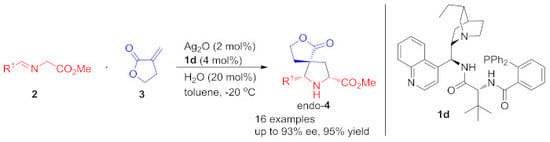
Endo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via Ag(I)/CAAA-Amidphos-Catalyzed 1,3-Dipolar Cycloaddition between Azomethine Ylides and α-Methylene-γ-Butyrolactone
Abstract
1. Introduction
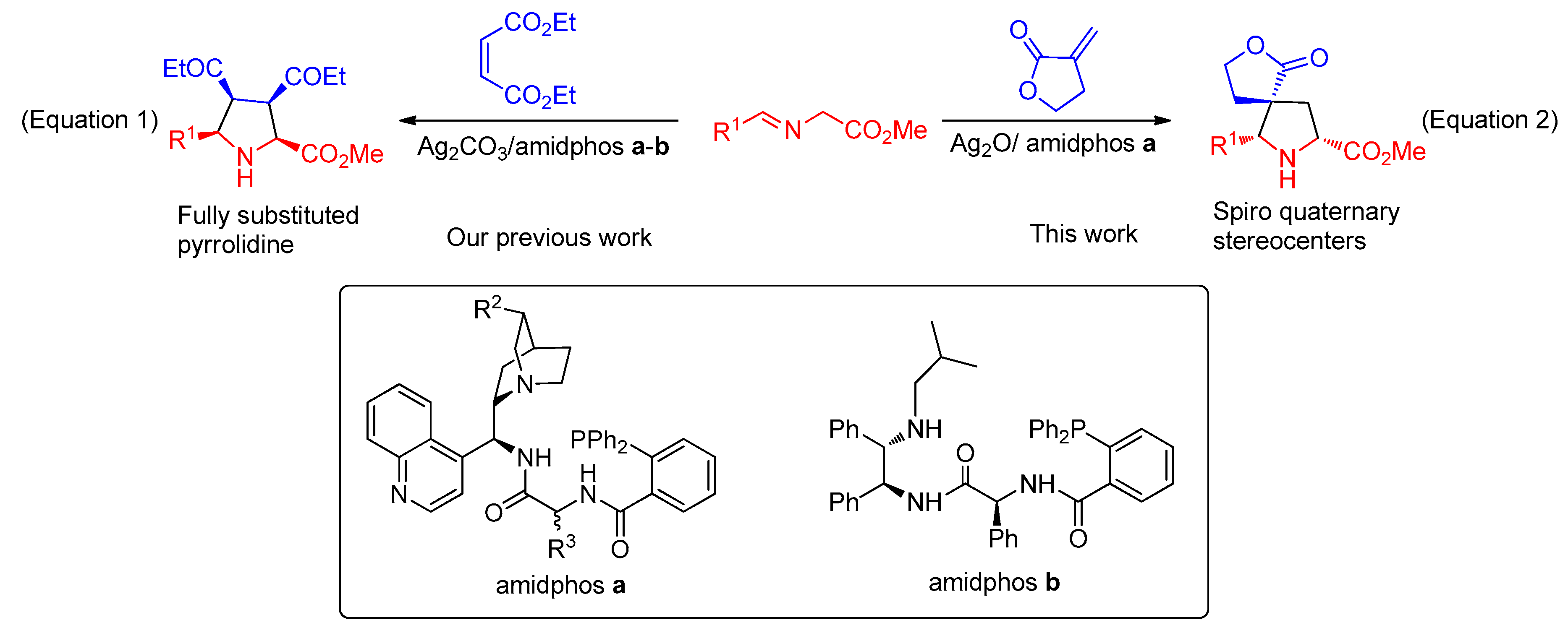
2. Results and Discussion
2.1. Optimization of the 1,3-Dipole Cycloaddition Reaction Conditions
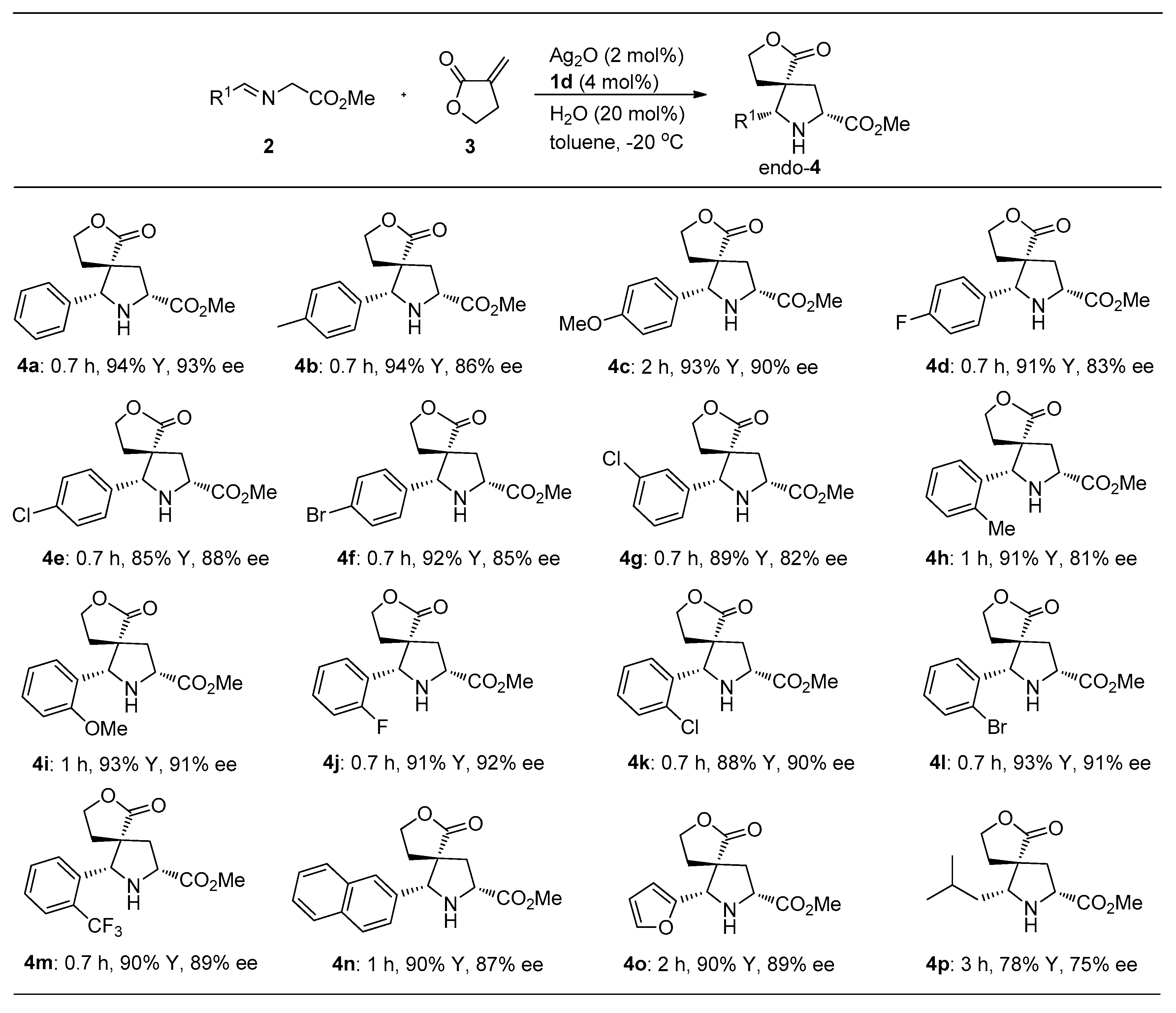
2.2. Study on Substrate Adaptability
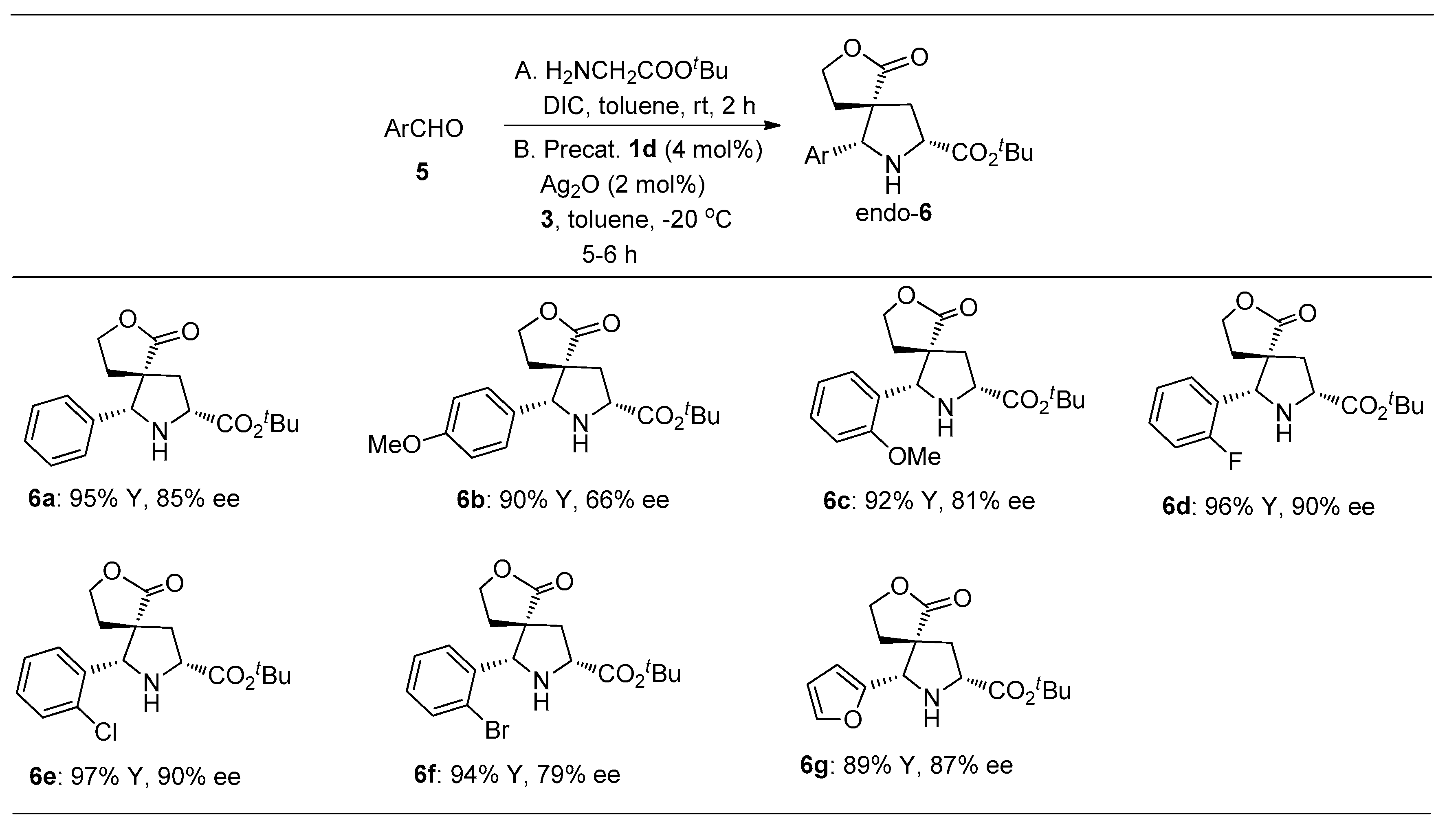
2.3. Study on the Construction of Spiro Pyrrolidines by the Three-Component One-Pot Method
3. Materials and Methods
3.1. Materials
3.2. General Synthesis Methods of 4a–4p
3.3. General Synthesis Methods of 6a–6g via Three-Component “One-Pot”
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Michael, J.P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2008, 25, 139–165. [Google Scholar] [CrossRef]
- Vitaku, E.D.; Smith, T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among USA. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Marti, C.; Carreira, E.M. Construction of Spiro [pyrrolidine-3, 3′-oxindoles]-Recent Applications to the Synthesis of Oxindole Alkaloids. Eur. J. Org. Chem. 2003, 12, 2209–2219. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef]
- Adrio, J.; Carretero, J.C. Recent Advances in the Catalytic Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides. Chem. Commun. 2014, 50, 12434–12446. [Google Scholar] [CrossRef] [PubMed]
- Bdiri, B.; Zhao, B.-J.; Zhou, Z.-M. Recent Advances in the Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Ylides and Dipolarophiles. Tetrahedron Asymmetry 2017, 28, 876–899. [Google Scholar] [CrossRef]
