Abstract
Construction of spirocyclic pyrrolidines bearing a spiro quaternary stereocenter is an enormous challenge in synthetic organic chemistry. In this report, we introduce a Ag(I)/CAAA-amidphos-catalyzed enantioselective 1,3-dipolar cycloaddition between azomethine ylides and α-methylene-γ-butyrolactone as an effective strategy for the construction of excellent endo-selective spiro-[butyrolactone-pyrrolidine] derivatives. Meanwhile, the catalytic system was also successfully applied in the three-component one-pot reaction of azomethine ylides formed in situ under the action of N,N′-diisopropylcarbodiimide serving as a dehydrator. Under the optimal conditions, endo-pyrrolidine derivatives bearing a spiro quaternary stereocenter were obtained with high to excellent yields (up to 95% yield) and enantioselectivities (up to 93% ee).
1. Introduction
Highly substituted pyrrolidine motifs widely exist in natural alkaloids and bioactive molecules [1,2]. In particular, spirocyclic pyrrolidine compounds bearing a spiro quaternary stereocenter at the 4-position are especially useful [3,4]. In this context, various synthetic methods have been utilized for the chiral synthesis of spirocyclic pyrrolidine skeletons in recent years. Among most approaches, the 1,3-dipolar cycloaddition between azomethine ylides and electron-deficient alkenes is one of the most effective tools for building such skeletons with high to excellent endo/exo-diastereo- and enantioselectivities [5,6,7,8]. Since the first asymmetric version of synthesis of spirooxindole-pyrrolidine derivatives was realized by Gong et al., via chiral BINOL-derived phosphoric acids, to catalyze the 1,3-dipolar cycloaddition of azomethine ylides formed in situ with N-Ac 2-oxoindolin-3-ylidenes [9]. A relatively broad range of dipolarophiles (such as 2-oxoindolin-3-ylidenes [10,11,12,13,14,15], cyclopropylidene acetates [16], 2-alkylidenecycloketones [17,18], α-alkylidene succinimides [19], and 5-alkylidene thia(oxa)zolidine-2,4-diones [20]) have been applied in the azomethine ylides-involved 1,3-dipolar cycloaddition to construct structurally and stereochemically rich spirocyclic pyrrolidine derivatives in the past decade. In contrast, little attention has been paid to α-methylene-γ-butyrolactone as a key electron-deficient alkene in the asymmetric 1,3-dipolar cycloaddition to construct the highly substituted spirocyclic [butyrolactone-pyrrolidine] skeletons with multiple contiguous stereocenters, although such skeletons with the lactone moiety have important biological activities. To the best of our knowledge, apart from a few of limited racemic examples [21,22,23], only an asymmetric version has been reported by Wang et al. on the construction of exo-selective spiro-[butyrolactone-pyrrolidine] via Cu(I)/DTBM-BIPHEP-catalyzed α-methylene-γ-butyrolactone-involved process so far [24]. It is thus necessary to realize the preparation of such medicinally useful and synthetically challenging molecules with different stereochemical selectivities.
