Eco-Friendly and Solvent-Less Mechanochemical Synthesis of ZrO2–MnCO3/N-Doped Graphene Nanocomposites: A Highly Efficacious Catalyst for Base-Free Aerobic Oxidation of Various Types of Alcohols
Abstract
:1. Introduction
2. Results and Discussion
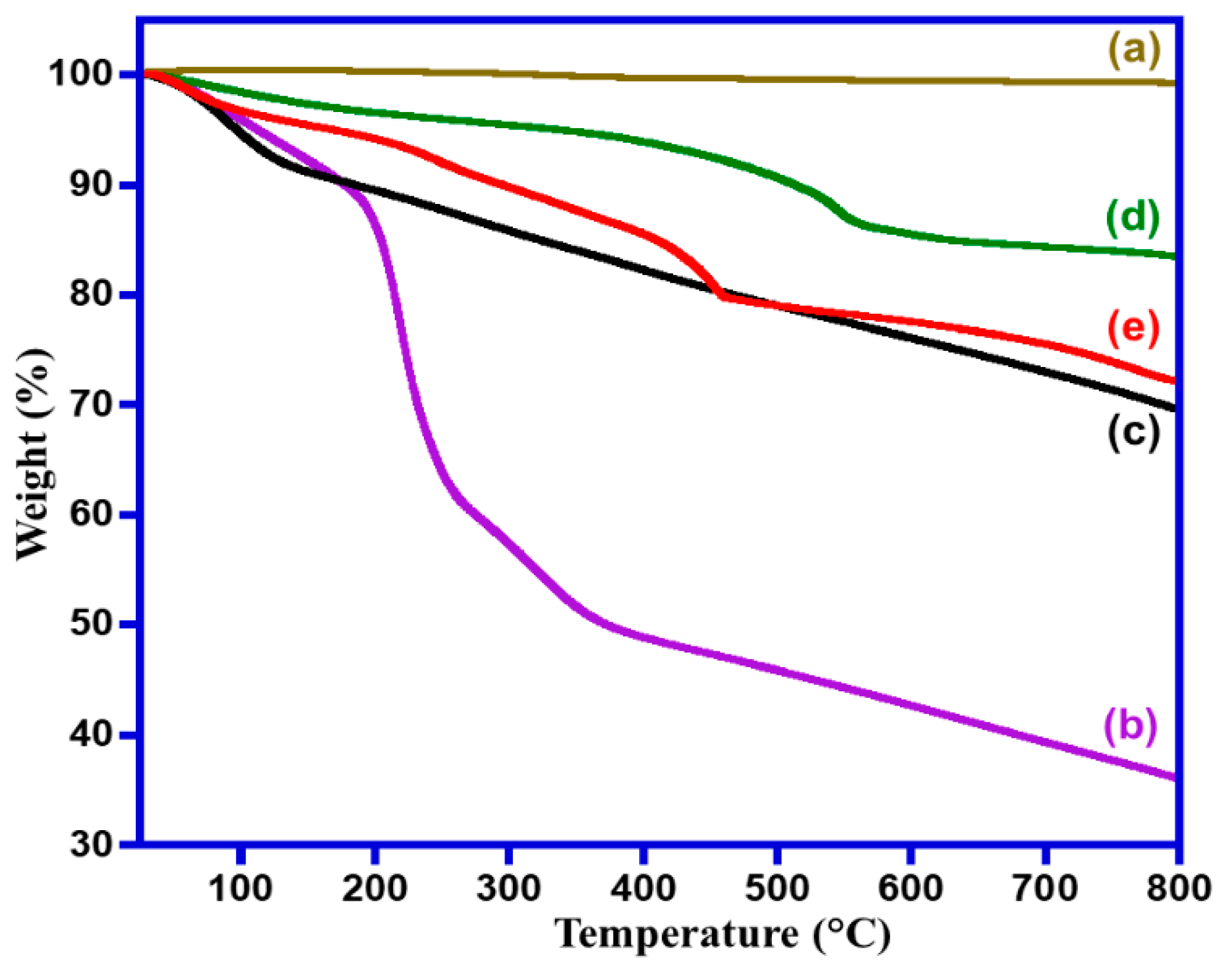
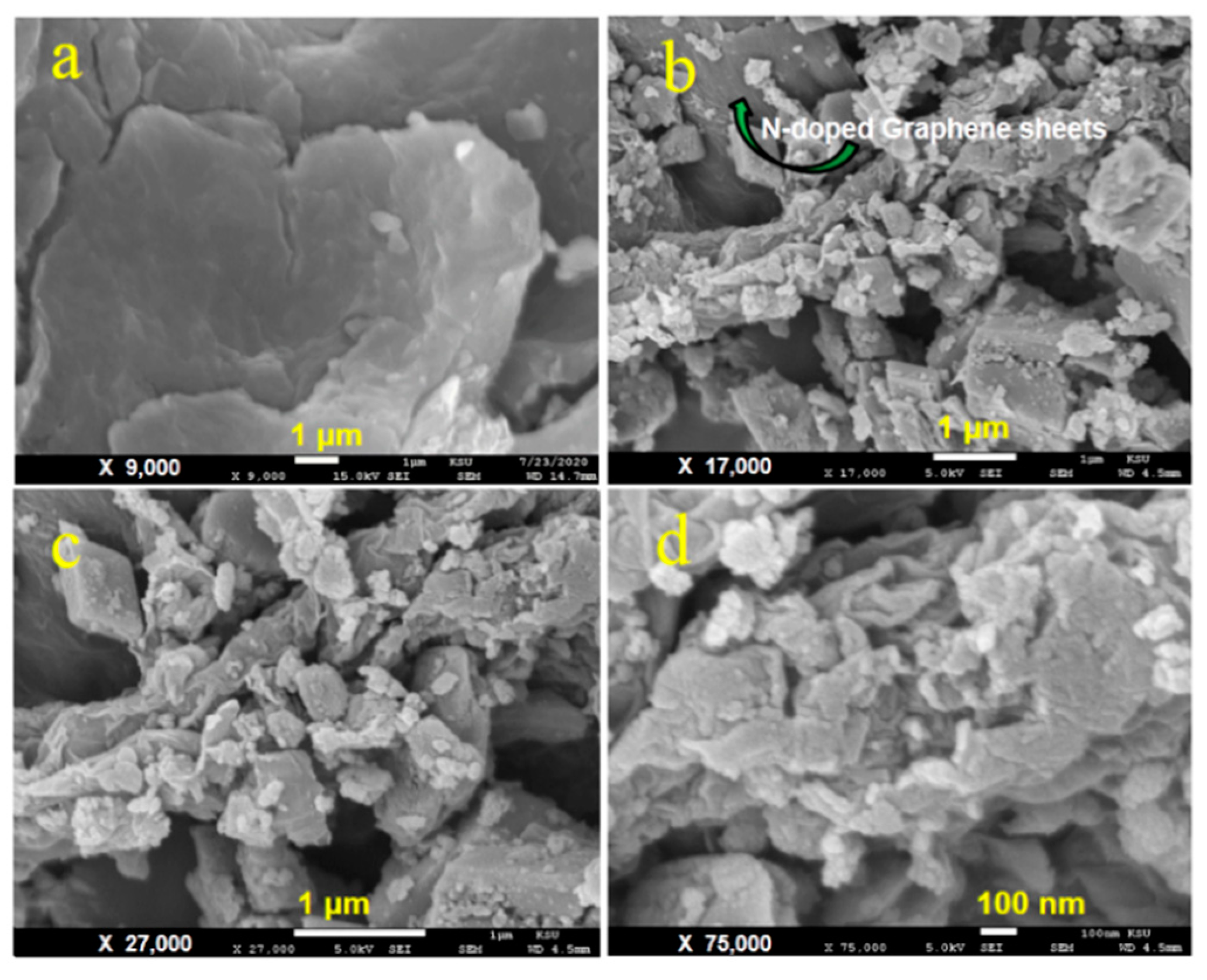
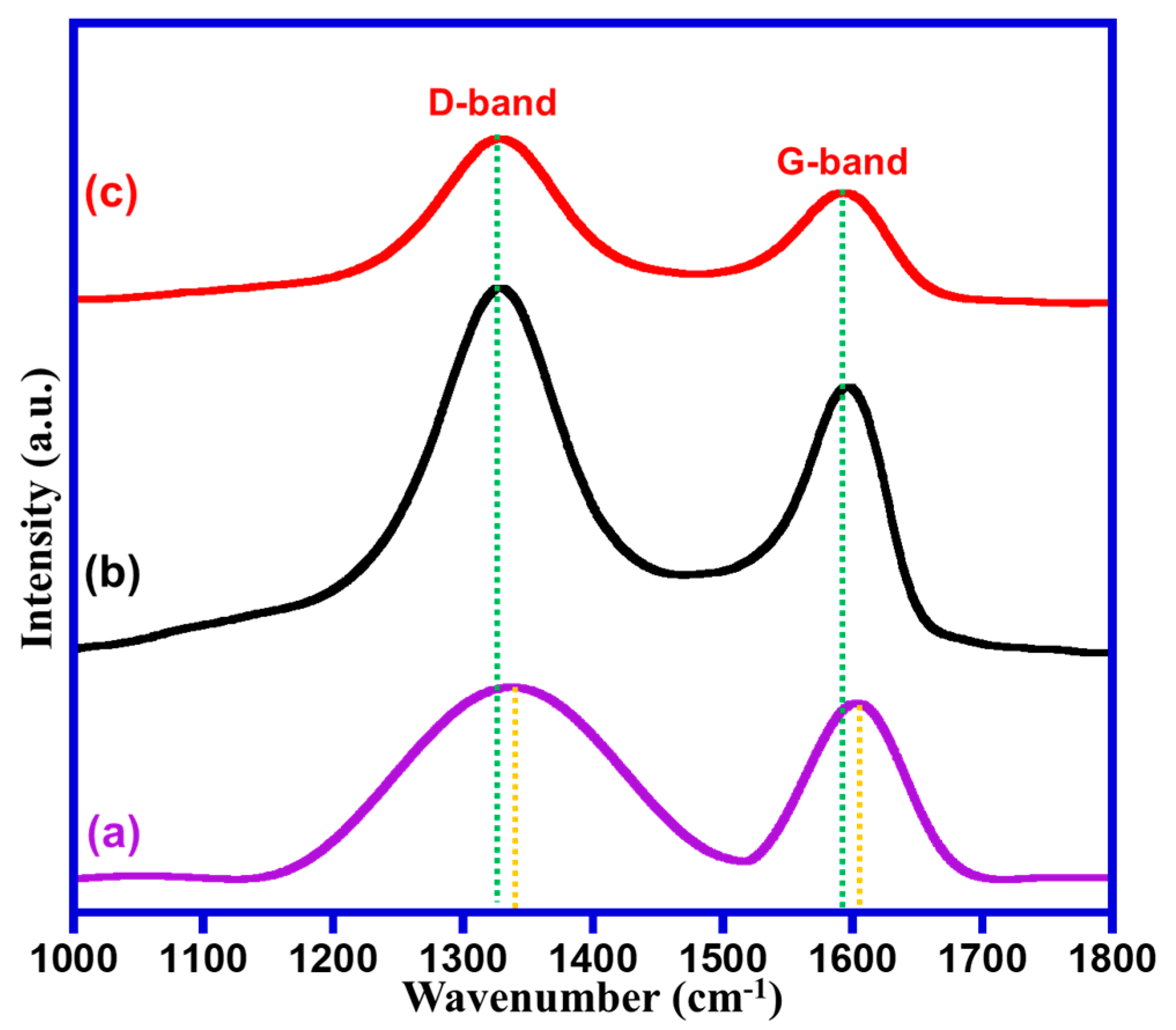
2.1. Characterizations
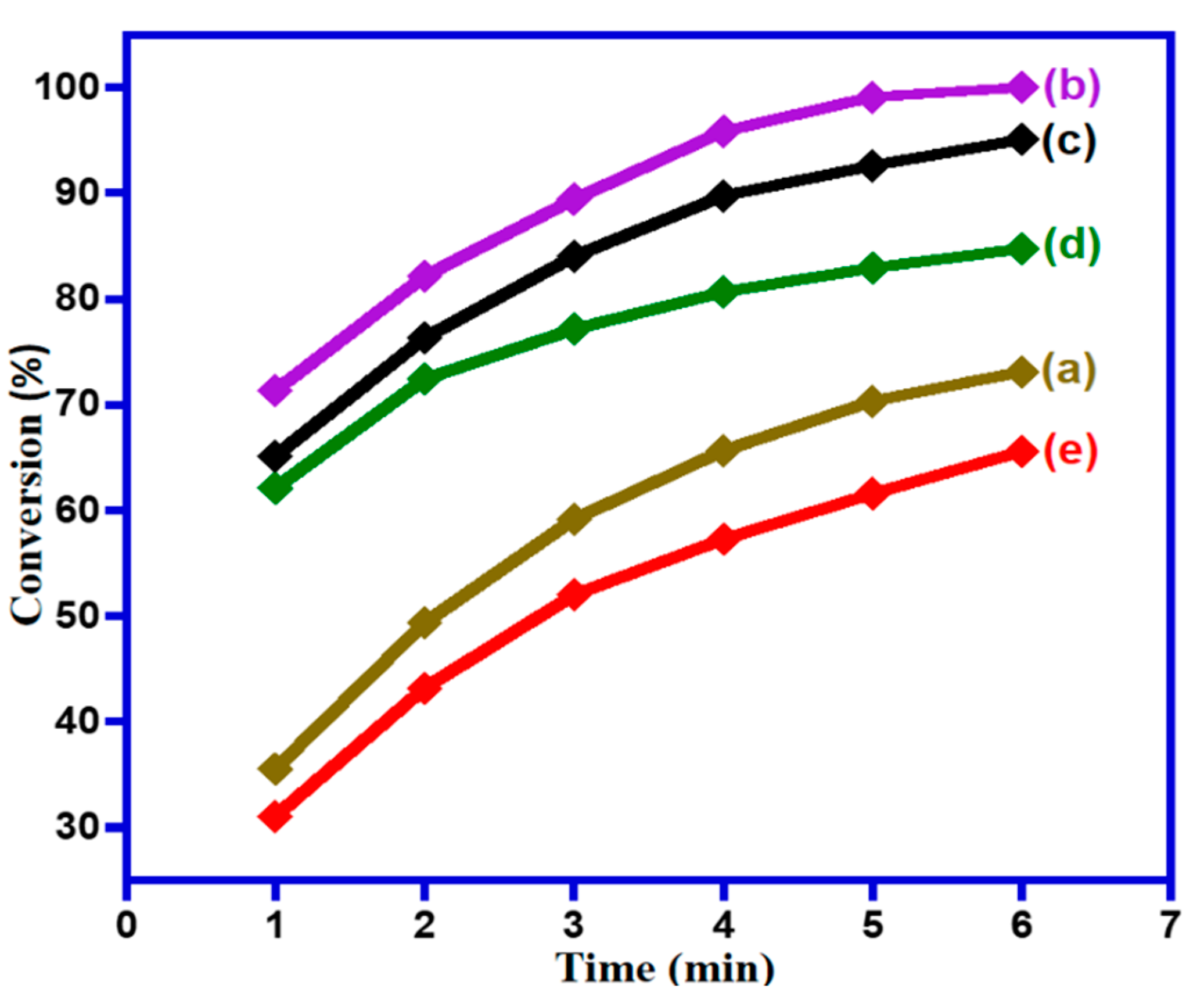
2.2. Catalytic Activities
2.2.1. Impact of wt.% of NDG
2.2.2. Effects of Various Graphene Derivatives
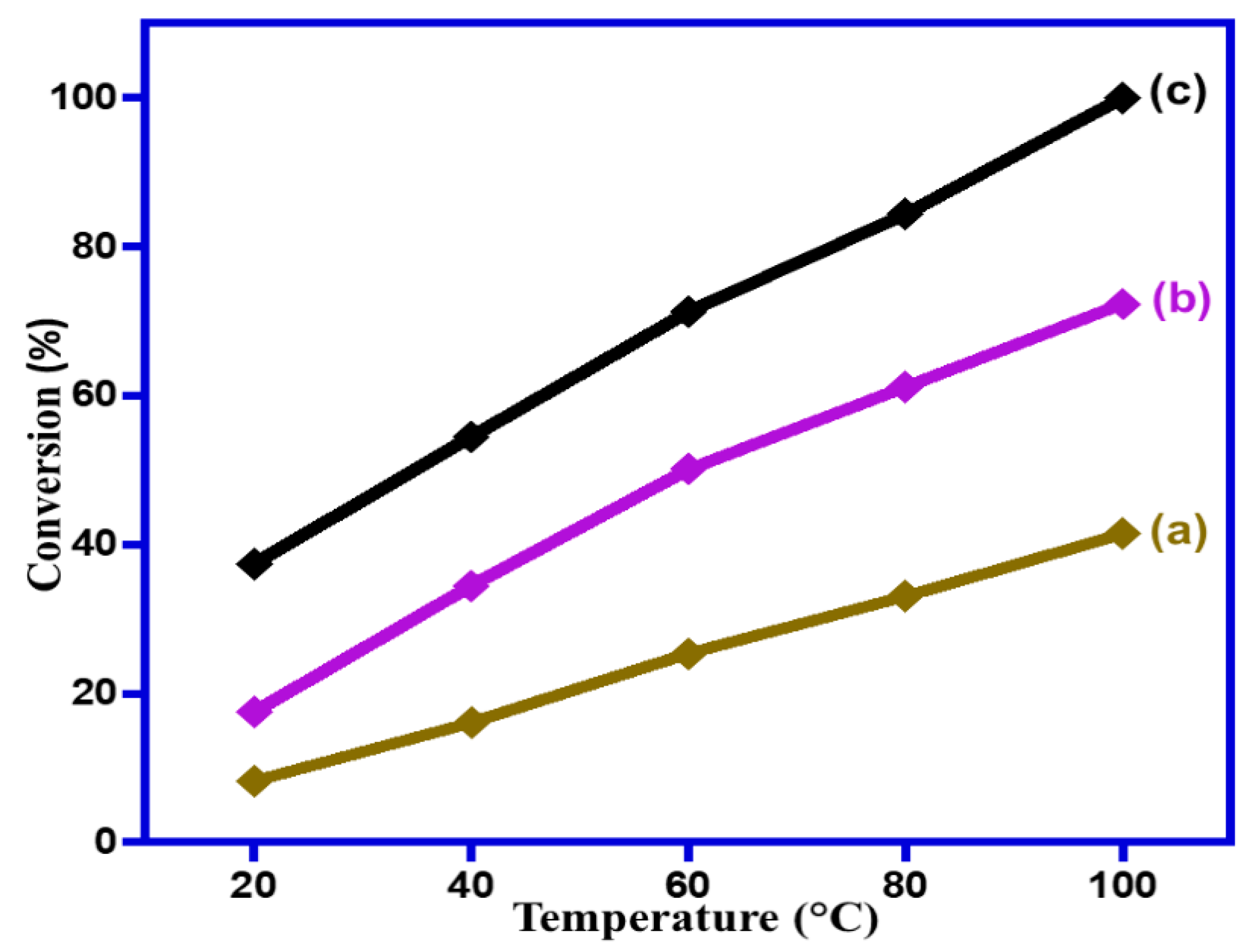
2.2.3. Impact of Operating Temperature
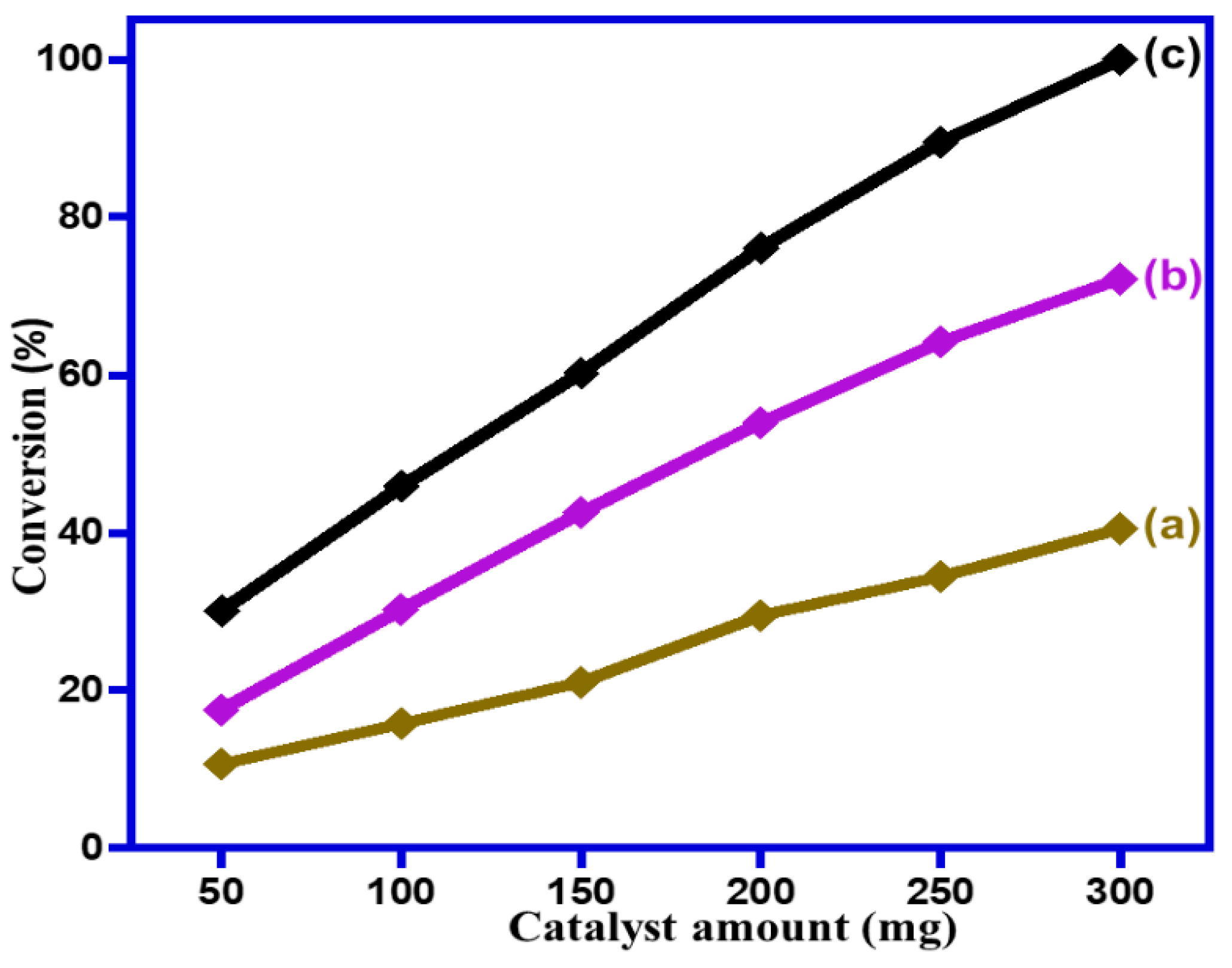
2.2.4. Impact of Catalyst Dose
2.3. Reusability Tests
3. Experimental Section
3.1. Preparation of GRO and NDG
3.2. Preparation of (X%)NDG Doped MnCO3–(1%)ZrO2 Nanocomposites
3.3. Characterizations
3.4. Catalytic Investigations
3.5. Recovery Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Mao, D.; Jia, M.; Qiu, J.; Zhang, X.-F.; Yao, J. N-Doped Porous Carbon Supported Au Nanoparticles for Benzyl Alcohol Oxidation. Catal. Lett. 2020, 150, 74–81. [Google Scholar] [CrossRef]
- Qiao, X.; Liao, S.; You, C.; Chen, R. Phosphorus and nitrogen dual doped and simultaneously reduced graphene oxide with high surface area as efficient metal-free electrocatalyst for oxygen reduction. Catalysts 2015, 5, 981–991. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, I.Y.; Zhang, S.; Zhang, L.; Choi, H.J.; Seo, J.M.; Xia, Z.; Dai, L.; Baek, J.B. Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: The electron spin effect. Adv. Mater. 2013, 25, 6138–6145. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Xu, G.; Wang, T.; Ma, D.; Yang, Z.; Yang, L. Single Pt atom stabilized on nitrogen doped graphene: CO oxidation readily occurs via the tri-molecular Eley–Rideal mechanism. PCCP 2015, 17, 20006–20013. [Google Scholar] [CrossRef]
- Gao, W.; Du, L.; Jiao, W.; Liu, Y. Oxidation of benzyl alcohols to ketones and aldehydes by O3 process enhanced using high-gravity technology. Chin. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Deng, B.; Zhuang, Y.; Li, D.; Liu, B.; Yang, W.; Li, Y. Ti3C2/TiO2 nanowires with excellent photocatalytic performance for selective oxidation of aromatic alcohols to aldehydes. J. Catal. 2020, 383, 1–12. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Mali, M.; Mastrorilli, P.; Cotugno, P.; Monopoli, A. Oxidation of Benzyl Alcohols to Aldehydes and Ketones under Air in Water Using a Polymer Supported Palladium Catalyst. J. Mol. Catal. A Chem. 2014, 386, 114–119. [Google Scholar] [CrossRef]
