ± 0.6 | 16 ± 1.2 | |
| 3 | 0.1 | 74 ± 1.5 | 95 ± 1.5 | 98 ± 0.6 | 98 ± 0.6 | 98 ± 0.0 |
| 0.5 | 51 ± 5.3 | 75 ± 2.6 | 88 ± 1.7 | 96 ± 0.6 | 97 ± 0.6 | |
| 1 | 23 ± 2.5 | 44 ± 1.5 | 51 ± 1.0 | 66 ± 7.0 | 77 ± 6.4 | |
| 2 | 11 ± 0.6 | 22 ± 1.0 | 26 ± 1.2 | 33 ± 3.2 | 48 ± 4.0 | |
| 5 | 4 ± 0.6 | 6 ± 1.2 | 8 ± 1.7 | 12 ± 3.0 | 22 ± 5.0 | |
| 4 | 0.1 | 91 ± 3.5 | 96 ± 0.6 | 99 ± 0.0 | 99 ± 0.0 | 99 ± 0.0 |
| 0.5 | 40 ± 3.6 | 50 ± 3.6 | 43 ± 2.6 | 44 ± 0.6 | 49 ± 1.2 | |
| 1 | 15 ± 1.5 | 26 ± 3.2 | 25 ± 3.5 | 25 ± 2.6 | 29 ± 2.0 | |
| 2 | 9 ± 1.2 | 13 ± 2.1 | 13 ± 1.5 | 14 ± 1.7 | 16 ± 2.5 | |
| 5 | 3 ± 0.6 | 4 ± 0.6 | 5 ± 0.6 | 6 ± 1.5 | 7 ± 1.5 | |
| Substrate | Product | Isolated Yield [%] | Substrate Retention Time [min] | Product Retention Time [min] |
|---|---|---|---|---|
| 2′-hydroxy-2”-methoxychalcone (1) | 9 | 47 | 11.93 | 10.58 |
| 2′-hydroxy-3”-methoxychalcone (2) | 10 | 33 | 11.90 | 10.87 |
| 2′-hydroxy-4”-methoxychalcone (3) | 11 | 40 | 12.24 | 11.03 |
| 2′-hydroxy-2”,5”-dimethoxychalcone (4) | 12 | 54 | 13.36 | 12.54 |
| 2′-hydroxy-3”,4”,5”-trimethoxychalcone (5) | 13 | 5 | 14.17 | 13.46 |
| 2′-hydroxy-4′,6′-dimethoxychalcone (6) | - | - | 13.83 | - |
| 2′-hydroxy-4′,6′,4”-trimethoxychalcone (7) | - | - | 14.69 | - |
| 2′-hydroxy-4′,6′,3”,4”,5”-penthamethoxychalcone (8) | - | - | 16.01 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łużny, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Highly Effective, Regiospecific Hydrogenation of Methoxychalcone by Yarrowia lipolytica Enables Production of Food Sweeteners. Catalysts 2020, 10, 1135. https://doi.org/10.3390/catal10101135
Łużny M, Kozłowska E, Kostrzewa-Susłow E, Janeczko T. Highly Effective, Regiospecific Hydrogenation of Methoxychalcone by Yarrowia lipolytica Enables Production of Food Sweeteners. Catalysts. 2020; 10(10):1135. https://doi.org/10.3390/catal10101135
Chicago/Turabian StyleŁużny, Mateusz, Ewa Kozłowska, Edyta Kostrzewa-Susłow, and Tomasz Janeczko. 2020. "Highly Effective, Regiospecific Hydrogenation of Methoxychalcone by Yarrowia lipolytica Enables Production of Food Sweeteners" Catalysts 10, no. 10: 1135. https://doi.org/10.3390/catal10101135
APA StyleŁużny, M., Kozłowska, E., Kostrzewa-Susłow, E., & Janeczko, T. (2020). Highly Effective, Regiospecific Hydrogenation of Methoxychalcone by Yarrowia lipolytica Enables Production of Food Sweeteners. Catalysts, 10(10), 1135. https://doi.org/10.3390/catal10101135