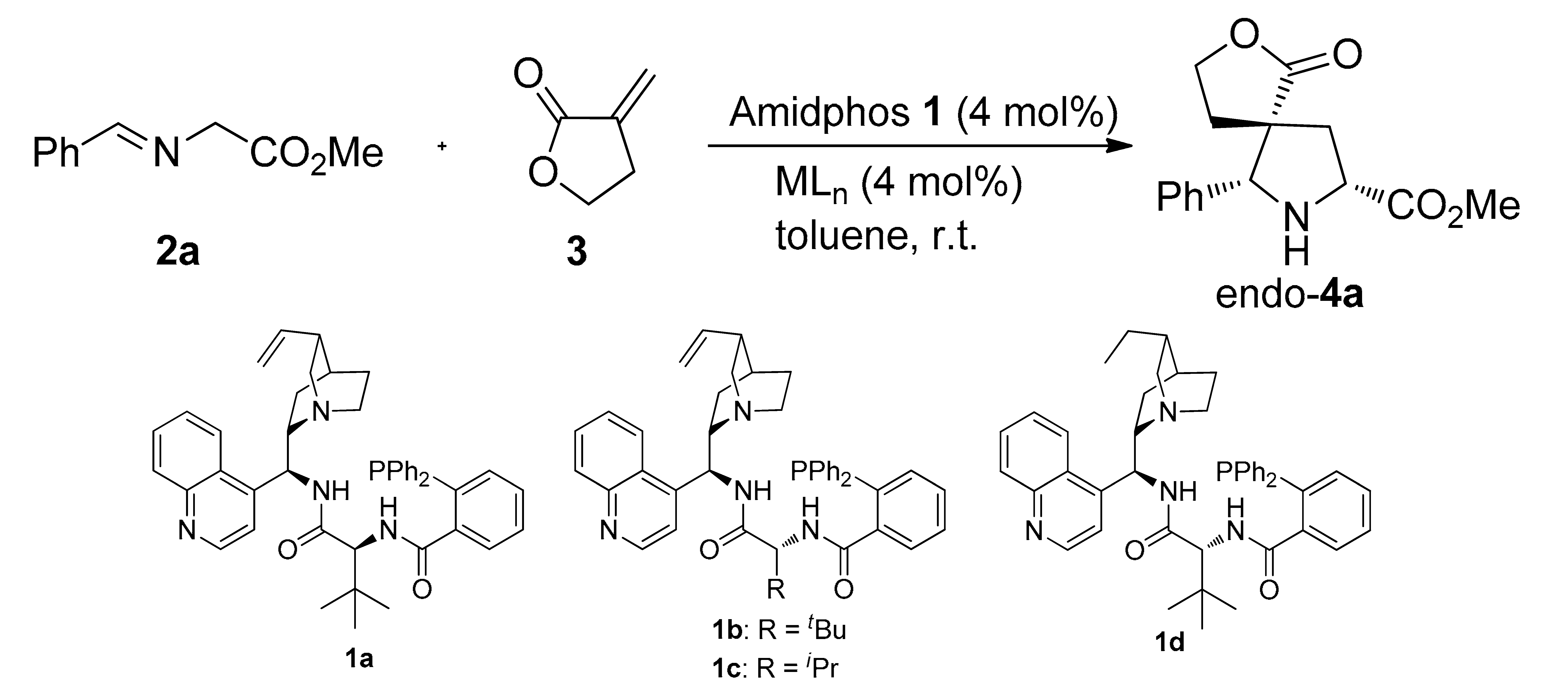
Recently, we have made a breakthrough by exploiting multifunctional Ag2CO3/amidphos catalytic system with different scaffolds of cinchona alkaloids, chiral 1,2-diphenylethylenediamines and α-amino acids to cooperatively catalyze the 1,3-dipolar cycloadditions between α-imino esters and diethyl maleate affording excellent endo-selective adducts with high enantioselectivities (Scheme 1, Equation (1)) [25,26,27,28]. (See Supplemental Materials). Encouraged by these findings, we envisaged that such a catalytic system may also be applied in the α-methylene-γ-butyrolactone-involved asymmetric 1,3-dipolar cycloaddition. Herein, we firstly described the Ag(I)/CAAA-amidphos-catalyzed 1,3-dipolar cycloaddition between α-imino esters and α-methylene-γ-butyrolactone as well as three-component one-pot reaction of α-imino esters formed in situ, affording excellent endo-selective spirocyclic [butyrolactone-pyrrolidine] with high levels of enantioselectivities (Scheme 1, Equation (2)).