- Wu, P.; Cao, Y.; Zhao, L.; Wang, Y.; He, Z.; Xing, W.; Bai, P.; Mintova, S.; Yan, Z. Formation of PdO on Au–Pd bimetallic catalysts and the effect on benzyl alcohol oxidation. J. Catal. 2019, 375, 32–43. [Google Scholar] [CrossRef]
- Bhavani, K.S.; Anusha, T.; Brahman, P.K. Fabrication and characterization of gold nanoparticles and fullerene-C60 nanocomposite film at glassy carbon electrode as potential electro-catalyst towards the methanol oxidation. Int. J. Hydrogen Energy 2019, 44, 25863–25873. [Google Scholar] [CrossRef]
- Xie, M.; Dai, X.; Meng, S.; Fu, X.; Chen, S. Selective oxidation of aromatic alcohols to corresponding aromatic aldehydes using In2S3 microsphere catalyst under visible light irradiation. Chem. Eng. J. 2014, 245, 107–116. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, S.M.; Tremel, W.; Tahir, M.N.; Adil, S.F. A highly reduced graphene oxide/ZrOx–MnCO3 or–Mn2O3 nanocomposite as an efficient catalyst for selective aerial oxidation of benzylic alcohols. RSC Adv. 2017, 7, 55336–55349. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Gong, X.; Zhao, P.; Chen, Y.; Yang, Y. Copper–manganese oxide catalysts supported on alumina: Physicochemical features and catalytic performances in the aerobic oxidation of benzyl alcohol. Appl. Catal. A 2010, 389, 101–107. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef] [Green Version]
- Basha, S.S.; Gnanakiran, M.; Kumar, B.R.; Bhadra, K.V.; Reddy, M.; Rao, M. Synthesis and spectral characterization on PVA/PVP: GO based blend polymer electrolytes. Rasayan J. Chem. 2017, 10, 1159–1166. [Google Scholar]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Alharthi, A.I.; Varala, R.; Siddiqui, M.R.H.; Adil, S.F. Ag2O nanoparticles/MnCO3,–MnO2 or–Mn2O3/highly reduced graphene oxide composites as an efficient and recyclable oxidation catalyst. Arab. J. Chem. 2019, 12, 54–68. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Alzahrani, A.Y.; Al-Warthan, A.; Siddiqui, M.R.H.; Adil, S.F. Mixed Zinc/Manganese on Highly Reduced Graphene Oxide: A Highly Active Nanocomposite Catalyst for Aerial Oxidation of Benzylic Alcohols. Catalysts 2017, 7, 391. [Google Scholar] [CrossRef] [Green Version]
