Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Parameters
2.2. Performance Indices
2.3. Catalyst Characterization
2.4. Ni Loading Effect
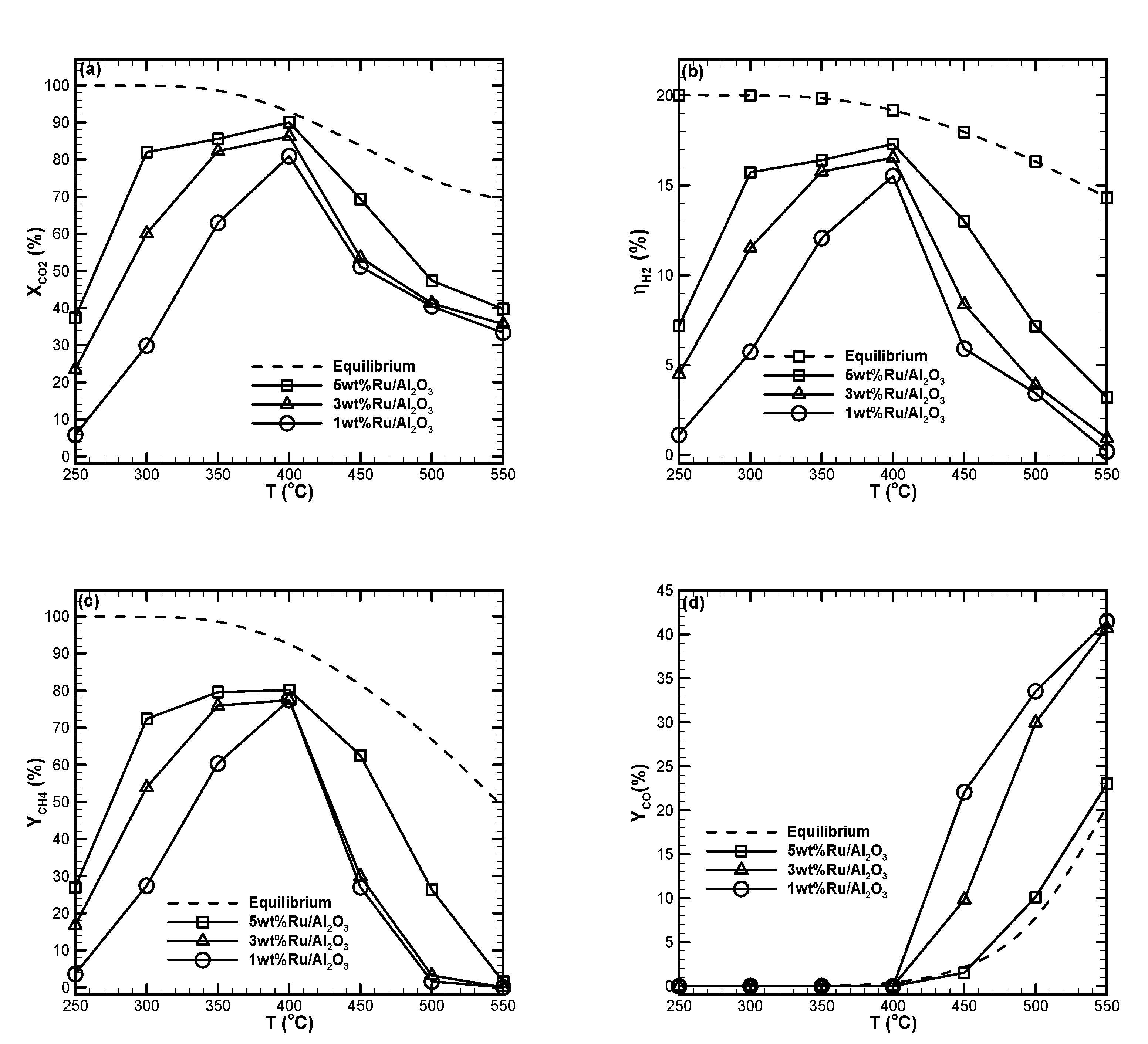