- Döndas, H.A.; de Gracia-Retamosa, M.; Sansano, J.M. Current Trends towards the Synthesis of Bioactive Heterocycles and Natural Products Using 1,3-Dipolar Cycloadditions (1,3-DC) with Azomethine Ylides. Synthesis 2017, 49, 2819–2851. [Google Scholar] [CrossRef]
- Chen, X.-H.; Wei, Q.; Luo, S.-W.; Xiao, H.; Gong, L.-Z. Organocatalytic Synthesis of Spiro [pyrrolidin-3,3′-oxindoles] with High Enantiopurity and Structural Diversity. J. Am. Chem. Soc. 2009, 131, 13819–13825. [Google Scholar] [CrossRef] [PubMed]
- Antonchick, A.P.; Gerding-Reimers, C.; Catarinella, M.; Schürmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Highly Enantioselective Synthesis and Cellular Evaluation of Spirooxindoles Inspired by Natural Products. Nat. Chem. 2010, 2, 735–740. [Google Scholar] [CrossRef]
- Awata, A.; Arai, T. Catalytic Asymmetric Exo′-Selective [3 + 2] Cycloaddition for Constructing Stereochemically Diversified Spiro [pyrrolidin-3,3′-oxindole] s. Chem. Eur. J. 2012, 18, 8278–8282. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-L.; He, Z.-L.; Tao, H.-Y.; Wang, C.-J. Stereoselective Construction of Spiro (butyrolactone pyrrolidines) by Highly Efficient Copper (I)/TF-BiphamPhos-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition. Chem. Eur. J. 2012, 18, 8042–8046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.-M.; Dong, W.-P.; Zhu, L.-P.; Wang, R. Efficient Construction of Highly Functionalized Spiro[γ-butyrolactone-pyrrolidin-3,3′-oxindole] Tricyclic Skeletons via an Organocatalytic 1,3-Dipolar Cycloaddition. Chem. Commun. 2013, 49, 3458–3460. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Ogawa, H.; Awata, A.; Sato, M.; Watabe, M.; Yamanaka, M. PyBidine-Cu (OTf) 2-Catalyzed Asymmetric [3 + 2] Cycloaddition with Imino Esters: Harmony of Cu-Lewis Acid and Imidazolidine-NH Hydrogen Bonding in Concerto Catalysis. Angew. Chem. Int. Ed. 2015, 54, 1595–1599. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Wang, H.-Y.; Jin, Q.-W.; Zheng, C.-W.; Zhao, G.; Shang, Y.-J. Thiourea-quaternary Ammonium Salt Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Imino Esters to Construct Spiro [pyrrolidin-3,3-oxindoles]. Org. Lett. 2016, 18, 4774–4777. [Google Scholar] [CrossRef]
- Liu, T.-L.; He, Z.-L.; Tao, H.-Y.; Cai, Y.-P.; Wang, C.-J. Stereoselective Construction of a 5-aza-Spiro [2, 4] Heptane Motif Viacatalytic Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides and Ethyl Cyclopropylidene Acetate. Chem. Commun. 2011, 47, 2616–2618. [Google Scholar] [CrossRef]
- Liu, T.-L.; He, Z.-L.; Wang, C.-J. Highly Efficient Construction of Spirocyclic Chromanone—Pyrrolidines via Cu (I)/TF-BiphamPhos-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition. Chem. Commun. 2011, 47, 9600–9602. [Google Scholar] [CrossRef]
- Liu, T.-L.; He, Z.-L.; Li, Q.-H.; Tao, H.-Y.; Wang, C.-J. Catalytic Asymmetric Construction of Spirocycles Containing Pyrrolidine Motifs and Spiro Quaternary Stereogenic Centers via 1,3-Dipolar Cycloaddition of Azomethine Ylides with 2-Alkylidene-Cycloketones. Adv. Synth. Catal. 2011, 353, 1713–1719. [Google Scholar] [CrossRef]