- Adil, S.F.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; AlOtaibi, N.M.; Khan, M.; Sharif, M.; Alam, M.M.; Al-Warthan, A.; Mohammed, J.A. A Facile Synthesis of ZrOx-MnCO3/Graphene Oxide (GRO) Nanocomposites for the Oxidation of Alcohols using Molecular Oxygen under Base Free Conditions. Catalysts 2019, 9, 759. [Google Scholar] [CrossRef] [Green Version]
- Mirza-Aghayan, M.; Tavana, M.M.; Boukherroub, R. Palladium Nanoparticles Supported on Reduced Graphene Oxide as an Efficient Catalyst for the Reduction of Benzyl Alcohol Compounds. Catal. Commun. 2015, 69, 97–103. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Song, H.; Chen, X.; Wu, B.; Zhou, J.; Wang, F. How graphene is exfoliated from graphitic materials: Synergistic effect of oxidation and intercalation processes in open, semi-closed, and closed carbon systems. J. Mater. Chem. 2012, 22, 22150–22154. [Google Scholar] [CrossRef]
- Yang, S.; Lin, Y.; Song, X.; Zhang, P.; Gao, L. Covalently Coupled Ultrafine H-TiO2 Nanocrystals/Nitrogen-Doped Graphene Hybrid Materials for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 17884–17892. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, G.; Xu, L.; Yang, J.; Zhang, B.; Xiang, X. Preparation of ternary Pd/CeO2-nitrogen doped graphene composites as recyclable catalysts for solvent-free aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2019, 471, 852–861. [Google Scholar] [CrossRef]
- Assal, M.E.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Nawaz Tahir, M.; Adil, S.F. Synthesis and comparative catalytic study of zirconia–MnCO3 or–Mn2O3 for the oxidation of benzylic alcohols. ChemOpen 2017, 6, 112–120. [Google Scholar]
- Santra, S.; Hota, P.K.; Bhattacharyya, R.; Bera, P.; Ghosh, P.; Mandal, S.K. Palladium Nanoparticles on Graphite Oxide: A Recyclable Catalyst for the Synthesis of Biaryl Cores. ACS Catal. 2013, 3, 2776–2789. [Google Scholar] [CrossRef]
- Metin, Ö.; Kayhan, E.; Özkar, S.; Schneider, J.J. Palladium Nanoparticles Supported on Chemically Derived Graphene: An Efficient and Reusable Catalyst for the Dehydrogenation of Ammonia Borane. Int. J. Hydrogen Energy 2012, 37, 8161–8169. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of Divalent Metal Ions from Aqueous Solutions Using Graphene Oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, K.H.; Park, K.; Kim, C.; Kim, S.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Amine-functionalized graphene/CdS composite for photocatalytic reduction of CO2. ACS Catal. 2017, 7, 7064–7069. [Google Scholar] [CrossRef]