2.5. Ru Loading Effect
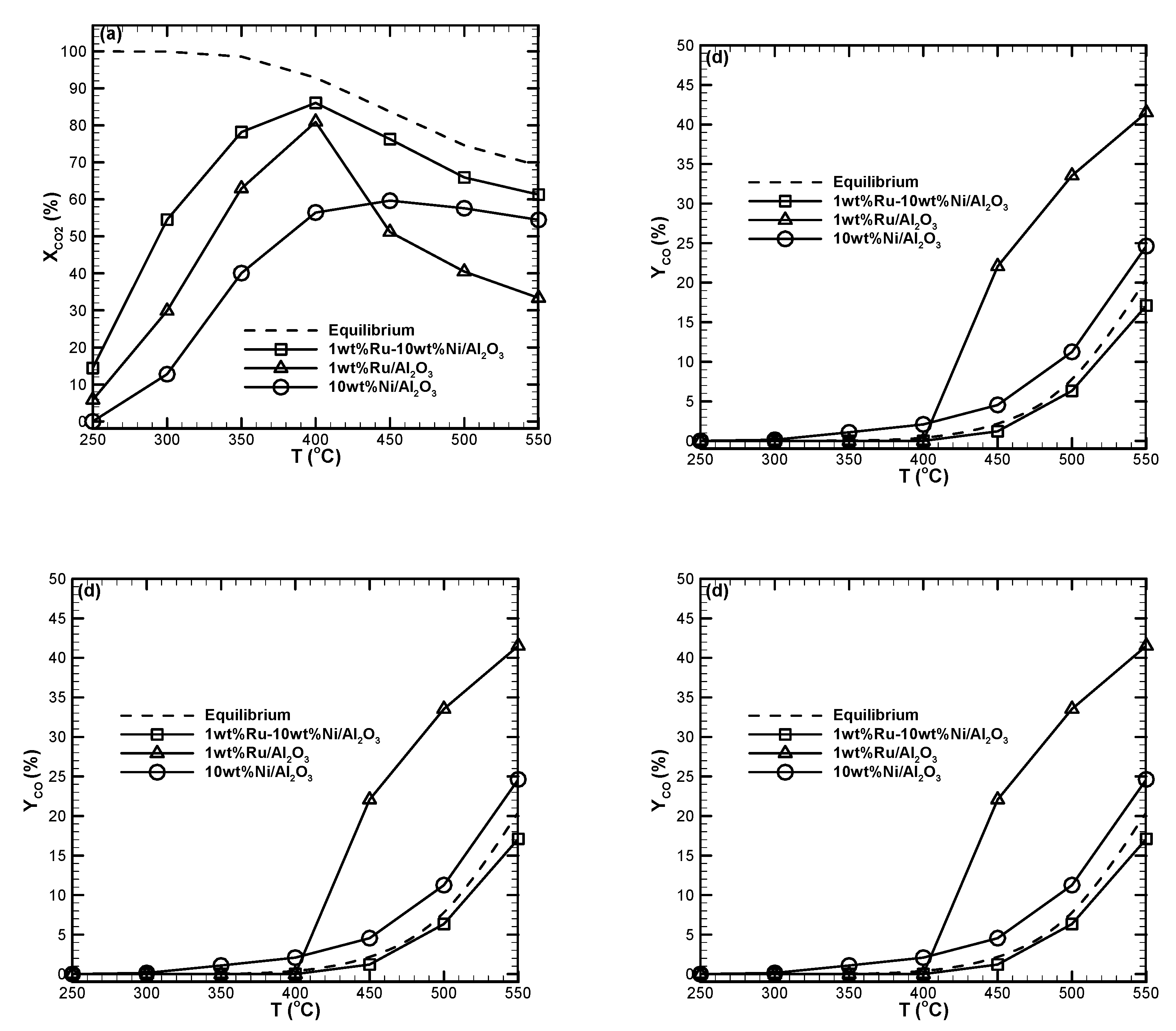
2.6. Ru-Ni Loading Effect
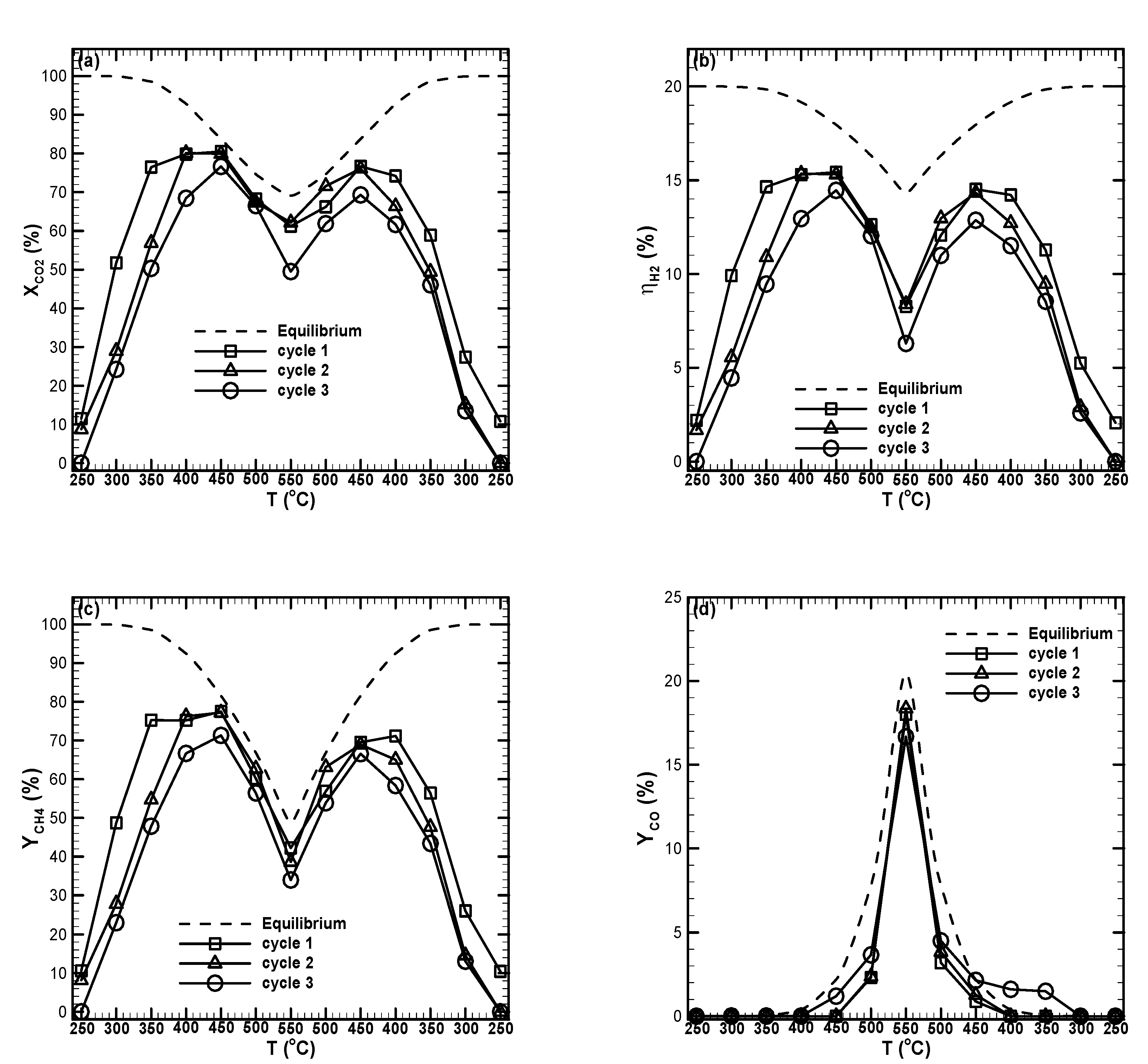
2.7. Stability Test
3. Experimental
