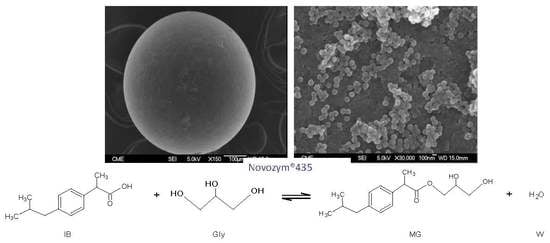
Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis
Abstract
1. Introduction
2. Results and Discussion
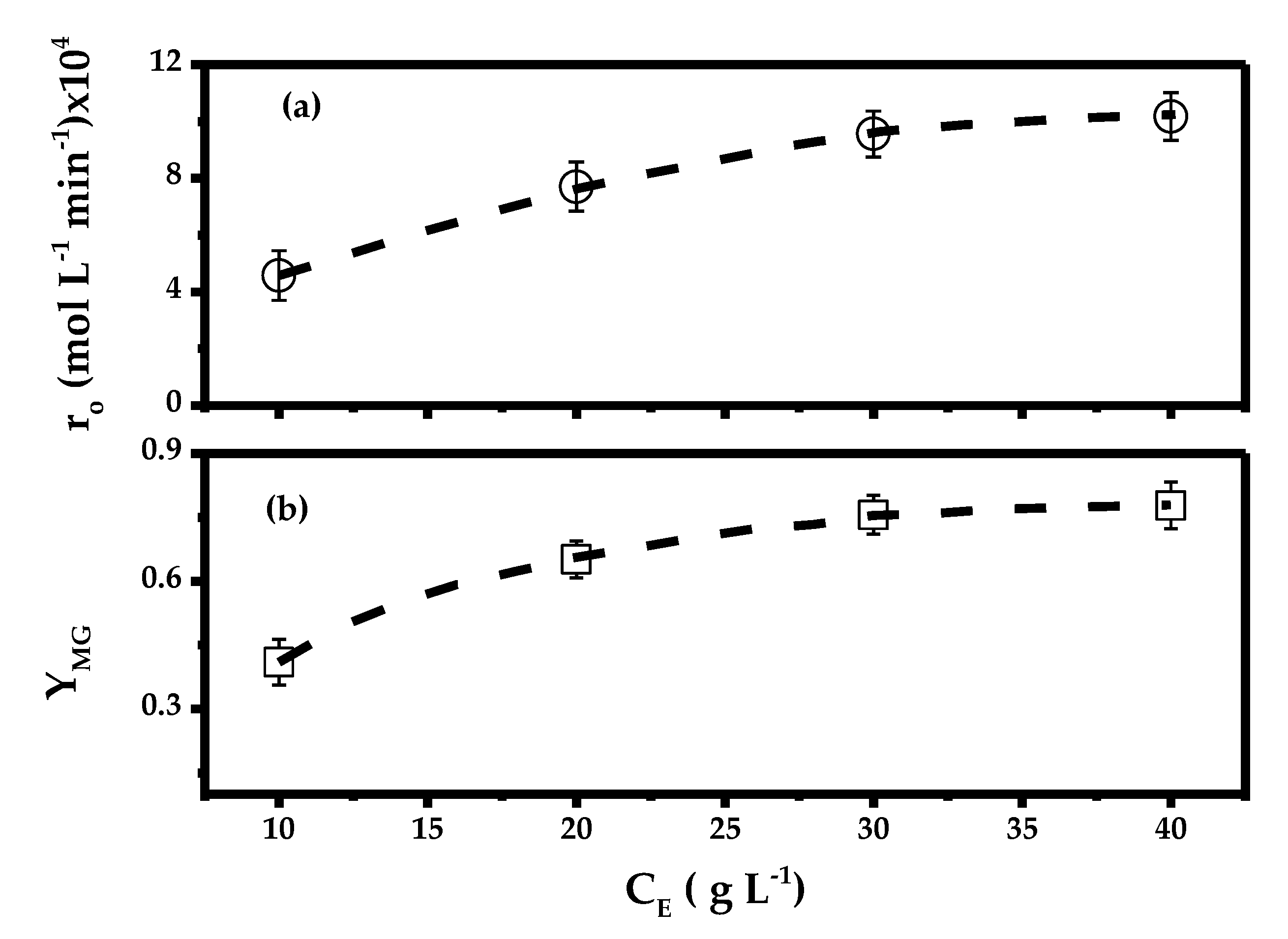
2.1. Effect of Biocatalyst Loading
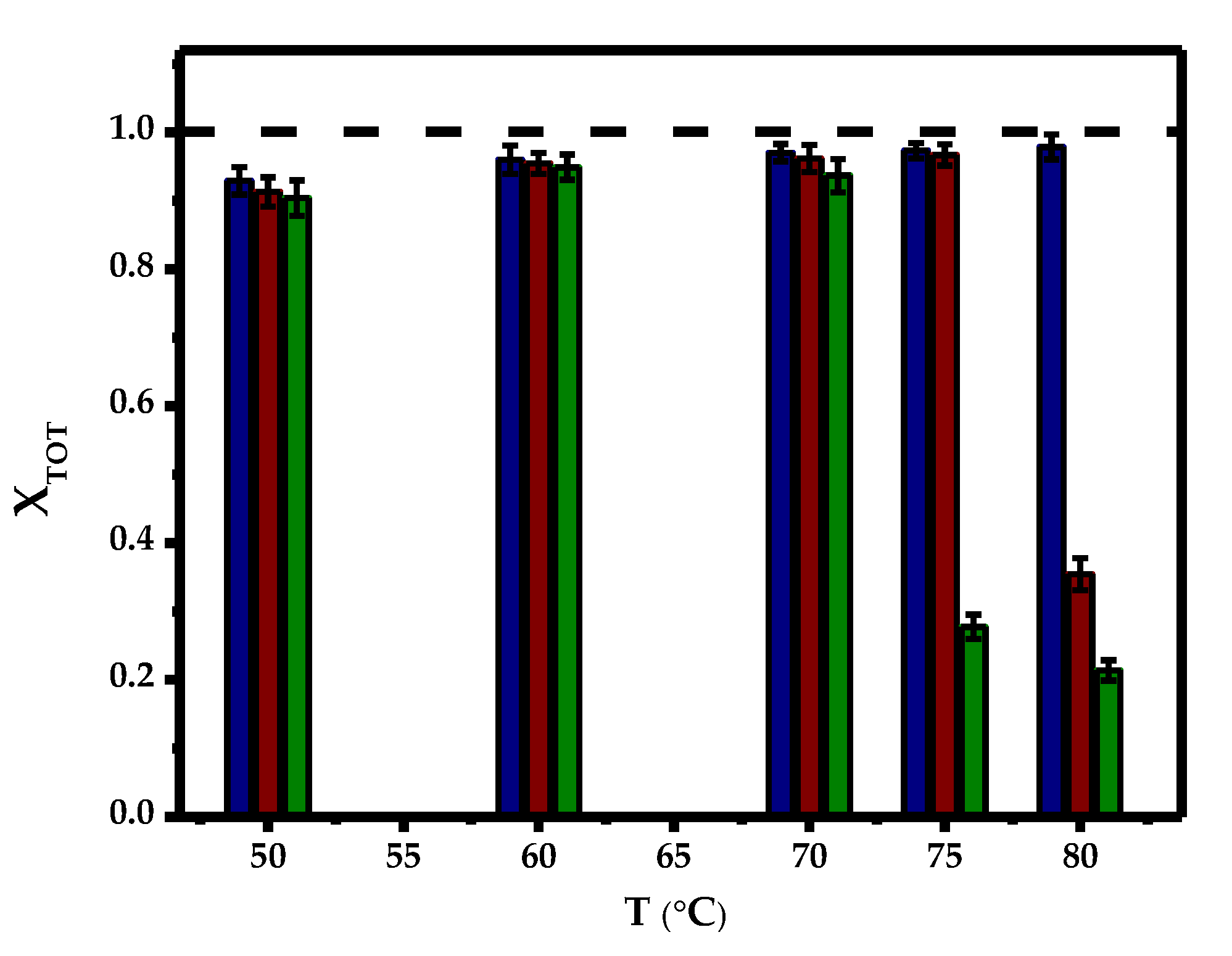
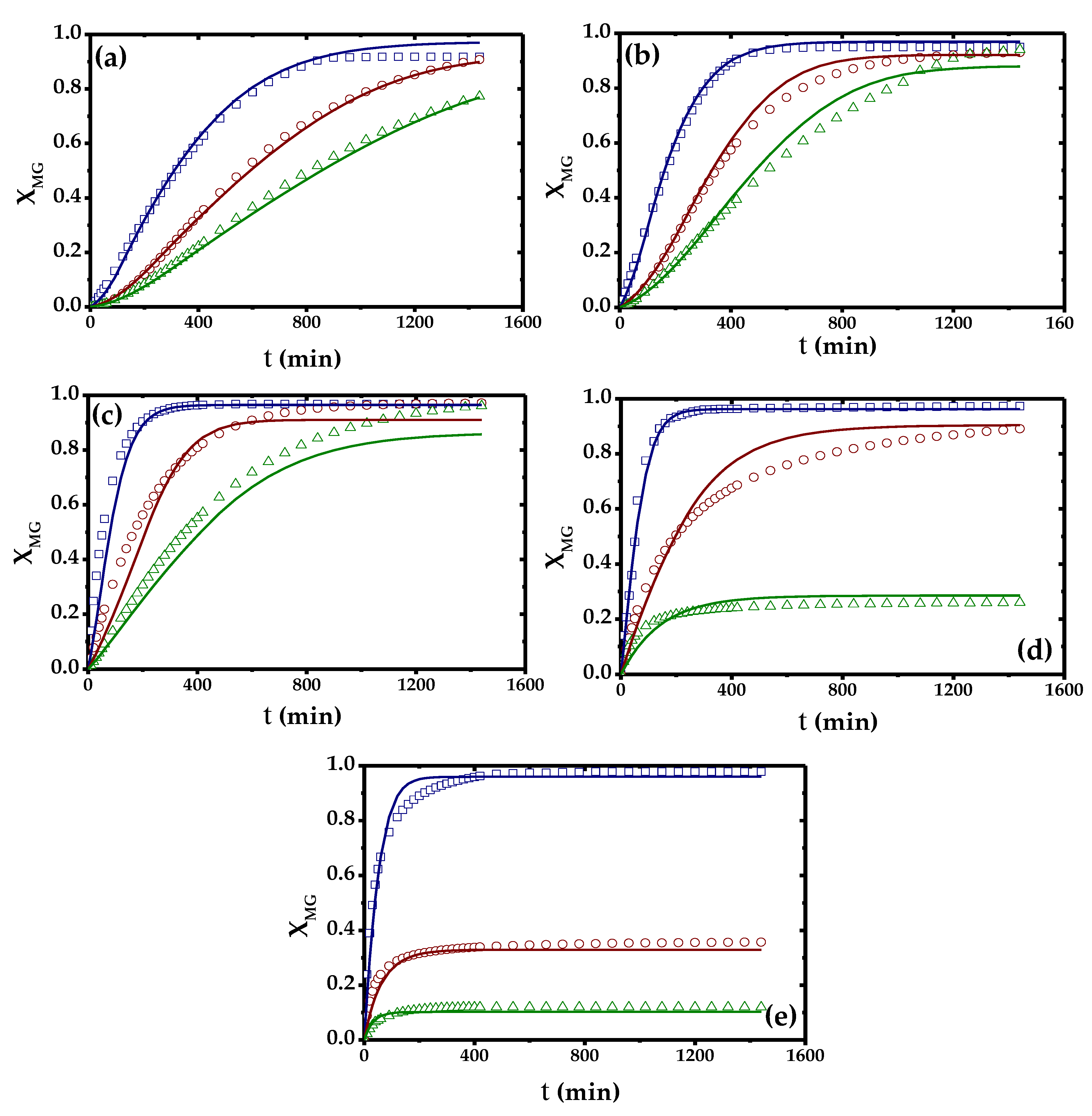
2.2. Effect of Temperature and Initial Concentration of Ibuprofen
2.3. Influence of Mass Transfer
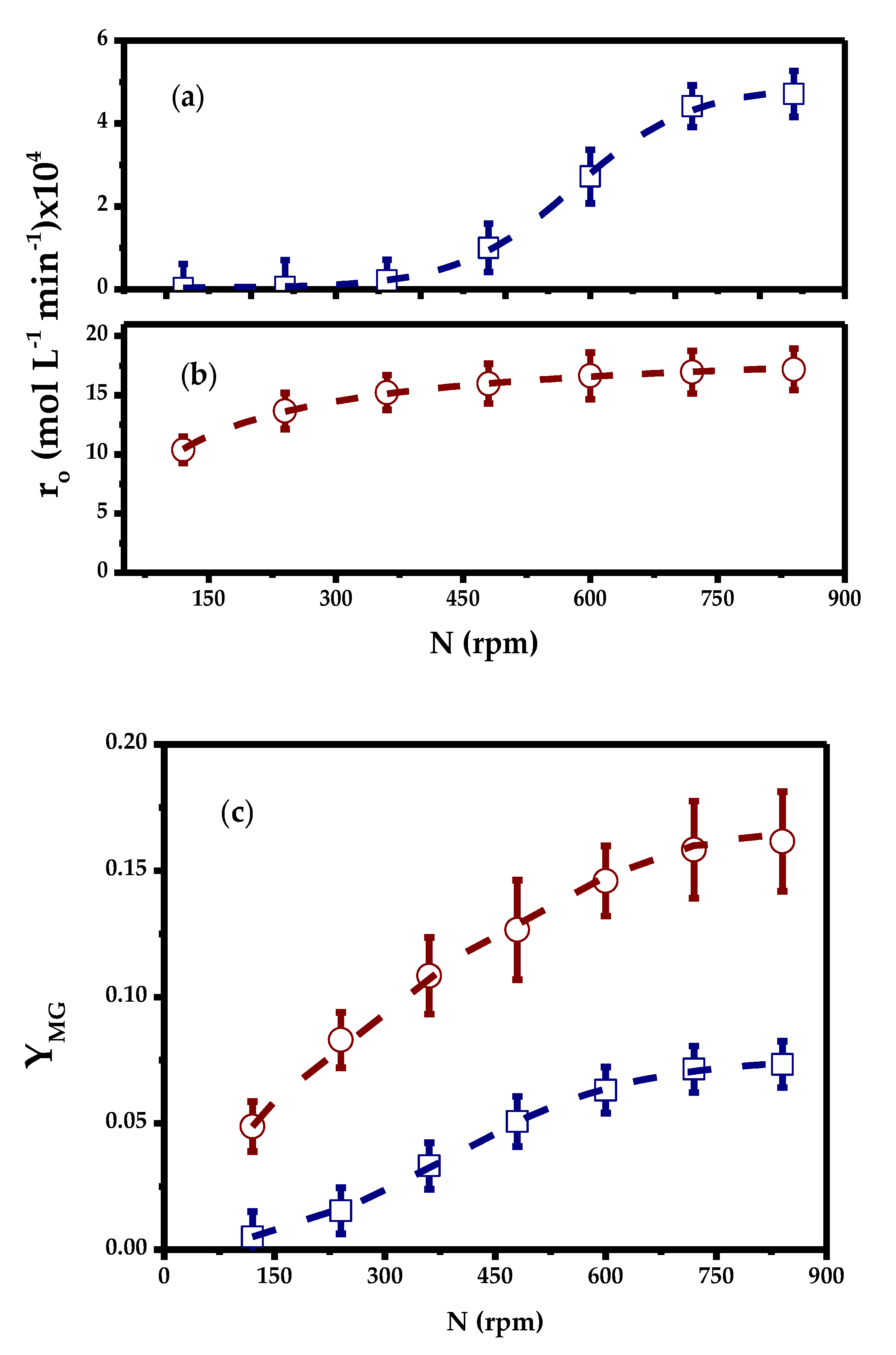
2.3.1. External Mass Transfer
2.3.2. Internal Mass Transfer
2.4. Kinetic Modelling
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Enzymatic Esterification of Ibuprofen Ester
3.2.2. Analytical Methods
3.2.3. Statistical Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AICc | Modified Akaike’s information criterion |
| aR | Remaining activity |
| BIC | Bayesian information criteria |
| CI0 | Initial concentration of ibuprofen (mol·L−1) |
| CE | Enzyme concentration (g·L−1) |
| Dij | Molecular diffusivity of substrate in the solvent (m2.s−1) |
| De | Effective diffusivity coefficient (m2.s−1) |
| Ea(ki) | Activation energy relative to constant ki (KJ mol−1) |
| F-value | Statistical Fisher’s F estimated considering Equation (2) |
| 1H-NMR | Proton nuclear magnetic resonance spectroscopy |
| HPLC | High-performance liquid chromatography |
| ki | Kinetic constant (mol L−1 min−1) |
| Ki | adsorption constant (mol L−1) |
| K | Number of parameters in kinetic model, Equations (2) to (5) |
| ki0 | Pre-exponential factor of the kinetic constant |
| kL | Mass transfer coefficient |
| n | Total number of components |
| N | Agitation rate (rpm) |
| Nt | Total number of data to which a model is fitted, Equations (2) to (5) |
| p-value | Value of the probability of getting a better model |
| RMG | Production rate of the monoglyceride (mol·L−1·min−1) |
| R | Ideal gas constant (J·mol−1·K−1) |
| RMSE | Square root of the mean of standard errors |