- Hu, H.; Xu, J.-y.; Yang, H.; Liang, J.; Yang, S.; Wu, H. Morphology-Controlled Hydrothermal Synthesis of MnCO3 Hierarchical Superstructures with Schiff Base as Stabilizer. Mater. Res. Bull. 2011, 46, 1908–1915. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, F.; Wang, Z.; Yang, M.; Xia, J.; Gui, R.; Xia, Y. Facile preparation of PtPdPt/graphene nanocomposites with ultrahigh electrocatalytic performance for methanol oxidation. J. Electroanal. Chem. 2016, 761, 55–61. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.-J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.V.S.; Adil, S.F.; Shaik, M.R.; Khan, M.; Assal, M.E.; Siddiqui, M.R.H.; Al-Warthan, A. One-Pot Synthesized Pd@N-Doped Graphene: An Efficient Catalyst for Suzuki–Miyaura Couplings. Catalysts 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Liu, S.; Tang, Z.-R.; Xu, Y.-J. Electrostatic self-assembly of CdS nanowires-nitrogen doped graphene nanocomposites for enhanced visible light photocatalysis. J. Energy Chem. 2015, 24, 145–156. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Shaik, M.R.; Kuniyil, M.; Sekou, D.; Dewidar, A.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Eco-friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols. Catalysts 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Mahyari, M.; Shaabani, A. Graphene Oxide-Iron Phthalocyanine Catalyzed Aerobic Oxidation of Alcohols. Appl. Catal. A 2014, 469, 524–531. [Google Scholar] [CrossRef]
- Ramirez-Barria, C.S.; Isaacs, M.; Parlett, C.; Wilson, K.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Ru nanoparticles supported on N-doped reduced graphene oxide as valuable catalyst for the selective aerobic oxidation of benzyl alcohol. Catal. Today 2019, in press. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Lv, X.; Mao, C.; Zhou, Y.; Wu, W.; Zhang, H.; Huang, Z. Synthesis of polymeric ionic liquids mircrospheres/Pd nanoparticles/CeO2 core-shell structure catalyst for catalytic oxidation of benzyl alcohol. J. Taiwan Inst. Chem. Eng. 2020, 107, 161–170. [Google Scholar] [CrossRef]