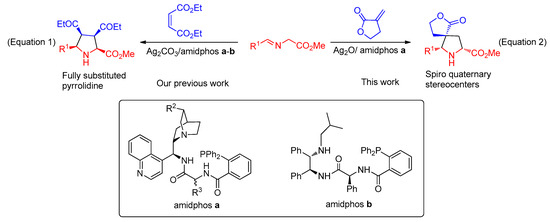
Scheme 1.
Azomethine ylides-involved the asymmetric 1,3-dipolar cycloadditions.
2. Results and Discussion
2.1. Optimization of the 1,3-Dipole Cycloaddition Reaction Conditions
We first investigated the 1,3-dipolar cycloaddition of α-imino ester 2a and α-methylene-γ-butyrolactone 3 catalyzed cooperatively by silver(I) oxide and different CAAA-Amidphos 1a−d (Table 1, entries 1−4). Through the screening of amidophosphanes, we found that the combination of Ag2O and the amidphos 1d gave relatively satisfactory reactivity and enantioselectivity (Table 1, entry 4). In order to achieve higher enantioselectivities in the process, different metal salts, such as Ag2CO3, AgF, AgOAc, AgOTf, and Cu(OTf)2, were screening for the process (entries 5−9). Disappointingly, the yields and enantioselectivities of endo-4a adduct have not been greatly improved when compared with the catalytic system Ag2O/1d. Next, when the reaction temperature was reduced to −20 °C, the enantioselectivity was increased to 92% ee, albeit with a slightly decreased yield and a prolonged reaction time (6 h, 90% yield, entry 10). Gratifyingly, when 20 mol% H2O was added, the reaction rate was accelerated to 0.7 h with a slightly increased enantioselectivity (93% ee, entry 11). We speculated the water (H2O) reacted with silver(I) oxide (Ag2O) to produce the strong base silver(I) hydroxide (AgOH), which could promote deprotonation of the α-imino esters to generate the azomethine ylide and accelerate the reaction process. Therefore, the optimal conditions for the asymmetric 1,3-dipolar cycloaddition of α-imino ester 2 and α-methylene-γ-butyrolactone 3 were established as 2 mol% Ag2O/4 mol% 1d/20 mol% H2O in toluene at −20 °C.

Table 1.
Optimization of the reaction conditions between α-imino ester 2a and α-methylene-γ-butyrolactone 3 a.
2.2. Study on Substrate Adaptability
Under the optimal reaction conditions, the adaptability of substrates was explored. As shown in Scheme 2, α-imino esters 2a−n from aromatic aldehydes with different electronic properties and steric hindrance reacted with α-methylene-γ-butyrolactone 3 to provide the exclusively endo-selective spirocyclic pyrrolidines 4a−n with high to excellent yields (85%−94%) and enantioselectivities (81%−93% ee) in the presence of the catalytic Ag2O/1d. Notably, the iminoesters derived from 2-substituted aromatic aldehydes afforded relatively high enantioselectivities except for the iminoester 2h. When R1 was the heteroaromatic group, the endo-4o was also successfully obtained with 90% yield and 89% enantioselectivity in 2 h. Delightedly, the primary alkyl iminoester 2p derived from 3-methylbutanal was also tolerated, affording the desired endo-4p adduct with moderate yield and enantioselectivity, albeit for requiring prolonged reaction time (4 h, 78% yield, 75% ee).
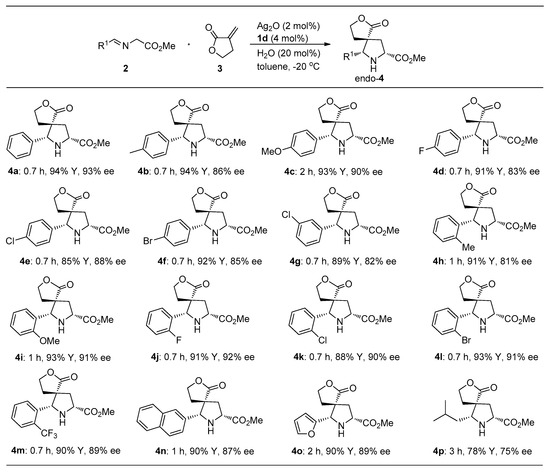
Scheme 2.