| SQR | Sum of quadratic residues |
| T | Temperature (°C) |
| X | Conversion, as defined by Equation (1) |
| Components | |
| d | Deactivation |
| E | Enzyme |
| I | Ibuprofen |
| Gly | Glycerol |
| MG | Ibuprofen monoglyceride |
| Tot | Total or final |
| Greek Letters | |
| ε | Porosity (-) |
| η | Effectiveness factor (-) |
| µ | Viscosity of solvent (kg·m−1·s−1) |
| µm | Viscosity of mix (kg·m−1·s−1) |
| ρ | Density (kg·L−1) |
| σ | Constriction factor (-) |
| τ | Tortuosity (-) |
| Subscripts | |
| 0 | Initial condition |
| 1 | kinetic direct constant |
| 2 | kinetic reverse constant |
| i | Relative to component i |
References
- Lari, G.M.; Pastore, G.; Haus, M.; Ding, Y.; Papadokonstantakis, S.; Mondelli, C.; Pérez-Ramírez, J. Environmental and economical perspectives of a glycerol biorefinery. Energy Environ. Sci. 2018, 11, 1012–1029. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Praveen Kumar, R.; Elavazhagan, S.; Barathiraja, B.; Jayakumar, M.; Varjani, S.J. Utilization of crude glycerol from biodiesel industry for the production of value-added bioproducts. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 65–82. [Google Scholar]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Dong, H.-P.; Wang, Y.-J.; Zheng, Y.-G. Enantioselective hydrolysis of diethyl 3-hydroxyglutarate to ethyl (S)-3-hydroxyglutarate by immobilized Candida antarctica lipase B. J. Mol. Catal. Enzym. 2010, 66, 90–94. [Google Scholar] [CrossRef]
- Rottici, D. Understanding and Engineering the Enantioselectivity Site of Candida Antarctica Lipase B towards Sec-Alcohols; Royal Institute of Technology: Stockholm, Sweden, 2000. [Google Scholar]
- Wang, S.-Z.; Wu, J.-P.; Xu, G.; Yang, L.-R. Kinetic modelling of lipase-catalyzed remote resolution of citalopram intermediate in solvent-free system. Biochem. Eng. J. 2009, 45, 113–119. [Google Scholar] [CrossRef]
