Photocatalysis as a Tool for in Vitro Drug Metabolism Simulation: Multivariate Comparison of Twelve Metal Oxides on a Set of Twenty Model Drugs
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Characterization of Metabolism Pathways
2.2. Chemometric Analysis
2.2.1. PCA
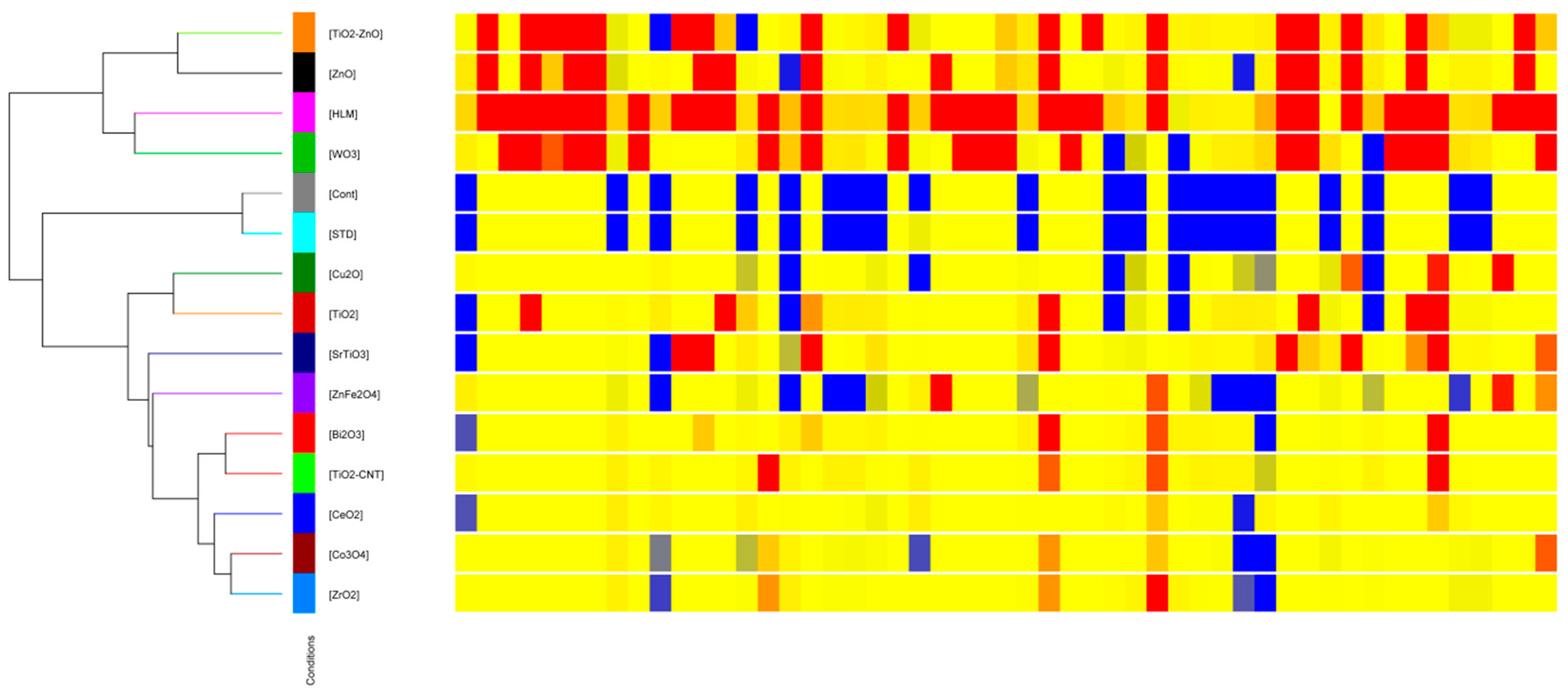
2.2.2. Hierarchical Cluster Analysis and Heatmap
3. Experimental
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Photocatalytic Simulation of Metabolism
3.4. HLM Metabolism Simulation
3.5. LC-MS Analysis
3.6. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mills, A.; Le Hunt, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl. Catal. B Environ. 2003, 42, 319–335. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; da Hora Machado, A.E.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Cáceres, J.; Fernández-Alba, A.R.; Agüera, A.; Rodrıguez, A. Photocatalytic treatment of water-soluble pesticides by photo-Fenton and TiO2 using solar energy. Catal. Today 2002, 76, 209–220. [Google Scholar] [CrossRef]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Kayano, S.; Yoshihiko, K.; Kazuhito, H.; Akira, F. Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Ohko, Y.; Utsumi, Y.; Niwa, C.; Tatsuma, T.; Kobayakawa, K.; Satoh, Y.; Kubota, Y.; Fujishima, A. Self-sterilizing and self-cleaning of silicone catheters coated with TiO2 photocatalyst thin films: A preclinical work. J. Biomed. Mater. Res. 2001, 58, 97–101. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic Activity, Antibacterial Effect, and Photoinduced Hydrophilicity of TiO2 Films Coated on a Stainless Steel Substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Lawler, D.F. Water Quality Engineering: Physical/Chemical Treatment Processes; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Pelaez, M.; de la Cruz, A.A.; Stathatos, E.; Falaras, P.; Dionysiou, D.D. Visible light-activated N-F-codoped TiO2 nanoparticles for the photocatalytic degradation of microcystin-LR in water. Catal. Today 2009, 144, 19–25. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2 New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Sorathiya, K.; Mishra, B.; Kalarikkal, A.; Reddy, K.P.; Gopinath, C.S.; Khushalani, D. Enhancement in Rate of Photocatalysis Upon Catalyst Recycling. Sci. Rep. 2016, 6, 35075. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Navío, J.A.; Melián, E.P.; Díaz, O.G.; Rodríguez, J.M.D. Highly photoactive ZnO by amine capping-assisted hydrothermal treatment. Appl. Catal. B Environ. 2008, 83, 30–38. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.-S. Effect of Electrolytes on the Selectivity and Stability of n-type WO3 Photoelectrodes for Use in Solar Water Oxidation. J. Phys. Chem. C 2012, 116, 7612–7620. [Google Scholar] [CrossRef]
- Hameed, A.; Gondal, M.A.; Yamani, Z.H. Effect of transition metal doping on photocatalytic activity of WO3 for water splitting under laser illumination: Role of 3d-orbitals. Catal. Commun. 2004, 11, 715–719. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; AGadri, A. Photocatalytic degradation of methylene blue dye by iron oxide (α-Fe2O3) nanoparticles under visible irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 8142–8152. [Google Scholar] [CrossRef]
- Calza, P.; Pelizzetti, E.; Brussino, M.; Baiocchi, C. Ion trap tandem mass spectrometry study of dexamethasone transformation products on light activated TiO2 surface. J. Am. Soc. Mass Spectrom. 2001, 12, 1286–1295. [Google Scholar] [CrossRef]