3.1. Catalyst Preparation
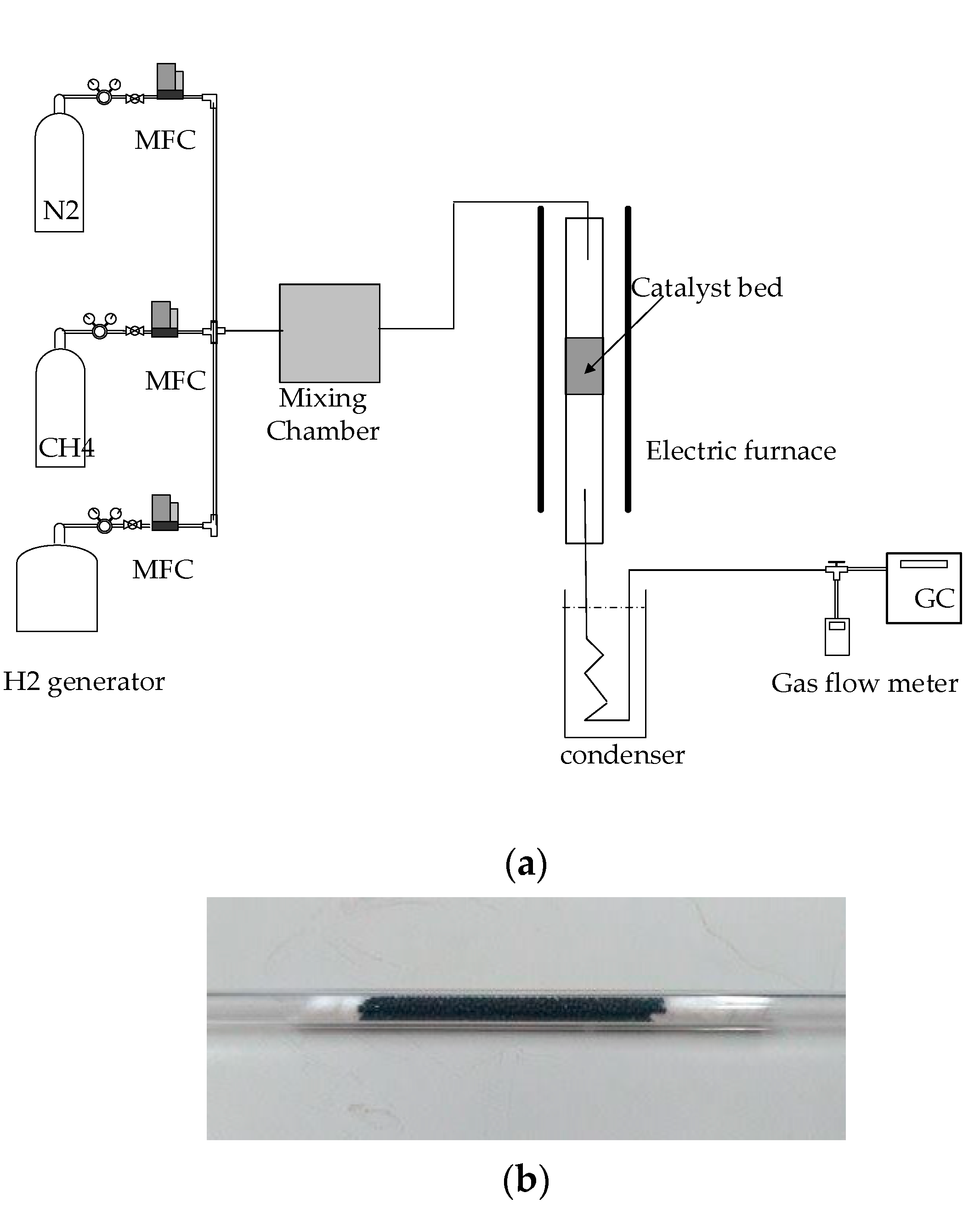
3.2. Experimental Setup
4. Conclusions
- (1)
- For the three types of catalyst studied, the optimum reaction temperature was found to be 400 °C. At this temperature, maximum CO2 conversion, maximum H2 efficiency, maximum CH4 yield, and minimum CO yield were obtained for all metal loadings studied.