- Morrone, R.; D’Antona, N.; Lambusta, D.; Nicolosi, G. Biocatalyzed irreversible esterification in the preparation of S-naproxen. J. Mol. Catal. Enzym. 2010, 65, 49–51. [Google Scholar] [CrossRef]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: Experimental and molecular modeling aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar] [CrossRef]
- José, C.; Briand, L. Deactivation of Novozym® 435 during the esterification of ibuprofen with ethanol: Evidences of the detrimental effect of the alcohol. React. Kinet. Mech. Catal. 2010, 99, 17–22. [Google Scholar] [CrossRef]
- Duan, G.; Ching, C.B. Preparative scale enantioseparation of flurbiprofen by lipase-catalysed reaction. Biochem. Eng. J. 1998, 2, 237–245. [Google Scholar] [CrossRef]
- Duan, G.; Ching, C.B.; Lim, E.; Ang, C.H. Kinetic study of enantioselective esterification of ketoprofen with n-propanol catalysed by an lipase in an organic medium. Biotechnol. Lett. 1997, 19, 1051–1055. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- José, C.; Austic, G.B.; Bonetto, R.D.; Burton, R.M.; Briand, L.E. Investigation of the stability of Novozym® 435 in the production of biodiesel. Catal. Today 2013, 213, 73–80. [Google Scholar] [CrossRef]
- Hong, W.P.; Park, J.Y.; Min, K.; Ko, M.J.; Park, K.; Yoo, Y.J. Kinetics of glycerol effect on biodiesel production for optimal feeding of methanol. Korean J. Chem. Eng. 2011, 28, 1908–1912. [Google Scholar] [CrossRef]
- Chen, B.; Miller, M.E.; Gross, R.A. Effects of porous polystyrene resin parameters on Candida antarctica lipase B adsorption, distribution, and polyester synthesis activity. Langmuir 2007, 23, 6467–6474. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.B.; Séguin, J.P.; Marquardt, D.; Harroun, T.A.; Zelisko, P.M. Synthesis of polyesters containing disiloxane subunits: Structural characterization, kinetics, and an examination of the thermal tolerance of Novozym-435. J. Mol. Catal. Enzym. 2013, 85–86, 149–155. [Google Scholar] [CrossRef]