- Moeini, S.S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized Palladium Supported on Ceria Nanorods for Catalytic Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde in Protic Solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Huo, Y.; Yang, J.; Chang, S.; Ma, Y.; Huang, W. Reduced Graphene Oxide Supported Au Nanoparticles as an Efficient Catalyst for Aerobic Oxidation of Benzyl Alcohol. Appl. Surf. Sci. 2013, 280, 450–455. [Google Scholar] [CrossRef]
- Sun, J.; Tong, X.; Liu, Z.; Liao, S.; Zhuang, X.; Xue, S. Gold-catalyzed selectivity-switchable oxidation of benzyl alcohol in the presence of molecular oxygen. Catal. Commun. 2016, 85, 70–74. [Google Scholar] [CrossRef]
- Yang, X.; Wu, S.; Hu, J.; Fu, X.; Peng, L.; Kan, Q.; Huo, Q.; Guan, J. Highly efficient N-doped magnetic cobalt-graphene composite for selective oxidation of benzyl alcohol. Catal. Commun. 2016, 87, 90–93. [Google Scholar] [CrossRef]
- Xie, X.; Long, J.; Xu, J.; Chen, L.; Wang, Y.; Zhang, Z.; Wang, X. Nitrogen-doped graphene stabilized gold nanoparticles for aerobic selective oxidation of benzylic alcohols. RSC Adv. 2012, 2, 12438–12446. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, Y.; Liu, J.; Wang, J.; Zhang, B.; Xiang, X. Ultrafine MnO2 nanoparticles decorated on graphene oxide as a highly efficient and recyclable catalyst for aerobic oxidation of benzyl alcohol. J. Colloid Interface Sci. 2016, 483, 26–33. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; An, Y.; Wang, X.; Xu, G.; Chen, Y.; Dai, L. Promotional synergistic effect of Sn doping into a novel bimetallic Sn-W oxides/graphene catalyst for selective oxidation of alcohols using aqueous H2O2 without additives. Appl. Catal. A 2018, 558, 26–33. [Google Scholar] [CrossRef]
- Zahed, B.; Hosseini-Monfared, H. A Comparative Study of Silver-Graphene Oxide Nanocomposites as a Recyclable Catalyst for the Aerobic Oxidation of Benzyl Alcohol: Support Effect. Appl. Surf. Sci. 2015, 328, 536–547. [Google Scholar] [CrossRef]
- Feng, X.; Lv, P.; Sun, W.; Han, X.; Gao, L.; Zheng, G. Reduced graphene oxide-supported Cu nanoparticles for the selective oxidation of benzyl alcohol to aldehyde with molecular oxygen. Catal. Commun. 2017, 99, 105–109. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Guan, N.; Li, L. Palladium on graphene as efficient catalyst for solvent-free aerobic oxidation of aromatic alcohols: Role of graphene support. Appl. Catal. B Environ. 2013, 136, 177–185. [Google Scholar] [CrossRef]
- Jha, A.; Mhamane, D.; Suryawanshi, A.; Joshi, S.M.; Shaikh, P.; Biradar, N.; Ogale, S.; Rode, C.V. Triple nanocomposites of CoMn2O4, Co3O4 and reduced graphene oxide for oxidation of aromatic alcohols. Catal. Sci. Technol. 2014, 4, 1771–1778. [Google Scholar] [CrossRef]