- Yang, W.-L.; Liu, Y.-Z.; Luo, S.; Yu, X.; Fossey, J.-S.; Deng, W.-P. The Copper-Catalyzed Asymmetric Construction of a Dispiropyrrolidine Skeleton via 1,3-Dipolar Cycloaddition of Azomethine Ylides to a-Alkylidene Succinimides. Chem. Commun. 2015, 51, 9212–9215. [Google Scholar] [CrossRef]
- Yang, W.-L.; Tang, F.-F.; He, F.-S.; Li, C.-Y.; Yu, X.; Deng, W.-P. Asymmetric Construction of Spirocyclic Pyrrolidine-Thia (oxa) Zolidinediones via N,O-Ligand/Cu (I) Catalyzed 1,3-Dipolar Cycloaddition of Azomethine Ylides with 5-Alkylidene Thia (oxa) Zolidine-2, 4-Diones. Org. Lett. 2015, 17, 4822–4825. [Google Scholar] [CrossRef]
- Castulik, J.; Marek, J.; Mazal, C. Synthesis of Spiropyrrolidines and Spiropyrrolizidines by 1,3-Dipolar Cycloadditions of Azomethine Ylides to Substituted α-Methylene-γ-Lactones. Tetrahedron 2001, 57, 8339–8347. [Google Scholar] [CrossRef]
- Jenkins, S.M.; Wadsworth, H.J.; Bromidge, S. Substituent Variation in Azabicyclic Triazole and Tetrazole-based Muscarinic Receptor Ligands. J. Med. Chem. 1992, 35, 2392–2406. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.D.; Peters, D.A.; Allway, P.A.; Beddoes, R.L. 1,3-Dipolar Cycloadditions to Oxidopyraziniums. Heterocycles 1995, 40, 331–347. [Google Scholar]
- Li, Q.-H.; Liu, T.-L.; Wei, L.; Zhou, X.; Tao, H.-Y.; Wang, C.-J. exo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via 1,3-Dipolar Cycloaddition of Azomethine Ylides with α-Methylene-γ-Butyrolactone Catalyzed by Cu (I)/DTBM-BIPHEP. Chem. Commun. 2013, 49, 9642–9644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, Q.; Zhou, Z.; Hu, S.; Liu, Z.; Zhou, L.-Y. Ag2CO3/CA-AA-AmidPhos Multifunctional Catalysis in the Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Ylides. Org. Lett. 2016, 18, 404–407. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, X.; Liu, J.; Li, J.; Wen, P.; Wang, H. Tert-Leucine-Derived AmidPhos-Silver (I) Chiral Complexes for the Asymmetric [3+2] Cycloaddition of Azomethine Ylides. Synlett 2017, 28, 999–1003. [Google Scholar]
- Hou, Y.; Zhou, Z.; Liu, P.; Wang, J.; Hou, Q.; Wen, P.; Wang, H. A Class of α-Amino Acids-derived Multifunctional Amidophosphane Precatalysts: Application to the Highly Enantio and Diastereoselective Silver (I)-Catalyzed 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Asymmetry 2017, 28, 930–938. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, Q.; Hou, Q.; Zhang, K.; Wen, P.; Hu, S.; Wang, H. A Chiral Secondary Amine-amidophosphane Precatalyst for Silver-catalyzed Asymmetric 1,3-Dipolar Cycloaddition Reactions. Synthesis 2018, 50, 2347–2358. [Google Scholar]
- Mancebo-Aracil, J.; Nájera, C.; Sansano, J.M. Multicomponent Synthesis of Unnatural Pyrrolizidines Using 1,3-Dipolar Cycloaddition of Proline Esters. Chem. Commun. 2013, 49, 11218–11220. [Google Scholar] [CrossRef]
- Mancebo-Aracil, J.; Nájera, C.; Castelló, L.M.; Sansano, J.M.; Larrañaga, O.; de Cózar, A.; Cossío, F.P. Regio and Diastereoselective Multicomponent 1,3-Dipolar Cycloadditions between Prolinate Hydrochlorides, Aldehydes and Dipolarophiles for the Direct Synthesis of Pyrrolizidines. Tetrahedron 2015, 71, 9645–9661. [Google Scholar] [CrossRef]

| Entry | Precat. | MLn | Temp. [°C] | Time [h] | Yield [%] b | Ee [%] c |
|---|---|---|---|---|---|---|
| 1 | 1a | Ag2O | rt | 2.5 | 74 | 14 |
| 2 | 1b | Ag2O | rt | 2 | 92 | 84 |
