Liquid Phase Furfural Oxidation under Uncontrolled pH in Batch and Flow Conditions: The Role of In Situ Formed Base
Abstract
1. Introduction
2. Results
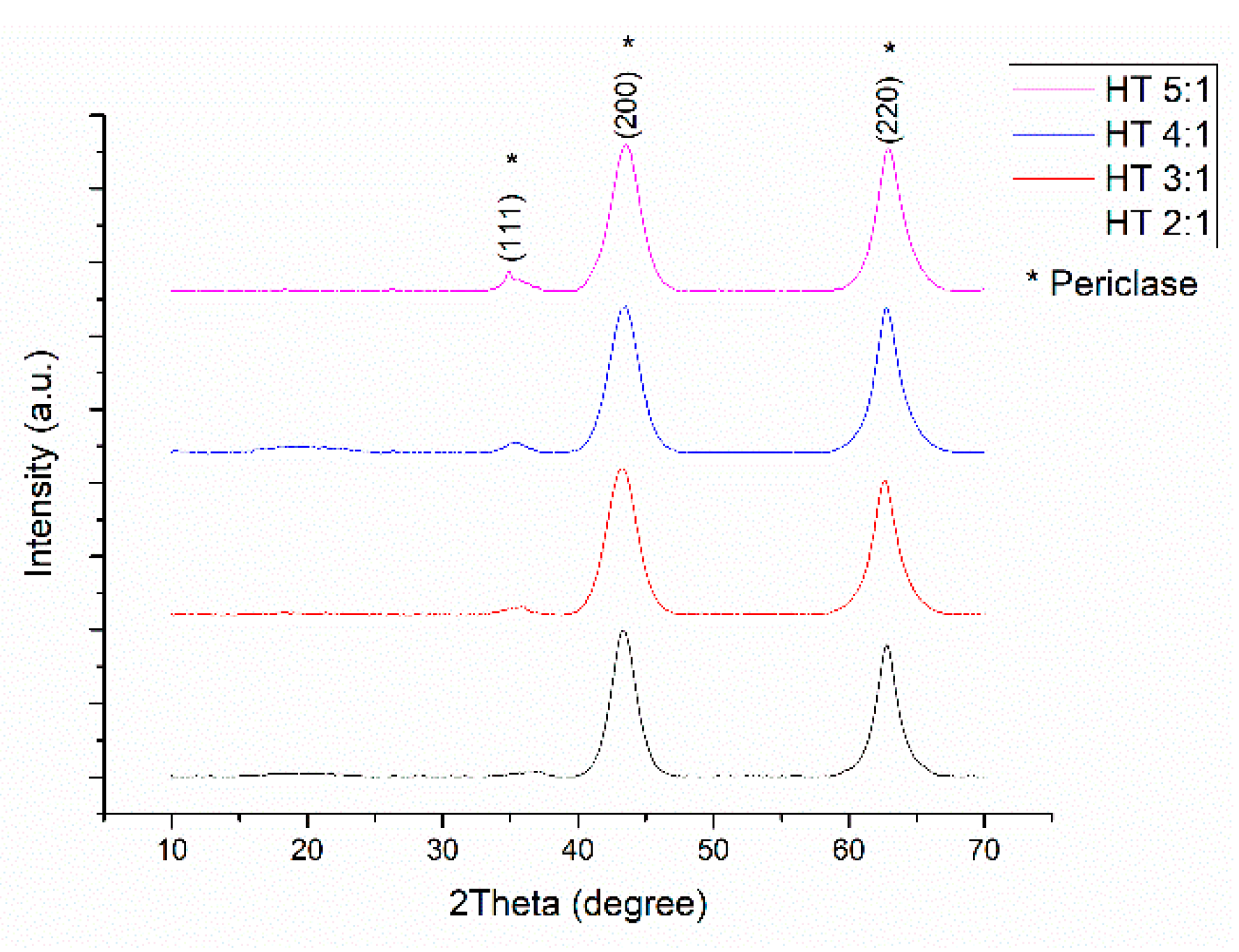
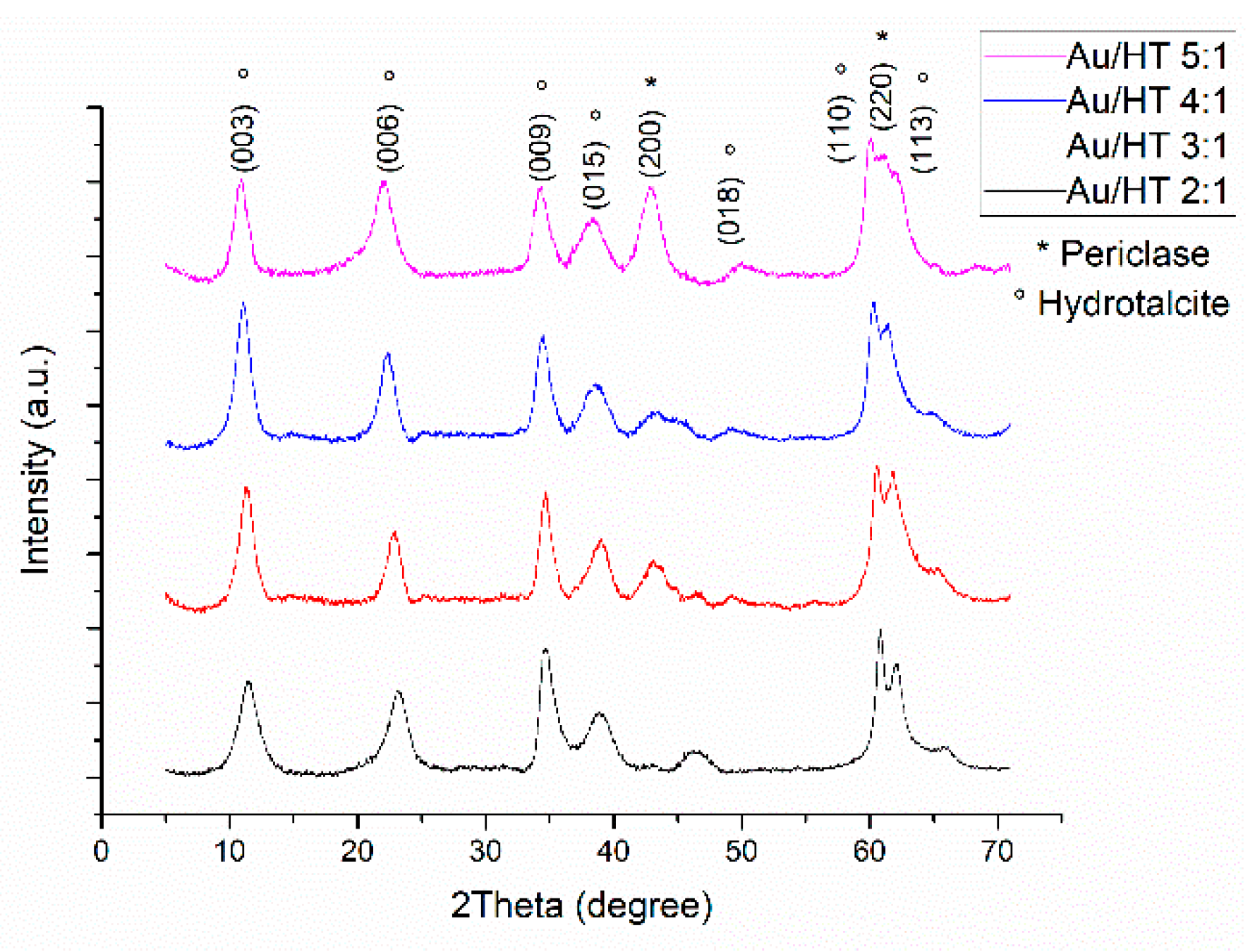
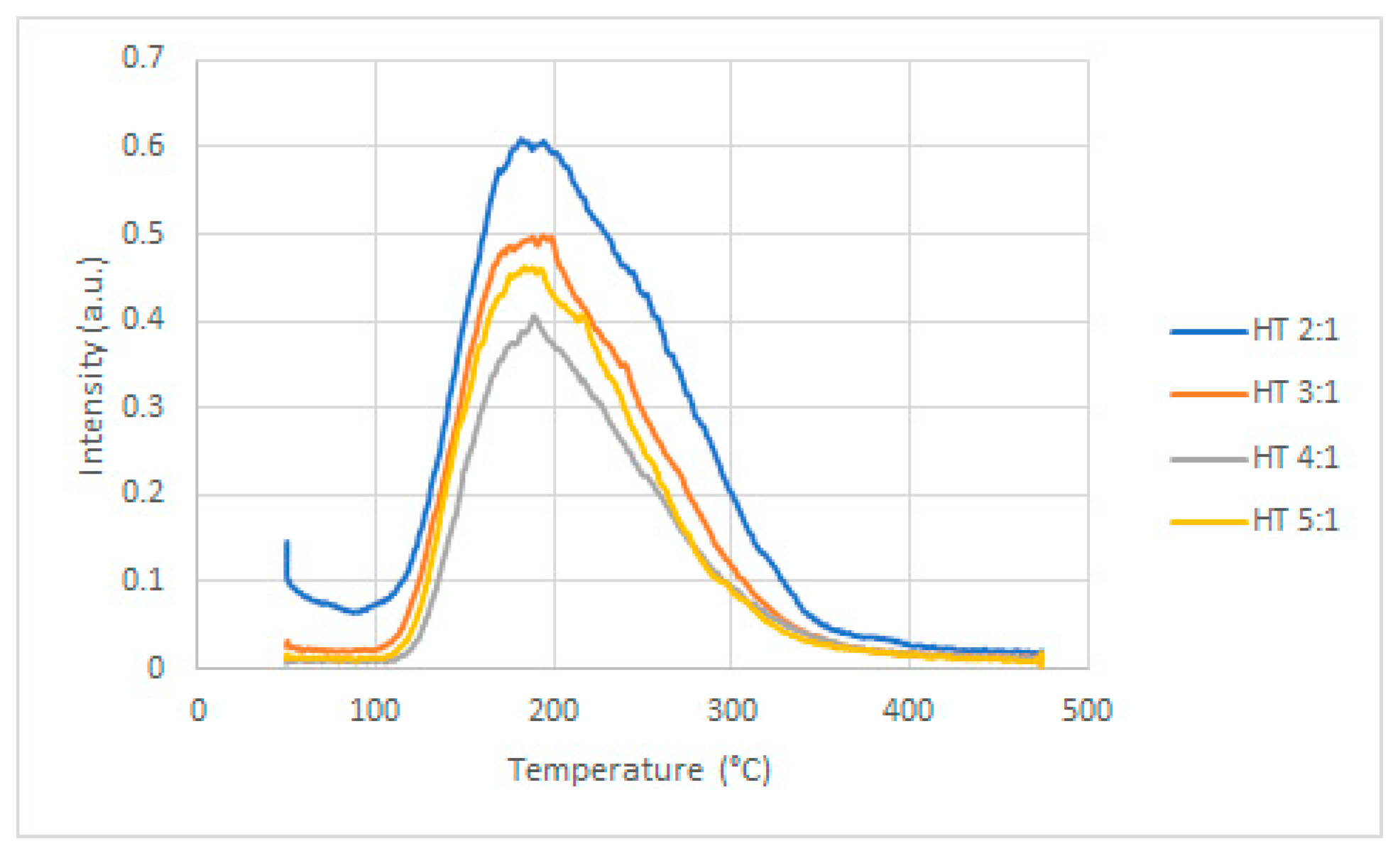
2.1. Catalysts Characterization
2.2. Blank Test with Hydrotalcite Supports Only
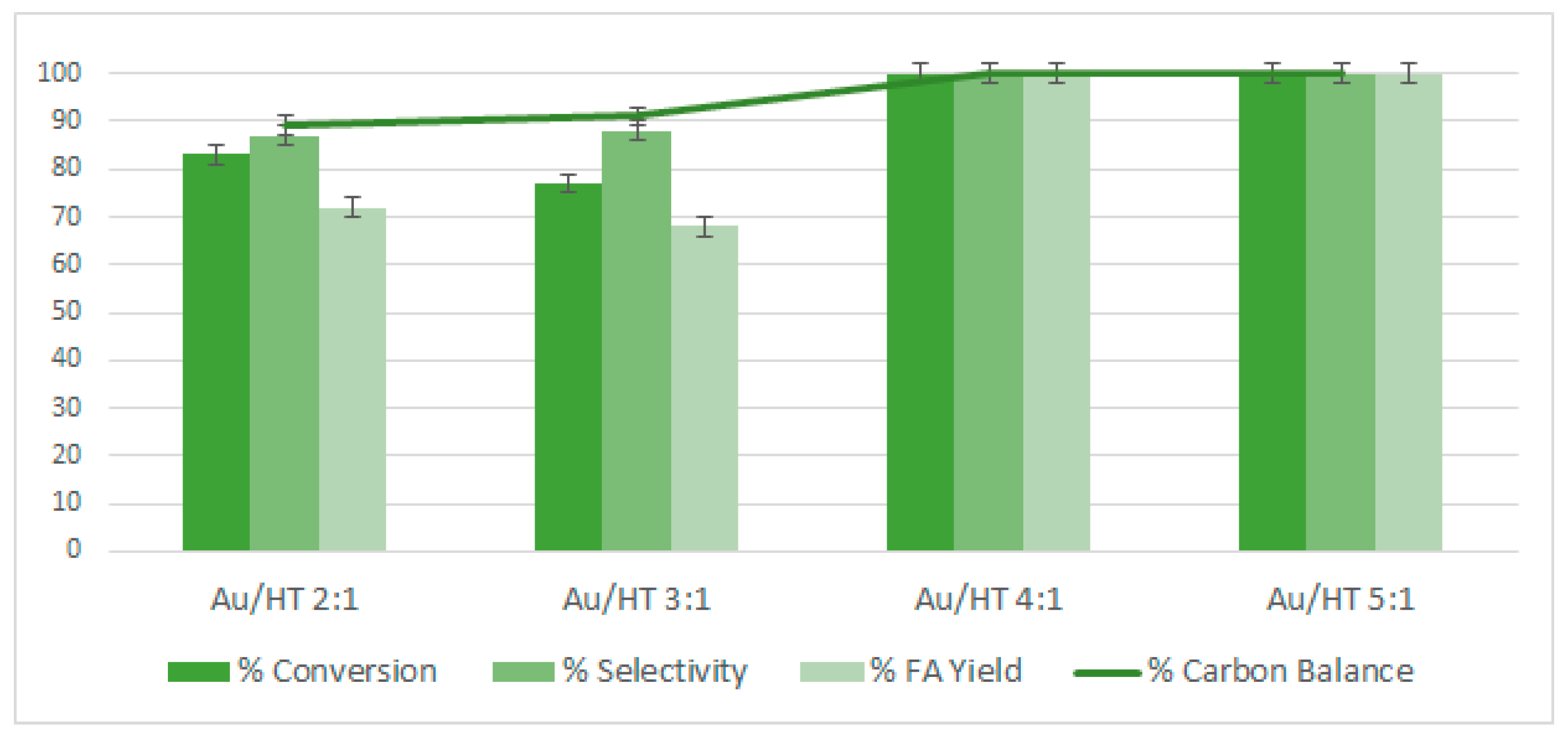
2.3. Reactivity of Gold Nanoparticles Supported on Hydrotalcites
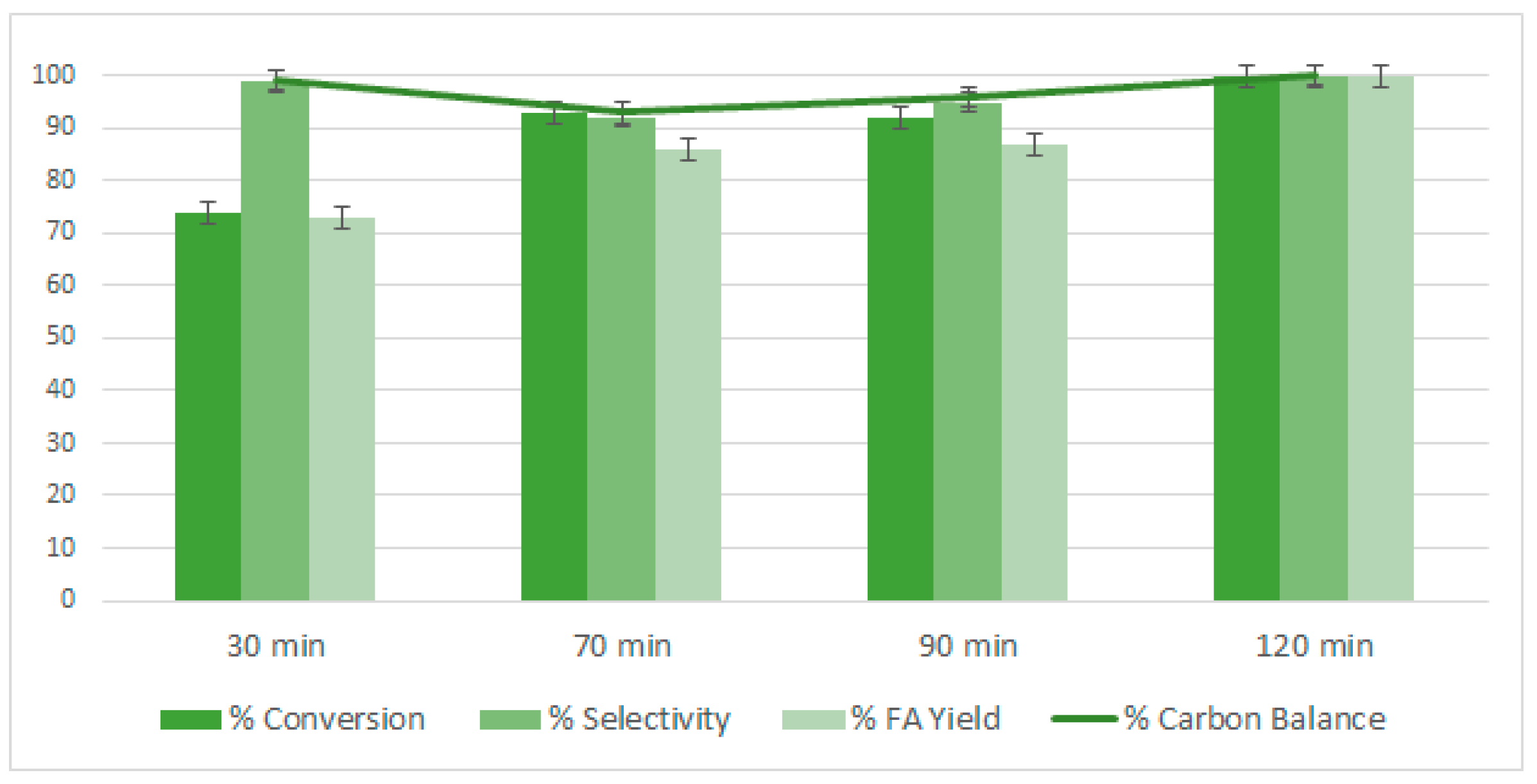
2.4. Study on the Effect of the Reaction Time
2.5. Study on the Effect of the Temperature
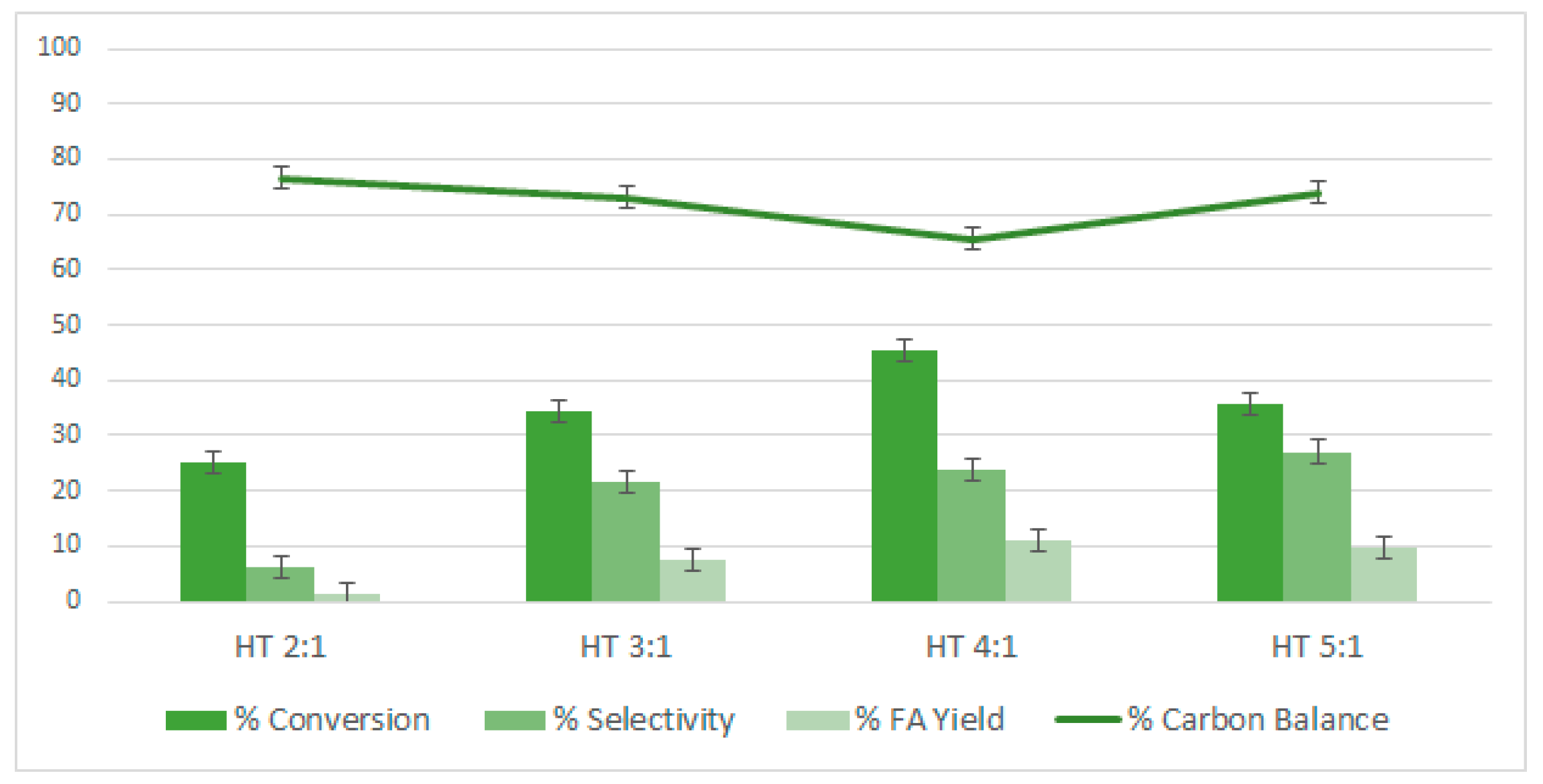
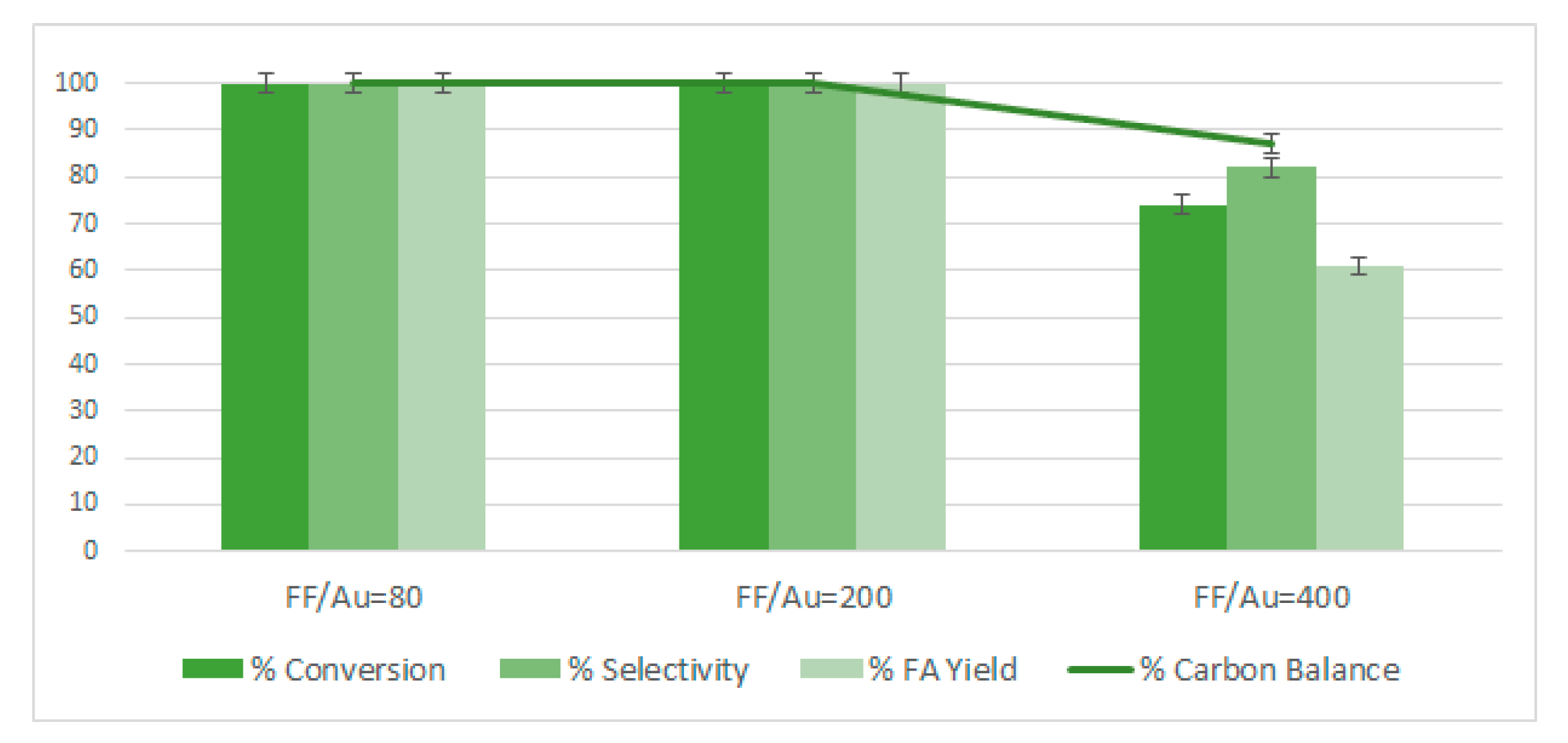
2.6. Study of the Effect of the FF/Au Molar Ratios
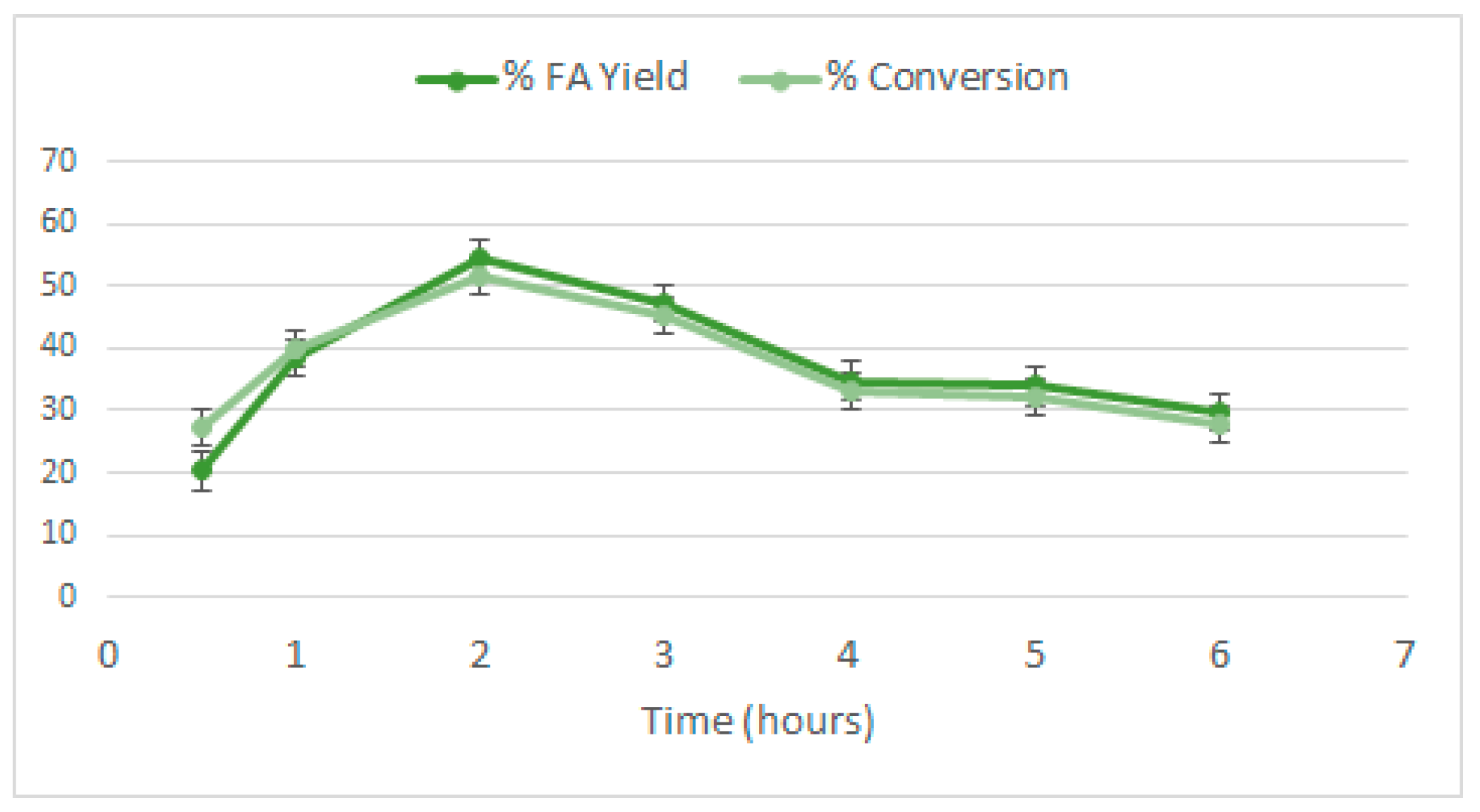
2.7. Stability of Au/HT 4:1 in a Continuous Reactor
3. Discussion
4. Materials and Methods
4.1. Hydrotalcite Supports Preparation
4.2. Gold NPs on Hydrotalcite Supports