- (2)
- CO2 methanation performance at low temperatures could be enhanced by increasing the catalyst loading for all the catalysts studied. Compared with Ni, Ru was more active in the low-temperature regime. At higher temperature regimes, CO2 methanation performance followed a variation trend resulting from the thermodynamic equilibrium of the Ni catalyst. The measured results indicated that Ru was active for the reverse water-gas shift reaction in the high-temperature regime. This led to a low CH4 yield and high CO yield.
- (3)
- The measured data indicated that the bimetallic Ru-Ni catalyst was more active compared with the monometallic Ni or Ru catalysts for CO2 methanation in a low-temperature regime.
- (4)
- The Ru-Ni catalyst showed good thermal stability, i.e., about 3% and 5% decreases in CO2 conversion and CH4 yield resulted after 74 h testing, respectively.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Centi, G.; Perathoner, S. CO2-based energy vectors for the storage of solar energy. Greenh. Gases Sci. Technol. 2011, 1, 21–35. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef] [Green Version]
- Suberua, M.Y.; Mustafa, M.Y.; Bashir, N. Energy storage systems for renewable energy power sector integration and mitigation of intermittency. Renew. Sustain. Energy Rev. 2014, 35, 499–514. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Schiebahn, S.; Grube, T.; Robinius, M.; Tietze, V.; Kumar, B.; Stolten, D. Power to gas: Technological overview, systems analysis and economic assessment for a case study in Germany. Int. J. Hydrog. Energy 2015, 40, 4285–4294. [Google Scholar] [CrossRef]
- Younas, M.; Kong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Eckle, S.; Anfang, H.G.; Behm, R.J. Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases. J. Phys. Chem. C 2011, 115, 1361–1367. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. A comparative study of CO and CO2 hydrogenation over Rh/SiO2. J. Catal. 1996, 162, 54–65. [Google Scholar] [CrossRef]
- Williams, K.J.; Boffa, A.B.; Salmeron, M.; Bell, A.T.; Somorjai, G.A. The kinetics of CO2 hydrogenation on a Rh foil promoted by titania overlayers. Catal. Lett. 1991, 9, 415–426. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “in situ” supply of hydrogen by Ni/activated carbon catalyst. Appl Catal B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Shanmugam, V; Neuberg, S; Zapf, R; Pennemann, H; Kolb, G. Effect of support and chelating ligand on the synthesis of Ni catalysts with high activity and stability for CO2 Methanation. Catalysts 2020, 10, 493. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.M.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299. [Google Scholar] [CrossRef]
- Pandey, D.; Ray, K.; Bhardwaj, R.; Bojja, S.; Chary, K.V.R.; Deo, G. Promotion of unsupported nickel catalyst using iron for CO2 methanation. Int. J. Hydrog. Energy 2018, 43, 4987–5000. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Shi, H.; Szanyi, J. Kinetic modeling and transient DRIFTS–MS studies of CO2 methanation over Ru/Al2O3 catalysts. J. Catal. 2016, 343, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsson, F.; Schaefer, A.; Skoglundh, M.; Carlsson, P. CO2 methanation over Rh/CeO2 studied with infrared modulation excitation spectroscopy and phase sensitive detection. Catalysts 2020, 10, 601. [Google Scholar] [CrossRef]