Ag2O/1d-catalyzed cycloaddition of various α-imino esters 2a–p with 3 a. a Reaction conditions: Imino ester 2 (0.30 mmol), 3 (0.20 mmol), Ag2O (2 mol%), precat. 1d (4 mol%), isolated yield, and ee value determined by HPLC.
2.3. Study on the Construction of Spiro Pyrrolidines by the Three-Component One-Pot Method
We next transferred our attention to evaluate the three-component one-pot 1,3-dipolar cycloaddition of the in situ generated α-iminoesters from tert-butyl glycinate and different aromatic aldehydes under the action of N,N’-Diisopropylcarbodiimide (DIC) serving as a dehydrator with α-methylene-γ-butyrolactone 3 [29,30]. As shown in Scheme 3, arene-substituents with different electronic properties and steric hindrance were well tolerated and the desired endo-6a−6f were obtained in one-pot process with excellent yields (92%–97%) and moderate to high enantioselectivities (66%–90% ee). Notably, when Ar was 2-furyl group, the corresponding sipro endo-6g was also obtained with 89% yield and 87% enantioselectivity.
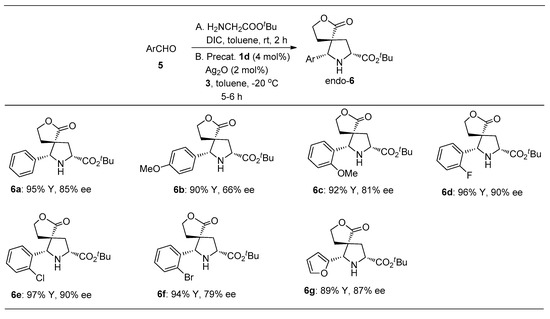
Scheme 3.
Ag2O/1d-catalyzed three-component reaction of α-imino esters generated in situ a. a Reaction conditions: Aldehyde 5 (0.30 mmol), tert-butyl glycinate (0.30 mmol), N,N’-Diisopropylcarbodiimide (DIC) (0.30 mmol), 3 (0.20 mmol), Ag2O (2 mol%), precat. 1d (4 mol%), isolated yield, ee value determined by HPLC.
3. Materials and Methods
3.1. Materials
Unless noted otherwise, 1H and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer (Bruker Biospin, Fällanden, Switzerland) in CDCl3. CDCl3 served as the internal standard (δ = 7.26) for 1H NMR and (δ = 77.0) for 13C NMR. Diastereomeric ratios were determined from crude 1H NMR. Chiral HPLC was performed on a Agilent 1260 apparatus (Agilent Technologies, California, US) equipped with a spectrophotometric detector (monitoring at 205–230 nm) with Daicel chiral AS-H and AD-H columns (Daicel Chemical Industries Co., Ltd., Tokyo, Japan). All solvents are used after reevaporation. The α-methylene-γ-butyrolactone were purchased from Adamas-beta® Co., Ltd. (Shanghai, China). Other commercially available reagents were not further purified. All reactions were monitored by thin-layer chromatography (TLC) plates (Qingdao Marine Chemistry Company, Qingdao, China). Flash column chromatography was completed by using silica gel 200–300 (particle size 0.0040–0.0750 mm) (Qingdao Marine Chemistry Company, Qingdao, China). The absolute configuration of endo-6e was determined by X-ray diffraction analysis (Bruker AXS, Karlsruhe, Germany), and those of other products were deduced on the basis of these results.