- Kumar, A.; Gross, R.A. Candida antarctica Lipase B-Catalyzed transesterification: New synthetic routes to copolyesters. J. Am. Chem. Soc. 2000, 122, 11767–11770. [Google Scholar] [CrossRef]
- Contesini, F.J.; de Oliveira Carvalho, P. Esterification of (RS)-Ibuprofen by native and commercial lipases in a two-phase system containing ionic liquids. Tetrahedron Asymmetry 2006, 17, 2069–2073. [Google Scholar] [CrossRef]
- Tamayo, J.J.; Ladero, M.; Santos, V.E.; Garcia-Ochoa, F. Esterification of benzoic acid and glycerol to alpha-monobenzoate glycerol in solventless media using an industrial free Candida antarctica lipase B. Process Biochem. 2012, 47, 243–250. [Google Scholar] [CrossRef]
- Ravelo, M.; Fuente, E.; Blanco, Á.; Ladero, M.; García-Ochoa, F. Esterification of glycerol and ibuprofen in solventless media catalyzed by free CALB: Kinetic modelling. Biochem. Eng. J. 2015, 101, 228–236. [Google Scholar] [CrossRef]
- Wolfson, A.; Atyya, A.; Dlugy, C.; Tavor, D. Glycerol triacetate as solvent and acyl donor in the production of isoamyl acetate with Candida antarctica lipase B. Bioprocess Biosyst. Eng. 2010, 33, 363–366. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P.; Schrebler, R.A. Remarkable stability of Candida antarctica lipase B immobilized via cross-linking aggregates (CLEA) in deep eutectic solvents. Biocatal. Biotransformation 2019, 37, 106–114. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Y.; Zhou, L.; Gao, J. Immobilization of Candida antarctica lipase B by adsorption in organic medium. New Biotechnol. 2010, 27, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Trubiano, G.; Borio, D.; Errazu, A. Influence of the operating conditions and the external mass transfer limitations on the synthesis of fatty acid esters using a Candida antarctica lipase. Enzyme Microb. Technol. 2007, 40, 716–722. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Itabaiana, I., Jr.; de Mariz e Miranda, L.S.; de Souza, R.O.M.A. Towards a continuous flow environment for lipase-catalyzed reactions. J. Mol. Catal. Enzym. 2013, 85–86, 1–9. [Google Scholar] [CrossRef]