- Calza, P.; Pazzi, M.; Medana, C.; Baiocchi, C.; Pelizzetti, E. The photocatalytic process as a tool to identify metabolitic products formed from dopant substances: The case of buspirone. J. Pharm. Biomed. Anal. 2004, 35, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Calza, P.; Giancotti, V.; Bello, F.D.; Pasello, E.; Montana, M.; Baiocchi, C. Horse metabolism and the photocatalytic process as a tool to identify metabolic products formed from dopant substances: The case of sildenafil. Drug Test. Anal. 2011, 3, 724–734. [Google Scholar] [CrossRef]
- Gawlik, M.; Trawiński, J.; Skibiński, R. Imitation of phase I metabolism reactions of MAO-A inhibitors by titanium dioxide photocatalysis. Eur. J. Pharm. Sci. 2018, 114, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ruokolainen, M.; Valkonen, M.; Sikanen, T.; Kotiaho, T.; Kostiainen, R. Imitation of phase I oxidative metabolism of anabolic steroids by titanium dioxide photocatalysis. Eur. J. Pharm. Sci. 2014, 65, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Nissilä, T.; Sainiemi, L.; Karikko, M.-M.; Kemell, M.; Ritala, M.; Franssila, S.; Kostiainen, R.; Ketola, R.A. Integrated photocatalytic micropillar nanoreactor electrospray ionization chip for mimicking phase I metabolic reactions. Lab. Chip. 2011, 11, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Raoof, H.; Mielczarek, P.; Michalow, K.A.; Rekas, M.; Silberring, J. Synthesis of metabolites of paracetamol and cocaine via photooxidation on TiO2 catalyzed by UV light. J. Photochem. Photobiol. B 2013, 118, 49–57. [Google Scholar] [CrossRef]
- Medana, C.; Calza, P.; Giancotti, V.; Bello, F.D.; Aragno, M.; Baiocchi, C. Study of the photocatalytic transformation of synephrine: A biogenic amine relevant in anti-doping analysis. Anal. Bioanal. Chem. 2013, 405, 1105–1113. [Google Scholar] [CrossRef]
- Ruokolainen, M.; Gul, T.; Permentier, H.; Sikanen, T.; Kostiainen, R.; Kotiaho, T. Comparison of TiO2 photocatalysis, electrochemically assisted Fenton reaction and direct electrochemistry for simulation of phase I metabolism reactions of drugs. Eur. J. Pharm. Sci. 2016, 83, 36–44. [Google Scholar] [CrossRef]
- Baillie, T.A.; Rettie, A.E. Role of Biotransformation in Drug-Induced Toxicity: Influence of Intra- and Inter-Species Differences in Drug Metabolism. Drug Metab. Pharm. 2011, 26, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Williams, D.P.; Naisbitt, D.J.; Kitteringham, N.R. MPirmohamed, Investigation of toxic metabolites during drug development. Toxicol. Appl. Pharmacol. 2005, 207, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Baillie, T.A. Metabolism and Toxicity of Drugs. Two Decades of Progress in Industrial Drug Metabolism. Chem. Res. Toxicol. 2008, 21, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The cost of drug development: A systematic review. Health Policy 2011, 100, 4–17. [Google Scholar] [CrossRef]
- Gawlik, M.; Skibiński, R. Identification of new metabolites of vardenafil with the use of HLM and photochemical methods by LC-ESI-HRMS combined with multivariate chemometric analysis. Int. J. Mass Spectrom. 2018, 433, 55–60. [Google Scholar] [CrossRef]