- Ronsch, S.; Schneider, J.; Matthischke, S.; Schluter, M.; Gotz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation-From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Martins, J.A.; Faria, A.C.; Soria, M.A.; Miguel, C.V.; Rodrigues, A.E.; Madeira, L.M. CO2 methanation over hydrotalcite-derived nickel/ruthenium and supported ruthenium catalysts. Catalysts 2019, 9, 1008. [Google Scholar] [CrossRef] [Green Version]
- Garbarino, G.; Bellotti, D.; Finocchio, E.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3: Catalytic activity and infrared study. Catal. Today 2016, 277, 21–28. [Google Scholar] [CrossRef]
- Duyar, M.S.; Ramachandran, A.; Wang, C.; Farrauto, R.J. Kinetics of CO2 methanation over Ru/γ-Al2O3 and implications for renewable energy storage applications. J. CO2 Util. 2015, 12, 27–33. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nano crystalline γ-Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 1346–1352. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, D.H.; Kim, T. Enhancement of methanation of carbon dioxide using dielectric barrier discharge on a ruthenium catalyst at atmospheric conditions. Catal. Today 2017, 293–294, 97–104. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrog. Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Abate, S.; Mebrahtu, C.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Catalytic performance of γ-Al2O3-ZrO2-TiO2-CeO2 composite oxide supported Ni-based catalysts for CO2 Methanation. Ind. Eng. Chem. Res. 2016, 55, 4451–4460. [Google Scholar] [CrossRef]
- Nawfal, M.; Gennequin, C.; Labaki, M.; Nsouli, B.; Abi-Aada, E. Hydrogen production by methane steam reforming over Ru supported on Ni–Mg–Al mixed oxides prepared via hydrotalcite route. Int. J. Hydrog. Energy 2015, 40, 1269–1277. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C.; De Felicis, D.; Basoli, F.; Battocchio, C.; Tuti, S.; Licoccia, S.; Di Bartolomeo, E. Ni supported on γ-Al2O3 promoted by Ru for the dry reforming of methane in packed and monolithic reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Tada, S.; Minori, D.; Otsuka, F.; Kikuchi, R.; Osada, K.; Akiyama, K.; Satokawa, S. Effect of Ru and Ni ratio on selective CO methanation over Ru–Ni/TiO2. Fuel 2014, 129, 219–224. [Google Scholar] [CrossRef]
- Gao, Z.; Cui, L.; Ma, H. Selective methanation of CO over Ni/Al2O3 catalyst: Effects of preparation method and Ru addition. Int. J. Hydrog. Energy 2016, 41, 5484–5493. [Google Scholar] [CrossRef]
- Polanski, J.; Siudyga, T.; Bartczaka, P.; Kapkowskia, M.; Ambrozkiewicz, W.; Nobis, A.; Sitko, R.; Klimontko, J.; Szade, J.; Lelatko, J. Oxide passivated Ni-supported Ru nanoparticles in silica: A new catalyst for low-temperature carbon dioxide methanation. Appl. Catal. B Environ. 2017, 206, 16–23. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO2 methanation on Ni–Ru/γ-Al2O3 via modulating impregnation sequence and controlling surface active species. RSC Adv. 2014, 4, 16472–16479. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Ru-Ni/MgAl2O4 structured catalyst for CO2 methanation. Renew. Energy 2020, 161, 120–132. [Google Scholar] [CrossRef]
- Froment, G.F.; Bischoff, K.B. Chemical Reactor Analysis and Design; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 Reduction on supported Ru/Al2O3 catalysts: Cluster size dependence of product selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Kim, M.J.; Youn, J.; Kim, H.J.; Seo, M.W.; Lee, D.; Go, K.S.; Lee, K.B.; Jeon, S.G. Effect of surface properties controlled by Ce addition on CO2 methanation over Ni/Ce/Al2O3 catalyst. Int. J. Hydrog. Energy 2020, 45, 24595–24603. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrog. Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Zhu, H.; Razzaq, R.; Li, C.; Muhmmad, Y.; Zhang, S. Catalytic methanation of carbon dioxide by active oxygen material CexZr1−xO2 supported Ni-Co bimetallic nanocatalysts. AICHE J. 2013, 59, 2567–2576. [Google Scholar] [CrossRef]