- Ladero, M.; Santos, A.; Garcia-Ochoa, F. Diffusion and chemical reaction rates with nonuniform enzyme distribution: An experimental approach. Biotechnol. Bioeng. 2001, 72, 458–467. [Google Scholar] [CrossRef]
- Ladero, M.; Santos, A.; García-Ochoa, F. Kinetic modeling of lactose hydrolysis with an immobilized β-galactosidase from Kluyveromyces fragilis. Enzyme Microb. Technol. 2000, 27, 583–592. [Google Scholar] [CrossRef]
- Sim, J.H.; Harun Kamaruddin, A.; Bhatia, S. Effect of Mass Transfer and Enzyme Loading on the Biodiesel Yield and Reaction Rate in the Enzymatic Transesterification of Crude Palm Oil. Energy Fuels 2009, 23, 4651–4658. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Nidetzky, B.; Bolivar, J.M.; López-Gallego, F. Single-Particle Studies to Advance the Characterization of Heterogeneous Biocatalysts. ChemCatChem 2018, 10, 654–665. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuo, T.; Kimura, Y.; Adachi, S. Thermal stability of immobilized lipase from Candida antarctica in glycerols with various water contents at elevated temperatures. J. Am. Oil Chem. Soc. 2008, 85, 1041–1044. [Google Scholar] [CrossRef]
- Yadav, G.D.; Borkar, I.V. Kinetic and mechanistic investigation of microwave-assisted lipase catalyzed synthesis of citronellyl acetate. Ind. Eng. Chem. Res. 2009, 48, 7915–7922. [Google Scholar] [CrossRef]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: Reaction optimization by response surface methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipase-catalyzed synthesis of isoamyl butyrate: A kinetic study. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2001, 1547, 262–267. [Google Scholar] [CrossRef]