- Gawlik, M.; Skibiński, R. Simulation of phase I metabolism reactions of clozapine by HLM and photocatalytic methods with the use of UHPLC-ESI-MS/MS. Biomed. Chromatogr. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.-F.; Guan, X.; Zhao, M.; Wang, J.; Li, F. Characterizing novel metabolic pathways of melatonin receptor agonist agomelatine using metabolomic approaches. Biochem. Pharmacol. 2016, 109, 70–82. [Google Scholar] [CrossRef]
- Bushee, J.L.; Dunne, C.E.; Argikar, U.A. An in vitro approach to investigate ocular metabolism of a topical, selective β1-adrenergic blocking agent, Betaxolol. Xenobiotica 2015, 45, 396–405. [Google Scholar] [CrossRef]
- Christensen, H. Chlorprothixene and its metabolites in blood, liver and urine from fatal poisoning. Acta Pharmacol. Toxicol. (Copenh.) 1974, 34, 16–26. [Google Scholar] [CrossRef]
- Yamahata, T.; Minaki, Y.; Okada, S.; Kohno, R.; Nishikawa, H.; Esumi, Y.; Jin, Y.; Okamura, Y.; Ishizaki, M.; Gunji, S.; et al. Metabolic Fate of Clonidine (V): Metabolism of Clonidine after Subcutaneous Administration of Clonidine to Rats, Dogs and Monkeys and Dermal Application of Clonidine Tape, M-5041T, to Rats. Drug Metab. Pharmacokinet. 1996, 11, 411–420. [Google Scholar] [CrossRef][Green Version]
- Lavrijsen, K.; van Houdt, J.; van Dyck, D.; Hendrickx, J.; Bockx, M.; Hurkmans, R.; Meuldermans, W.; le Jeune, L.; Lauwers, W.; Heykants, J. Comparative metabolism of flunarizine in rats, dogs and man: An in vitro study with subcellular liver fractions and isolated hepatocytes. Xenobiotica Fate Foreign Compd. Biol. Syst. 1992, 22, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Wo, S.K.; Zuo, Z. Investigation of the disposition of loxapine, amoxapine and their hydroxylated metabolites in different brain regions, CSF and plasma of rat by LC-MS/MS. J. Pharm. Biomed. Anal. 2012, 58, 83–93. [Google Scholar] [CrossRef]
- Hendrickx, J.; Bockx, M.; Zwijsen, C.; Borgmans, C.; Mannens, G.; Meuldermans, W.; Heykants, J. Location of the hydroxyl functions in hydroxylated metabolites of nebivolol in different animal species and human subjects as determined by on-line high-performance liquid chromatography-diode-array detection. J. Chromatogr. A 1996, 729, 341–354. [Google Scholar] [CrossRef]
- Davies, B.J.; Herbert, M.K.; Coller, J.K.; Somogyi, A.A.; Milne, R.W.; Sallustio, B.C. Determination of the 4-monohydroxy metabolites of perhexiline in human plasma, urine and liver microsomes by liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 843, 302–309. [Google Scholar] [CrossRef]
- Fisher, D.S.; Handley, S.A.; Taylor, D.; Flanagan, R.J. Measurement of quetiapine and four quetiapine metabolites in human plasma by LC-MS/MS. Biomed. Chromatogr. BMC 2012, 26, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Fleishaker, J.C. Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clin. Pharm. 2000, 39, 413–427. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Y.-M.; Yao, T.; Shi, H.-L.; Li, J.; Zhao, Z.; Qin, Y.-C. Identification of Major Metabolites of Salbutamol in Swine Urine and Plasma Using Ultra-High Performance Liquid Chromatography-Electrospray Time of Flight Mass Spectrometry. Chin. J. Anal. Chem. 2014, 42, 1692–1696. [Google Scholar] [CrossRef]
- Yamato, C.; Takahashi, T.; Fujita, T. Studies on metabolism of trazodone. III Species differences. Xenobiotica Fate Foreign Compd. Biol. Syst. 1976, 6, 295–306. [Google Scholar] [CrossRef]
- von Moltke, L.L.; Greenblatt, D.J.; Granda, B.W.; Duan, S.X.; Grassi, J.M.; Venkatakrishnan, K.; Harmatz, J.S.; Shader, R.I. Zolpidem metabolism in vitro: Responsible cytochromes, chemical inhibitors, and in vivo correlations. Br. J. Clin. Pharmacol. 1999, 48, 89–97. [Google Scholar] [CrossRef]