- Darvishi, K.; Amani, K.; Rezaei, M. Preparation, characterization and heterogeneous catalytic applications of GO/Fe3O4/HPW nanocomposite in chemoselective and green oxidation of alcohols with aqueous H2O2. Appl. Organomet. Chem. 2018, 32, e4323. [Google Scholar] [CrossRef]
- Geng, L.; Wu, S.; Zou, Y.; Jia, M.; Zhang, W.; Yan, W.; Liu, G. Correlation between the Microstructures of Graphite Oxides and Their Catalytic Behaviors in Air Oxidation of Benzyl Alcohol. J. Colloid Interface Sci. 2014, 421, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Sarvari, M.; Ataee-Kachouei, T.; Moeini, F. A Novel and Active Catalyst Ag/ZnO for Oxidant-Free Dehydrogenation of Alcohols. Mater. Res. Bull. 2015, 72, 98–105. [Google Scholar] [CrossRef]
- Assady, E.; Yadollahi, B.; Riahi Farsani, M.; Moghadam, M. Zinc Polyoxometalate on Activated Carbon: An Efficient Catalyst for Selective Oxidation of Alcohols with Hydrogen Peroxide. Appl. Organomet. Chem. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]





| Entry | Catalyst | S. A. (m2/g) | Con. (%) | Specific Activity (mmol·g−1·h−1) | Sel. (%) |
|---|---|---|---|---|---|
| 1 | NDG | 485.6 | 2.7 | 1.8 | >99 |
| 2 | MnCO3 | 70.5 | 41.6 | 27.7 | >99 |
| 3 | MnCO3–(1%)ZrO2 | 133.6 | 73.1 | 48.8 | >99 |
| 4 | (1%)NDG/MnCO3–(1%)ZrO2 | 243.7 | 100.0 | 66.7 | >99 |
| 5 | (1%)GRO/MnCO3–(1%)ZrO2 | 229.5 | 96.8 | 64.6 | >99 |
| 6 | (1%)HRG/MnCO3–(1%)ZrO2 | 211.0 | 89.3 | 59.6 | >99 |
| Entry | Catalyst | Conv. (%) | Specific Activity (mmol·g−1·h−1) | Sel. (%) |
|---|---|---|---|---|
| 1 | NDG | 2.7 | 1.8 | >99 |
| 2 | MnCO3–(1%)ZrO2 | 73.1 | 48.8 | >99 |
| 3 | (1%)NDG/MnCO3–(1%)ZrO2 | 100.0 | 66.7 | >99 |
| 4 | (3%)NDG/MnCO3–(1%)ZrO2 | 95.1 | 63.4 | >99 |
| 5 | (5%)NDG/MnCO3–(1%)ZrO2 | 84.8 | 56.6 | >99 |
| 6 | (7%)NDG/MnCO3–(1%)ZrO2 | 65.6 | 43.8 | >99 |
| Catalyst | T. (°C) | Time (h) | Conv. (%) | Sel. (%) | Specific Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|
| (1%)NDG/MnCO3–(1%)ZrO2 | 100 | 0.1 | 100 | >99 | 66.7 | Herein |
| (1%)GRO/MnCO3–(1%)ZrO2 | 100 | 0.1 | 100 | >99 | 64.6 | [20] |
| (1%)HRG/MnCO3–(1%)ZrO2 | 100 | 0.1 | 100 | >99 | 59.6 | [14] |
| 4%Ru (CO)/NDG | 90 | 24 | 46 | >99 | 6.4 | [38] |
| MCG-700 | 100 | 8 | 89.5 | 97.3 | 4.5 | [43] |
| Au–Pd/Fe–Gr | 110 | 4 | 89.1 | 87.5 | 6.7 | [42] |