- Penkova, A.; Bobadilla, L.; Ivanova, S.; Domínguez, M.I.; Romero-Sarria, F.; Roger, A.C.; Centeno, M.A.; Odriozola, J.A. Hydrogen production by methanol steam reforming on NiSn/MgO–Al2O3 catalysts: The role of MgO addition. Appl. Catal. A Gen. 2011, 392, 184–191. [Google Scholar] [CrossRef]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrog. Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2019, 60, 661–668. [Google Scholar] [CrossRef]
- Marconi, E.; Tuti, S.; Luisetto, I. Structure-sensitivity of CO2 Methanation over nanostructured Ni Supported on CeO2 Nanorods. Catalysts 2019, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Park, J.N.; McFarland, E.W. A highly dispersed Pd-Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Li, X.; Hao, Q.; Chen, H.; Ma, X. Ni-based catalysts prepared for CO2 reforming and decomposition of methane. Energy Convers. Manag. 2020, 205, 112419. [Google Scholar] [CrossRef]
- Janke, C.; Duyar, M.S.; Hoskins, M.; Farrauto, R. Catalytic and adsorption studies for the hydrogenation of CO2 to methane. Appl. Catal. B Environ. 2014, 152–153, 184–191. [Google Scholar] [CrossRef]
- Sharma, S.; Hu, Z.; Zhang, P.; McFarland, E.W.; Metiu, H. CO2 methanation on Ru-doped ceria. J. Catal. 2011, 278, 297–309. [Google Scholar] [CrossRef]
- Skriver, H.L.; Rosengaard, N.M. Surface energy and work function of elemental metals. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 46, 7157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostrup-Nielsen, J.R.; Hansen, J.H.B. CO2-reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Jalama, K. Carbon dioxide hydrogenation over nickel-, ruthenium-, and copper-based catalysts: Review of kinetics and mechanism. Catal. Rev. 2017, 59, 95–164. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Wissing, L.; Skjoth-Rasmussen, M.S. High temperature methanation: Catalyst considerations. Catal. Today 2013, 215, 233–238. [Google Scholar] [CrossRef]
- Yeung, C.; Tsang, S.C. Some optimization in preparing core-shell Pt–ceria catalysts for water gas shift reaction. J. Mol. Catal. A Chem. 2010, 322, 17–25. [Google Scholar] [CrossRef]






| Catalyst Type | Catalyst |
|---|---|
| Ni catalyst | 5 wt% Ni/Al2O3 |
| 10 wt% Ni/Al2O3 | |
| 15 wt% Ni/Al2O3 | |
| 20 wt% Ni/Al2O3 | |
| Ru catalyst | 1 wt% Ru/Al2O3 |
| 3 wt% Ru/Al2O3 | |
| 5 wt% Ru/Al 2O3 | |
| Bimetallic catalyst | 1 wt% Ru-10 wt% Ni/Al2O3 |
| 1 wt% Ru-15 wt% Ni/Al2O3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chein, R.-Y.; Wang, C.-C. Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts. Catalysts 2020, 10, 1112. https://doi.org/10.3390/catal10101112
Chein R-Y, Wang C-C. Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts. Catalysts. 2020; 10(10):1112. https://doi.org/10.3390/catal10101112
Chicago/Turabian StyleChein, Rei-Yu, and Chih-Chang Wang. 2020. "Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts" Catalysts 10, no. 10: 1112. https://doi.org/10.3390/catal10101112
APA StyleChein, R.-Y., & Wang, C.-C. (2020). Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts. Catalysts, 10(10), 1112. https://doi.org/10.3390/catal10101112