- Becquemont, L.; Mouajjah, S.; Escaffre, O.; Beaune, P.; Funck-Brentano, C.; Jaillon, P. Cytochrome P-450 3A4 and 2C8 Are Involved in Zopiclone Metabolism. Drug Metab. Dispos. 1999, 27, 1068–1073. [Google Scholar] [PubMed]
- Frey, H.H.; Scherkl, R. Clorazepate, correlation between metabolism and anticonvulsant activity. Eur. J. Pharmacol. 1988, 158, 213–216. [Google Scholar] [CrossRef]

| Name Structure | m/z [M+H]+ | Metabolism Reaction | HLM | Bi2O3 | CeO2 | Co3O4 | Cu2O | SrTiO3 | TiO2 | TiO2-CNTs | TiO2-ZnO | WO3 | ZnFe2O4 | ZnO | ZrO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Chlorprothixene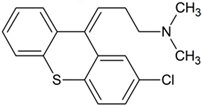 | 302.0764 | demethylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 332.0870 | S-oxidation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 318.0714 | demethylation S-oxidation | + | - | - | - | - | - | + | - | + | + | - | - | - | |
| 348.0819 | S-oxidation N-oxidation | + | - | - | - | - | - | - | - | - | - | - | - | - | |
Reboxetine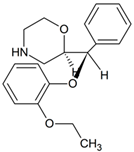 | 286.1437 | deethylation | + | - | + | - | - | + | + | + | + | + | - | + | + |
| 330.1699 | hydroxylation | + | - | - | - | - | - | - | - | - | + | - | - | - | |
| 328.1543 | oxidation | + | - | - | - | - | - | + | - | + | + | - | - | - | |
| 344.1492 | hydroxylation oxidation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Trazodone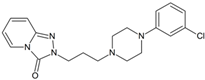 | 192.0767 | dealkylation oxidation | - | + | + | - | + | + | + | + | + | + | - | + | + |
| 388.1534 | hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 406.1640 | dihydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Quetiapine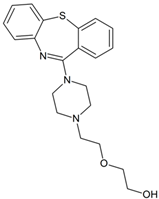 | 340.1478 | dealkylation | + | + | + | - | + | - | + | + | + | + | + | + | + |
| 296.1215 | dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 400.1689 | hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Loxapine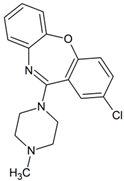 | 344.1160 | hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 314.1054 | demethylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Agomelatine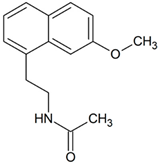 | 260.1287 | hydroxylation | + | - | - | + | - | - | + | + | + | - | - | + | - |
| 230.1181 | demethylation | + | - | - | - | - | - | + | - | - | - | + | + | - | |
| 246.1130 | demethylation hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 276.1236 | dihydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 262.1079 | demethylation dihydroxylation | + | - | - | - | - | - | + | - | + | - | - | + | - | |
Salbutamol | 254.1386 | N-oxidation | + | + | - | - | + | + | + | + | + | + | - | - | + |
Orciprenaline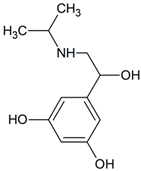 | 228.1230 | hydroxylation | + | - | - | - | - | - | - | - | - | - | - | - | - |
Nebivolol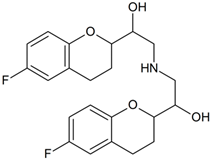 | 420.1671 | oxidation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 422.1773 | hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 212.1081 | N-dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Toloxatone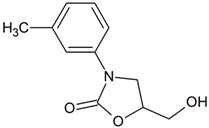 | 224.0917 | aliphatic hydroxylation | + | - | - | - | - | - | - | - | - | - | - | - | - |
| 224.0917 | aromatic hydroxylation | + | - | - | - | - | - | - | - | - | - | - | - | - | |