4.3. Catalysts Characterization
4.4. Catalytic Tests
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sousa-Aguiar, E.F.; Appel, L.G.; Zonetti, P.C.; do Couto Fraga, A.; Bicudo, A.A.; Fonseca, I. Some important catalytic challenges in the bioethanol integrated biorefinery. Catal. Today 2014, 234, 13–23. [Google Scholar] [CrossRef]
- Dunlop, A.P. Furfural formation and behavior. Ind. Eng. Chem. 1948, 40, 204–209. [Google Scholar] [CrossRef]
- Brownlee, H.J.; Miner, C.S. Industrial development of furfural. Ind. Eng. Chem. 1948, 40, 201–204. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Asano, T.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Selective Hydrodeoxygenation of 2-Furancarboxylic Acid to Valeric Acid over Molybdenum-Oxide-Modified Platinum Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 6253–6257. [Google Scholar] [CrossRef]
- Asano, T.; Takagi, H.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Selective hydrogenolysis of 2-furancarboxylic acid to 5-hydroxyvaleric acid derivatives over supported platinum catalysts. Green Chem. 2019, 21, 6133–6145. [Google Scholar] [CrossRef]
- Li, X.; Lan, X.; Wang, T. Selective oxidation of furfural in a bi-phasic system with homogeneous acid catalyst. Catal. Today 2016, 276, 97–104. [Google Scholar] [CrossRef]
- Dunlop, A.P. Process for Manufacturing Furoic Acid and Furoic Acid Salts. U.S. Patent No. 2/407/066, 3 September 1946. [Google Scholar]
- Tian, Q.; Shi, D.; Sha, Y. CuO and Ag2O/CuO catalyzed oxidation of aldehydes to the corresponding carboxylic acids by molecular oxygen. Molecules 2008, 13, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, P.; Merat, N.; Gaset, A. Lead/platinum on charcoal as catalyst for oxidation of furfural. Effect of main parameters. Appl. Catal. A Gen. 1994, 112, 1–11. [Google Scholar] [CrossRef]