- Yadav, G.D.; Borkar, I.V. Lipase-catalyzed hydrazinolysis of phenyl benzoate: Kinetic modeling approach. Process Biochem. 2010, 45, 586–592. [Google Scholar] [CrossRef]
- Yadav, G.D.; Borkar, I.V. Kinetic modeling of microwave-assisted chemoenzymatic epoxidation of styrene. AIChE J. 2006, 52, 1235–1247. [Google Scholar] [CrossRef]
- Peesa, J.P.; Yalavarthi, P.R.; Rasheed, A.; Mandava, V.B.R. A perspective review on role of novel NSAID prodrugs in the management of acute inflammation. J. Acute Dis. 2016, 5, 364–381. [Google Scholar] [CrossRef]
- Ong, A.L.; Kamaruddin, A.H.; Bhatia, S.; Long, W.S.; Lim, S.T.; Kumari, R. Performance of free Candida antarctica lipase B in the enantioselective esterification of (R)-ketoprofen. Enzyme Microb. Technol. 2006, 39, 924–929. [Google Scholar] [CrossRef]
- Carvalho, P.D.O.; Contesini, F.J.; Ikegaki, M. Enzymatic resolution of (R, S)-ibuprofen and (R, S)-ketoprofen by microbial lipases from native and commercial sources. Braz. J. Microbiol. 2006, 37, 329–337. [Google Scholar] [CrossRef]
- Xin, J.Y.; Chen, L.L.; Zhang, Y.X.; Zhang, S.; Xia, C.G. Lipase-catalyzed transesterification of ethyl ferulate with triolein in solvent-free medium. Food Bioprod. Process. 2011, 89, 457–462. [Google Scholar] [CrossRef]
- Guauque Torres, M.D.P.; Foresti, M.L.; Ferreira, M.L. Cross-linked enzyme aggregates (CLEAs) of selected lipases: A procedure for the proper calculation of their recovered activity. AMB Express 2013, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Wu, P.-L. Synthesis of triglycerides of phenylbutyric acids by lipase-catalyzed glycerolysis in a solvent-free system. J. Mol. Catal. Enzym. 2009, 61, 117–122. [Google Scholar] [CrossRef]
- Mears, D.E. Tests for transport limitations in experimental catalytic reactors. Ind. Eng. Chem. Process Des. Dev. 1971, 10, 541–547. [Google Scholar] [CrossRef]