| Au NPs/NG | 70 | 6 | 67 | 40 | 0.4 | [44] |
| MnO2/GRO | 110 | 3 | 97 | 100 | 1.6 | [45] |
| Sn–W/RGO | 80 | 3 | 94.0 | 94.4 | 15.7 | [46] |
| Ag NPs/GOSH | 80 | 24 | 61 | 58 | 5.1 | [47] |
| Cu NPs@rGO | 80 | 16 | <99 | 98.6 | 8.3 | [48] |
| Pd NPs/GRO | 110 | 6 | 36 | 34.1 | 1.0 | [49] |
| 1 wt.% RGO/MnCoO | 140 | 2 | 78 | 100 | 12.6 | [50] |
| Au NPs/RGO | 100 | 8 | 65 | 93 | 5.4 | [41] |
| GRO/Fe3O4/HPW | 70 | 3 | 99 | 100 | 16.7 | [51] |
| GO-100 | 80 | 5 | 100 | 100 | 1.1 | [52] |
| S. No. | Reactant | Product | Time (min) | Conv. (%) | Sel. (%) |
|---|---|---|---|---|---|
| 1 |  | 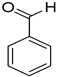 | 6 | 100 | >99.9 |
| 2 | 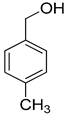 |  | 7 | 100 | >99.9 |
| 3 |  |  | 6 | 100 | >99.9 |
| 4 | 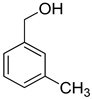 | 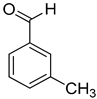 | 8 | 100 | >99.9 |
| 5 | 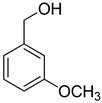 |  | 10 | 100 | >99.9 |
| 6 | 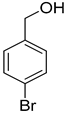 | 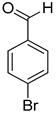 | 9 | 100 | >99.9 |
| 7 |  | 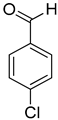 | 11 | 100 | >99.9 |
| 8 | 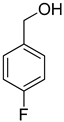 |  | 10 | 100 | >99.9 |
| 9 |  |  | 11 | 100 | >99.9 |
| 10 |  | 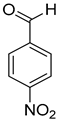 | 12 | 100 | >99.9 |
| 11 | 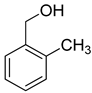 | 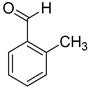 | 14 | 100 | >99.9 |
| 12 | 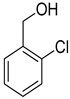 |  | 14 | 100 | >99.9 |
| 13 | 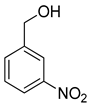 | 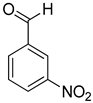 | 17 | 100 | >99.9 |
| 14 |  |  | 15 | 100 | >99.9 |
| 15 | 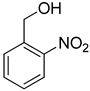 | 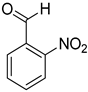 | 20 | 100 | >99.9 |
| 16 |  | 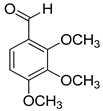 | 17 | 100 | >99.9 |
| 17 | 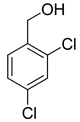 | 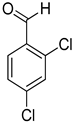 | 18 | 100 | >99.9 |
| 18 |  | 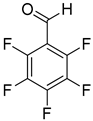 | 24 | 100 | >99.9 |
| 19 | 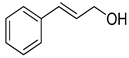 | 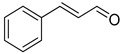 | 12 | 100 | >99.9 |
| 20 | 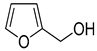 | 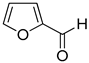 | 25 | 100 | >99.9 |
| 21 | 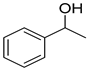 |  | 8 | 100 | >99.9 |
| 22 | 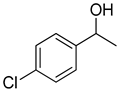 | 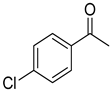 | 10 | 100 | >99.9 |