3.2. General Synthesis Methods of 4a–4p
To a solution of precat. 1d (5.57 mg, 0.008 mmol) in toluene (1.4 mL) was added Ag2O (0.92 mg, 0.004 mmol), followed by water dropwise (0.72 mg, 0.04 mmol) at room temperature under N2 atmosphere. After the reaction mixture was stirred for 1 h, α-imino ester 2 (0.30 mmol) and α-methylene-γ-butyrolactone 3 (19.6 mg, 0.20 mmol) were successively added at −20 °C. The reaction was monitored by TLC plate, and the residue was purified by column chromatography on silica gel (gradient, ethyl acetate:petroleum ether, 20:80 to 30:70) to afford the spiro endo-4 adducts.
3.3. General Synthesis Methods of 6a–6g via Three-Component “One-Pot”
To a solution of tert-butyl glycine (39.3 mg, 0.30 mmol) in toluene (1.0 mL) was added the aldehyde 5 (0.30 mmol) and N,N′-diisopropylcarbodiimide (37.8 mg, 0.30 mmol), which was stirred for 2 h at room temperature. Then, a solution of precat. 1d (5.57 mg, 0.008 mmol) and Ag2O (0.92 mg, 0.004 mmol) in toluene (0.5 mL) stirred for 1 h, was mixed with the above solution, followed by α-methylene-γ-butyrolactone 3 dropwise (19.6 mg, 0.20 mmol) at −20 °C. The reaction was monitored by TLC plate, and the residue was purified by column chromatography on silica gel (gradient, ethyl acetate:petroleum ether, 20:80 to 30:70) to afford the spiro endo-6 adducts.
4. Conclusions
In conclusion, we have developed the Ag(I)/CAAA-amidphos 1d-catalyzed 1,3-dipolar cycloaddition between azomethine ylides and α-methylene-γ-butyrolactone, achieving excellent endo-selective Spiro-[butyrolactone-pyrrolidine] derivatives in moderate to excellent yields (75%–94%) and enantioselectivities (78%–93%) under mild conditions. Furthermore, the three-component one-pot reaction of α-imino esters formed in situ, and α-methylene-γ-butyrolactone was also successfully realized by the catalytic system under the action of DIC. Further investigations on mechanistic aspects and other applications are in progress.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/1/28/s1, experimental procedures and detailed characterization data of all new compounds.
Author Contributions
H.W. and Q.H. designed the experiments of the project; Y.Y., X.S., Y.W. and K.C. performed the experiments; Y.Y. drafted this manuscript; H.W. proofread the manuscript and supervised the studies. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Nature Science Foundation of China (Nos. 21202042, 51374103, 51674114) and the Hunan Provincial Natural Science Foundation of China (Nos. 2017JJ2067), and the Zhuzhou Municipal Science and Technology Program and Undergraduate Innovation Fund of Hunan Province.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Michael, J.P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2008, 25, 139–165. [Google Scholar] [CrossRef]
- Vitaku, E.D.; Smith, T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among USA. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Marti, C.; Carreira, E.M. Construction of Spiro [pyrrolidine-3, 3′-oxindoles]-Recent Applications to the Synthesis of Oxindole Alkaloids. Eur. J. Org. Chem. 2003, 12, 2209–2219. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef]
- Adrio, J.; Carretero, J.C. Recent Advances in the Catalytic Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides. Chem. Commun. 2014, 50, 12434–12446. [Google Scholar] [CrossRef] [PubMed]