- Doran, P.M. 12—Heterogeneous Reactions; Academic Press: London, UK, 1995; pp. 297–332. [Google Scholar]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Perry, R.; Green, D. Perry’s Chemical Engineer’s Handbook; McGraw-Hill Education: New York, NY, USA, 2007. [Google Scholar]
- Tamayo, J. Producción Enzimática de Monoglicéridos por Esterificación de Glicerina con Ácido Benzoico y α−Metoxicinámico. Ph.D.Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Haigh, K.F.; Abidin, S.Z.; Vladisavljević, G.T.; Saha, B. Comparison of Novozyme 435 and Purolite D5081 as heterogeneous catalysts for the pretreatment of used cooking oil for biodiesel production. Fuel 2013, 111, 186–193. [Google Scholar] [CrossRef]
- Chesterfield, D.M.; Rogers, P.L.; Al-Zaini, E.O.; Adesina, A.A. Production of biodiesel via ethanolysis of waste cooking oil using immobilised lipase. Chem. Eng. J. 2012, 207–208, 701–710. [Google Scholar] [CrossRef]
- Ladero, M.; Santos, A.; Garcia-Ochoa, F. Hindered diffusion of proteins and polymethacrylates in controlled-pore glass: An experimental approach. Chem. Eng. Sci. 2007, 62, 666–678. [Google Scholar] [CrossRef]
- Cha, S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J. Biol. Chem. 1968, 243, 820–825. [Google Scholar]
- Gekko, K.; Timasheff, S.N. Mechanism of protein stabilization by glycerol: Preferential hydration in glycerol-water mixtures. Biochemistry 1981, 20, 4667–4676. [Google Scholar] [CrossRef]
- Nian, B.; Cao, C.; Liu, Y. How Candida antarctica lipase B can be activated in natural deep eutectic solvents: Experimental and molecular dynamics studies. J. Chem. Technol. Biotechnol. 2020, 95, 86–93. [Google Scholar] [CrossRef]
 ) and 80 °C (∗).
) and 80 °C (∗).
 ) and 80 °C (∗).
) and 80 °C (∗).
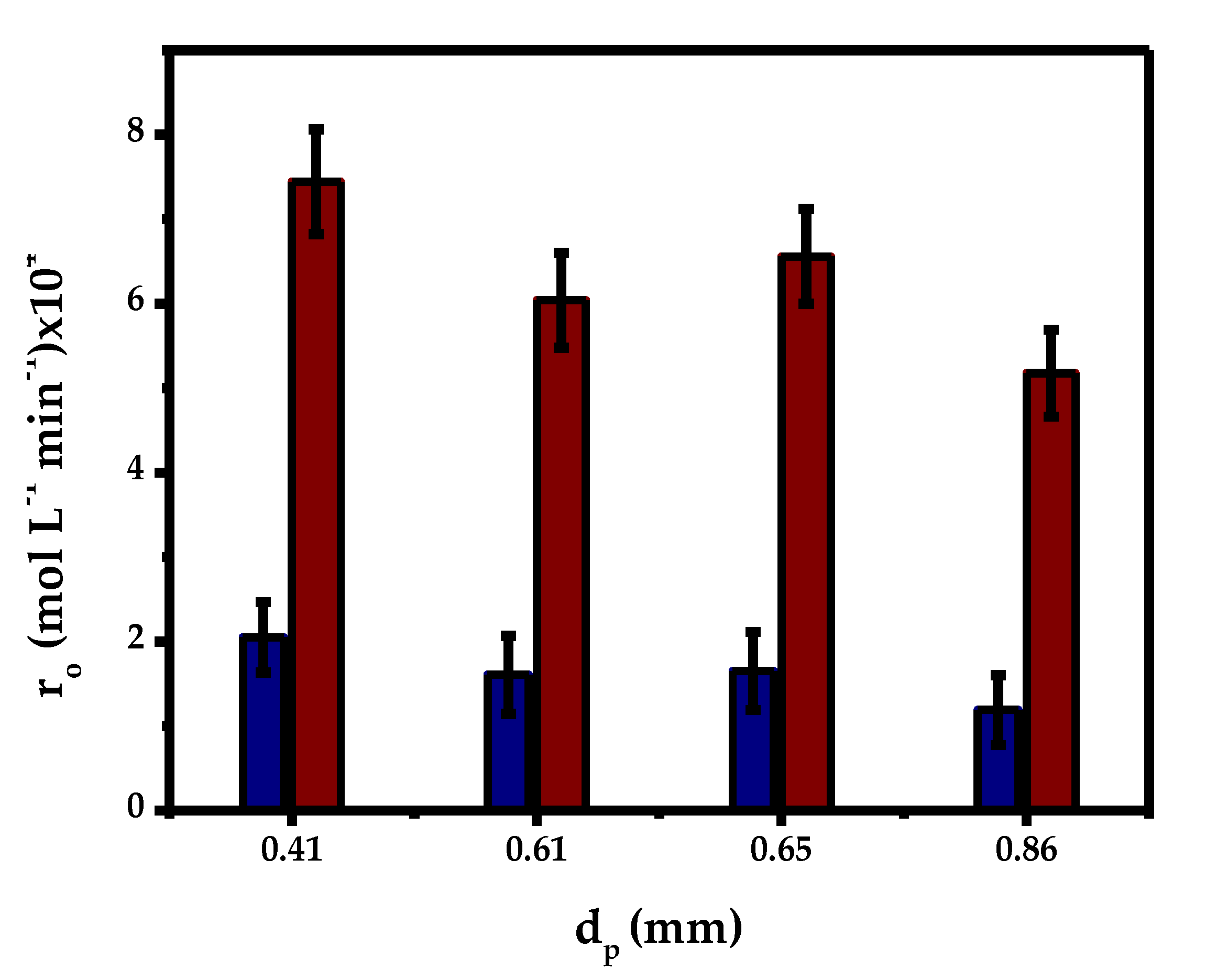
| T (°C) | Dij × 1011 (m2 s−1) | De × 1013 (m2 s−1) | Size Fraction % w/w | Size Particle (mm) | RMG × 104 Novozym®435 (mol L−1 min−1) | η | We-Pt |
|---|---|---|---|---|---|---|---|