| 23 |  | 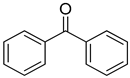 | 10 | 100 | >99.9 |
| 24 |  |  | 15 | 100 | >99.9 |
| 25 |  | 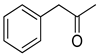 | 14 | 100 | >99.9 |
| 26 | 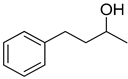 |  | 18 | 100 | >99.9 |
| 27 |  |  | 30 | 100 | >99.9 |
| 28 |  | 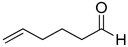 | 100 | 100 | >99.9 |
| 29 |  | 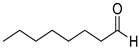 | 110 | 100 | >99.9 |
| 30 |  | 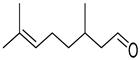 | 130 | 100 | >99.9 |
| 31 |  | 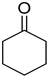 | 40 | 100 | >99.9 |
| 32 |  | 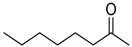 | 120 | 100 | >99.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuniyil, M.; Kumar, J.V.S.; Adil, S.F.; Assal, M.E.; Shaik, M.R.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, A.; Bilal, M.; et al. Eco-Friendly and Solvent-Less Mechanochemical Synthesis of ZrO2–MnCO3/N-Doped Graphene Nanocomposites: A Highly Efficacious Catalyst for Base-Free Aerobic Oxidation of Various Types of Alcohols. Catalysts 2020, 10, 1136. https://doi.org/10.3390/catal10101136
Kuniyil M, Kumar JVS, Adil SF, Assal ME, Shaik MR, Khan M, Al-Warthan A, Siddiqui MRH, Khan A, Bilal M, et al. Eco-Friendly and Solvent-Less Mechanochemical Synthesis of ZrO2–MnCO3/N-Doped Graphene Nanocomposites: A Highly Efficacious Catalyst for Base-Free Aerobic Oxidation of Various Types of Alcohols. Catalysts. 2020; 10(10):1136. https://doi.org/10.3390/catal10101136
Chicago/Turabian StyleKuniyil, Mufsir, J. V. Shanmukha Kumar, Syed Farooq Adil, Mohamed E. Assal, Mohammed Rafi Shaik, Mujeeb Khan, Abdulrahman Al-Warthan, Mohammed Rafiq H. Siddiqui, Aslam Khan, Muhammad Bilal, and et al. 2020. "Eco-Friendly and Solvent-Less Mechanochemical Synthesis of ZrO2–MnCO3/N-Doped Graphene Nanocomposites: A Highly Efficacious Catalyst for Base-Free Aerobic Oxidation of Various Types of Alcohols" Catalysts 10, no. 10: 1136. https://doi.org/10.3390/catal10101136
APA StyleKuniyil, M., Kumar, J. V. S., Adil, S. F., Assal, M. E., Shaik, M. R., Khan, M., Al-Warthan, A., Siddiqui, M. R. H., Khan, A., Bilal, M., Iqbal, H. M. N., & Al-Masry, W. A. (2020). Eco-Friendly and Solvent-Less Mechanochemical Synthesis of ZrO2–MnCO3/N-Doped Graphene Nanocomposites: A Highly Efficacious Catalyst for Base-Free Aerobic Oxidation of Various Types of Alcohols. Catalysts, 10(10), 1136. https://doi.org/10.3390/catal10101136