- Bdiri, B.; Zhao, B.-J.; Zhou, Z.-M. Recent Advances in the Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Ylides and Dipolarophiles. Tetrahedron Asymmetry 2017, 28, 876–899. [Google Scholar] [CrossRef]
- Döndas, H.A.; de Gracia-Retamosa, M.; Sansano, J.M. Current Trends towards the Synthesis of Bioactive Heterocycles and Natural Products Using 1,3-Dipolar Cycloadditions (1,3-DC) with Azomethine Ylides. Synthesis 2017, 49, 2819–2851. [Google Scholar] [CrossRef]
- Chen, X.-H.; Wei, Q.; Luo, S.-W.; Xiao, H.; Gong, L.-Z. Organocatalytic Synthesis of Spiro [pyrrolidin-3,3′-oxindoles] with High Enantiopurity and Structural Diversity. J. Am. Chem. Soc. 2009, 131, 13819–13825. [Google Scholar] [CrossRef] [PubMed]
- Antonchick, A.P.; Gerding-Reimers, C.; Catarinella, M.; Schürmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Highly Enantioselective Synthesis and Cellular Evaluation of Spirooxindoles Inspired by Natural Products. Nat. Chem. 2010, 2, 735–740. [Google Scholar] [CrossRef]
- Awata, A.; Arai, T. Catalytic Asymmetric Exo′-Selective [3 + 2] Cycloaddition for Constructing Stereochemically Diversified Spiro [pyrrolidin-3,3′-oxindole] s. Chem. Eur. J. 2012, 18, 8278–8282. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-L.; He, Z.-L.; Tao, H.-Y.; Wang, C.-J. Stereoselective Construction of Spiro (butyrolactone pyrrolidines) by Highly Efficient Copper (I)/TF-BiphamPhos-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition. Chem. Eur. J. 2012, 18, 8042–8046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.-M.; Dong, W.-P.; Zhu, L.-P.; Wang, R. Efficient Construction of Highly Functionalized Spiro[γ-butyrolactone-pyrrolidin-3,3′-oxindole] Tricyclic Skeletons via an Organocatalytic 1,3-Dipolar Cycloaddition. Chem. Commun. 2013, 49, 3458–3460. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Ogawa, H.; Awata, A.; Sato, M.; Watabe, M.; Yamanaka, M. PyBidine-Cu (OTf) 2-Catalyzed Asymmetric [3 + 2] Cycloaddition with Imino Esters: Harmony of Cu-Lewis Acid and Imidazolidine-NH Hydrogen Bonding in Concerto Catalysis. Angew. Chem. Int. Ed. 2015, 54, 1595–1599. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Wang, H.-Y.; Jin, Q.-W.; Zheng, C.-W.; Zhao, G.; Shang, Y.-J. Thiourea-quaternary Ammonium Salt Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Imino Esters to Construct Spiro [pyrrolidin-3,3-oxindoles]. Org. Lett. 2016, 18, 4774–4777. [Google Scholar] [CrossRef]
- Liu, T.-L.; He, Z.-L.; Tao, H.-Y.; Cai, Y.-P.; Wang, C.-J. Stereoselective Construction of a 5-aza-Spiro [2, 4] Heptane Motif Viacatalytic Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides and Ethyl Cyclopropylidene Acetate. Chem. Commun. 2011, 47, 2616–2618. [Google Scholar] [CrossRef]
- Liu, T.-L.; He, Z.-L.; Wang, C.-J. Highly Efficient Construction of Spirocyclic Chromanone—Pyrrolidines via Cu (I)/TF-BiphamPhos-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition. Chem. Commun. 2011, 47, 9600–9602. [Google Scholar] [CrossRef]
- Liu, T.-L.; He, Z.-L.; Li, Q.-H.; Tao, H.-Y.; Wang, C.-J. Catalytic Asymmetric Construction of Spirocycles Containing Pyrrolidine Motifs and Spiro Quaternary Stereogenic Centers via 1,3-Dipolar Cycloaddition of Azomethine Ylides with 2-Alkylidene-Cycloketones. Adv. Synth. Catal. 2011, 353, 1713–1719. [Google Scholar] [CrossRef]