| 50 | 1.69 | 1.35 | 13 | 0.32–0.5 | 2.05 | 0.16 | 3.7 |
| 52 | 0.5–0.7 | 1.61 | 0.12 | 6.2 | |||
| 35 | 0.7–1 | 1.19 | 0.09 | 9.3 | |||
| Average | 0.3–1 | 1.65 | 0.12 | 7.4 | |||
| 70 | 5.03 | 4.03 | 13 | 0.32–0.5 | 7.44 | 0.11 | 4.4 |
| 52 | 0.5–0.7 | 6.04 | 0.09 | 7.9 | |||
| 35 | 0.7–1 | 5.18 | 0.08 | 10.6 | |||
| Average | 0.3–1 | 6.56 | 0.09 | 9.8 |
| Model | Rate Equations | Parameters | Value | Error |
|---|---|---|---|---|
| 1 | lnk′10 | 13.14 | 0.32 | |
| Eak′1/R | 6999 | 107 | ||
| lnk′20 | 22.57 | 3.88 | ||
| Eak′2/R | 10,679 | 1311 | ||
| lnkd0 | 85.43 | 1.86 | ||
| Eakd/R | 33,111 | 654 | ||
| KI | 17.97 | 1.02 | ||
| 2 | Si CI ≥ SIR ⇒ CIR=SIR Si CI < SIR ⇒ CIR=CI | lnk′10 | 15.45 | 0.44 |
| Eak′1/R | 7800 | 148 | ||
| lnk′20 | 19.58 | 2.35 | ||
| Eak′2/R | 9562 | 794 | ||
| lnks0 | −31.3 | 2.45 | ||
| Eaks/R | −10,610 | 812 | ||
| lnkd0 | 89.13 | 1.97 | ||
| Eakd/R | 32408 | 690 | ||
| KI | 11.14 | 0.58 |
| Model | F-Value | SQR | AICc | BIC | RMSE | VE (%) |
|---|---|---|---|---|---|---|
| 1 | 10863 | 1.18 | −3833 | −3847 | 0.06 | 98.43 |
| 2 | 13995 | 0.88 | −4010 | −4028 | 0.04 | 98.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravelo, M.; Wojtusik, M.; Ladero, M.; García-Ochoa, F. Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis. Catalysts 2020, 10, 76. https://doi.org/10.3390/catal10010076
Ravelo M, Wojtusik M, Ladero M, García-Ochoa F. Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis. Catalysts. 2020; 10(1):76. https://doi.org/10.3390/catal10010076
Chicago/Turabian StyleRavelo, Marianela, Mateusz Wojtusik, Miguel Ladero, and Félix García-Ochoa. 2020. "Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis" Catalysts 10, no. 1: 76. https://doi.org/10.3390/catal10010076
APA StyleRavelo, M., Wojtusik, M., Ladero, M., & García-Ochoa, F. (2020). Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis. Catalysts, 10(1), 76. https://doi.org/10.3390/catal10010076