| 3 | 1c | Ag2O | rt | 2 | 91 | 76 |
| 4 | 1d | Ag2O | rt | 2 | 93 | 87 |
| 5 | 1d | Ag2CO3 | rt | 2 | 78 | 84 |
| 6 | 1d | AgF | rt | 2 | 80 | 45 |
| 7 | 1d | AgOAc | rt | 18 | 61 | 4 |
| 8 | 1d | AgOTf | rt | 24 | trace | - |
| 9 | 1d | Cu(OTf)2 | rt | 24 | trace | - |
| 10 | 1d | Ag2O | −20 | 6 | 90 | 92 |
| 11 d | 1d | Ag2O | −20 | 0.7 | 94 | 93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Q.; You, Y.; Song, X.; Wang, Y.; Chen, K.; Wang, H. Endo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via Ag(I)/CAAA-Amidphos-Catalyzed 1,3-Dipolar Cycloaddition between Azomethine Ylides and α-Methylene-γ-Butyrolactone. Catalysts 2020, 10, 28. https://doi.org/10.3390/catal10010028
Hou Q, You Y, Song X, Wang Y, Chen K, Wang H. Endo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via Ag(I)/CAAA-Amidphos-Catalyzed 1,3-Dipolar Cycloaddition between Azomethine Ylides and α-Methylene-γ-Butyrolactone. Catalysts. 2020; 10(1):28. https://doi.org/10.3390/catal10010028
Chicago/Turabian StyleHou, Qinglin, Yaoyao You, Xinluo Song, Yingxia Wang, Ke Chen, and Haifei Wang. 2020. "Endo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via Ag(I)/CAAA-Amidphos-Catalyzed 1,3-Dipolar Cycloaddition between Azomethine Ylides and α-Methylene-γ-Butyrolactone" Catalysts 10, no. 1: 28. https://doi.org/10.3390/catal10010028
APA StyleHou, Q., You, Y., Song, X., Wang, Y., Chen, K., & Wang, H. (2020). Endo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via Ag(I)/CAAA-Amidphos-Catalyzed 1,3-Dipolar Cycloaddition between Azomethine Ylides and α-Methylene-γ-Butyrolactone. Catalysts, 10(1), 28. https://doi.org/10.3390/catal10010028