- Yang, W.-L.; Liu, Y.-Z.; Luo, S.; Yu, X.; Fossey, J.-S.; Deng, W.-P. The Copper-Catalyzed Asymmetric Construction of a Dispiropyrrolidine Skeleton via 1,3-Dipolar Cycloaddition of Azomethine Ylides to a-Alkylidene Succinimides. Chem. Commun. 2015, 51, 9212–9215. [Google Scholar] [CrossRef]
- Yang, W.-L.; Tang, F.-F.; He, F.-S.; Li, C.-Y.; Yu, X.; Deng, W.-P. Asymmetric Construction of Spirocyclic Pyrrolidine-Thia (oxa) Zolidinediones via N,O-Ligand/Cu (I) Catalyzed 1,3-Dipolar Cycloaddition of Azomethine Ylides with 5-Alkylidene Thia (oxa) Zolidine-2, 4-Diones. Org. Lett. 2015, 17, 4822–4825. [Google Scholar] [CrossRef]
- Castulik, J.; Marek, J.; Mazal, C. Synthesis of Spiropyrrolidines and Spiropyrrolizidines by 1,3-Dipolar Cycloadditions of Azomethine Ylides to Substituted α-Methylene-γ-Lactones. Tetrahedron 2001, 57, 8339–8347. [Google Scholar] [CrossRef]
- Jenkins, S.M.; Wadsworth, H.J.; Bromidge, S. Substituent Variation in Azabicyclic Triazole and Tetrazole-based Muscarinic Receptor Ligands. J. Med. Chem. 1992, 35, 2392–2406. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.D.; Peters, D.A.; Allway, P.A.; Beddoes, R.L. 1,3-Dipolar Cycloadditions to Oxidopyraziniums. Heterocycles 1995, 40, 331–347. [Google Scholar]
- Li, Q.-H.; Liu, T.-L.; Wei, L.; Zhou, X.; Tao, H.-Y.; Wang, C.-J. exo-Selective Construction of Spiro-[butyrolactone-pyrrolidine] via 1,3-Dipolar Cycloaddition of Azomethine Ylides with α-Methylene-γ-Butyrolactone Catalyzed by Cu (I)/DTBM-BIPHEP. Chem. Commun. 2013, 49, 9642–9644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, Q.; Zhou, Z.; Hu, S.; Liu, Z.; Zhou, L.-Y. Ag2CO3/CA-AA-AmidPhos Multifunctional Catalysis in the Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Ylides. Org. Lett. 2016, 18, 404–407. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, X.; Liu, J.; Li, J.; Wen, P.; Wang, H. Tert-Leucine-Derived AmidPhos-Silver (I) Chiral Complexes for the Asymmetric [3+2] Cycloaddition of Azomethine Ylides. Synlett 2017, 28, 999–1003. [Google Scholar]
- Hou, Y.; Zhou, Z.; Liu, P.; Wang, J.; Hou, Q.; Wen, P.; Wang, H. A Class of α-Amino Acids-derived Multifunctional Amidophosphane Precatalysts: Application to the Highly Enantio and Diastereoselective Silver (I)-Catalyzed 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Asymmetry 2017, 28, 930–938. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, Q.; Hou, Q.; Zhang, K.; Wen, P.; Hu, S.; Wang, H. A Chiral Secondary Amine-amidophosphane Precatalyst for Silver-catalyzed Asymmetric 1,3-Dipolar Cycloaddition Reactions. Synthesis 2018, 50, 2347–2358. [Google Scholar]
- Mancebo-Aracil, J.; Nájera, C.; Sansano, J.M. Multicomponent Synthesis of Unnatural Pyrrolizidines Using 1,3-Dipolar Cycloaddition of Proline Esters. Chem. Commun. 2013, 49, 11218–11220. [Google Scholar] [CrossRef]
- Mancebo-Aracil, J.; Nájera, C.; Castelló, L.M.; Sansano, J.M.; Larrañaga, O.; de Cózar, A.; Cossío, F.P. Regio and Diastereoselective Multicomponent 1,3-Dipolar Cycloadditions between Prolinate Hydrochlorides, Aldehydes and Dipolarophiles for the Direct Synthesis of Pyrrolizidines. Tetrahedron 2015, 71, 9645–9661. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).