| 224.0917 | N-oxidation | + | - | - | - | - | - | - | - | - | - | - | - | - | |
Vardenafil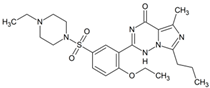 | 461.1966 | N-dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 393.1227 | dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 477.1915 | N-dealkylation aliphatic hydroxylation | + | - | - | - | - | - | - | - | - | - | - | - | - | |
Dapoxetine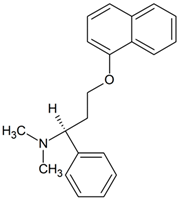 | 322.1802 | N-oxidation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 322.1802 | aromatic hydroxylation | + | - | - | - | - | - | - | - | - | + | - | - | - | |
| 292.1696 | N-dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 308.1645 | oxidation | + | - | - | - | - | - | - | - | - | - | - | - | - | |
| 278.1539 | N,N-dealkylation | + | + | + | + | + | + | + | + | + | + | - | + | + | |
Tramadol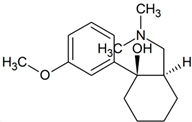 | 250.1801 | N-demethylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 250.1801 | O-demethylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Zopiclone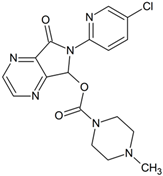 | 375.0966 | demethylation | + | + | + | + | + | + | - | + | + | + | + | - | + |
| 405.1072 | N-oxidation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Zolpidem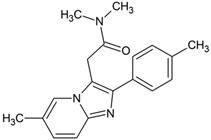 | 324.1706 | aliphatic hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
Betaxolol | 254.1750 | O-dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 266.1750 | N-dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Clorazepate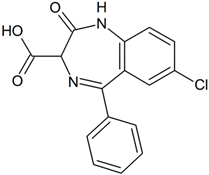 | 271.0632 | decarboxylation | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 287.0581 | decarboxylation hydroxylation | + | - | + | - | + | - | + | + | + | + | - | + | - | |
Clonidine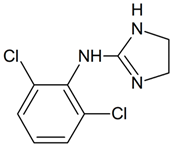 | 228.0089 | dehydrogenation | + | + | + | + | - | + | + | + | + | + | + | + | + |
Perhexiline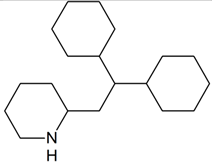 | 294.2791 | aliphatic hydroxylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 292.2634 | oxidation | + | + | + | + | + | + | + | + | + | + | - | + | + | |
Flunarizine | 421.2085 | aromatic hydroxylation | + | - | - | - | - | - | - | - | - | - | - | - | - |
| 439.2191 | dihydroxylation | + | - | - | - | - | - | - | - | - | - | - | - | - | |
| 289.1510 | dealkylation | + | + | + | + | + | + | + | + | + | + | + | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlik, M.; Trawiński, J.; Skibiński, R. Photocatalysis as a Tool for in Vitro Drug Metabolism Simulation: Multivariate Comparison of Twelve Metal Oxides on a Set of Twenty Model Drugs. Catalysts 2020, 10, 26. https://doi.org/10.3390/catal10010026
Gawlik M, Trawiński J, Skibiński R. Photocatalysis as a Tool for in Vitro Drug Metabolism Simulation: Multivariate Comparison of Twelve Metal Oxides on a Set of Twenty Model Drugs. Catalysts. 2020; 10(1):26. https://doi.org/10.3390/catal10010026
Chicago/Turabian StyleGawlik, Maciej, Jakub Trawiński, and Robert Skibiński. 2020. "Photocatalysis as a Tool for in Vitro Drug Metabolism Simulation: Multivariate Comparison of Twelve Metal Oxides on a Set of Twenty Model Drugs" Catalysts 10, no. 1: 26. https://doi.org/10.3390/catal10010026
APA StyleGawlik, M., Trawiński, J., & Skibiński, R. (2020). Photocatalysis as a Tool for in Vitro Drug Metabolism Simulation: Multivariate Comparison of Twelve Metal Oxides on a Set of Twenty Model Drugs. Catalysts, 10(1), 26. https://doi.org/10.3390/catal10010026