- Verdeguer, P.; Merat, N.; Rigal, L.; Gaset, A. Optimization of experimental conditions for the catalytic oxidation of furfural to furoic acid. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1994, 61, 97–102. [Google Scholar] [CrossRef]
- Douthwaite, M.; Huang, X.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Kondrat, S.A.; Edwards, J.K.; Sankar, M.; Knight, D.W.; Bethell, D.; et al. The controlled catalytic oxidation of furfural to furoic acid using AuPd/Mg (OH) 2. Catal. Sci. Technol. 2017, 7, 5284–5293. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, P.; Gao, J.; Lu, X.; Hou, Z.; Zheng, X. Pt/solid-base: A predominant catalyst for glycerol hydrogenolysis in a base-free aqueous solution. Catal. Lett. 2009, 130, 261–265. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid under atmospheric oxygen pressure. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Tongsakul, D.; Nishimura, S.; Ebitani, K. Platinum/gold alloy nanoparticles-supported hydrotalcite catalyst for selective aerobic oxidation of polyols in base-free aqueous solution at room temperature. ACS Catal. 2013, 3, 2199–2207. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Zieliński, M.; Pietrowski, M.; Heyte, S.; Dumeignil, F.; Rossi, L.M.; Wojcieszak, R. Influence of Support Basic Sites in Green Oxidation of Biobased Substrates Using Au-Promoted Catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16332–16340. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Da Silva, A.G.M.; Rodrigues, T.S.; Camargo, P.H.C.; Paul, S.; Wojcieszak, R. Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances. Appl. Sci. 2018, 8, 1246. [Google Scholar] [CrossRef]
- Zhao, R.; Yin, C.; Zhao, H.; Liu, C. Synthesis, characterization, and application of hydotalcites in hydrodesulfurization of FCC gasoline. Fuel Process. Technol. 2003, 81, 201–209. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Ferraz, C.P.; Sha, J.; Houda, S.; Rossi, L.M.; Paul, S. Advances in base-free oxidation of bio-based compounds on supported gold catalysts. Catalysts 2017, 7, 352. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.; Carley, A.; Prati, L.; Hutchings, G.J. Solvent free liquid phase oxidation of benzyl alcohol using Au supported catalysts prepared using a sol immobilization technique. Catal. Today 2007, 122, 317–324. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Garcia, M.A.S.; Teixeira-Neto, É.; Rossi, L.M. Oxidation of benzyl alcohol catalyzed by gold nanoparticles under alkaline conditions: Weak vs. strong bases. RSC Adv. 2016, 6, 25279–25285. [Google Scholar] [CrossRef]



| Catalyst | Au (w/w %) | Al (w/w %) | Mg (w/w %) | Mg/Al Ratio |
|---|---|---|---|---|
| Au/HT 2:1 | 1.3% | 13.8% | 23.0% | 1.7 |
| Au/HT 3:1 | 1.7% | 11.1% | 27.8% | 2.5 |
| Au/HT 4:1 | 1.2% | 8.7% | 27.5% | 3.2 |
| Au/HT 5:1 | 1.4% | 7.6% | 30.0% | 4 |
| Catalyst | BET Surface Area (m2g−1) | Pore Volume (cm3g−1) |
|---|---|---|
| Au/HT 2:1 | 73 | 0.17 |
| Au/HT 3:1 | 25 | 0.07 |
| Au/HT 4:1 | 30 | 0.08 |
| Au/HT 5:1 | 25 | 0.07 |
| Support | NH3 (a.u.g−1) 1 | CO2 (a.u.g−1) 1 | Mg/Al Ratio |
|---|---|---|---|
| HT 2:1 | 90 | 33 | 1.7 |
| HT 3:1 | 63 | 68 | 2.5 |
| HT 4:1 | 48 | − | 3.2 |
| HT 5:1 | 56 | 105 | 4 |
| Time | [Au] mgL−1 | [Mg] mgL−1 | [Mg]leach./[Mg]total w/w % | pH |
|---|---|---|---|---|
| 0 | − | − | 0 | 3 |
| 30 | − | 13.4 | 1.55% | 7 |
| 70 | − | 17.7 | 2.04% | 7 |
| 90 | − | 19.8 | 2.29% | 7 |
| 120 | − | 23.0 | 2.66% | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roselli, A.; Carvalho, Y.; Dumeignil, F.; Cavani, F.; Paul, S.; Wojcieszak, R. Liquid Phase Furfural Oxidation under Uncontrolled pH in Batch and Flow Conditions: The Role of In Situ Formed Base. Catalysts 2020, 10, 73. https://doi.org/10.3390/catal10010073
Roselli A, Carvalho Y, Dumeignil F, Cavani F, Paul S, Wojcieszak R. Liquid Phase Furfural Oxidation under Uncontrolled pH in Batch and Flow Conditions: The Role of In Situ Formed Base. Catalysts. 2020; 10(1):73. https://doi.org/10.3390/catal10010073
Chicago/Turabian StyleRoselli, Alessandra, Yuri Carvalho, Franck Dumeignil, Fabrizio Cavani, Sébastien Paul, and Robert Wojcieszak. 2020. "Liquid Phase Furfural Oxidation under Uncontrolled pH in Batch and Flow Conditions: The Role of In Situ Formed Base" Catalysts 10, no. 1: 73. https://doi.org/10.3390/catal10010073
APA StyleRoselli, A., Carvalho, Y., Dumeignil, F., Cavani, F., Paul, S., & Wojcieszak, R. (2020). Liquid Phase Furfural Oxidation under Uncontrolled pH in Batch and Flow Conditions: The Role of In Situ Formed Base. Catalysts, 10(1), 73. https://doi.org/10.3390/catal10010073









