Abstract
The acyl Sonogashira reaction represents an extension of Sonogashira cross-coupling to acid chlorides which replace aryl or vinyl halides, while terminal acetylenes are used as coupling partners in both reactions. The introduction of a carbonyl functional group on the alkyne backbone determines a radical change in the reactivity of the products. Indeed, α,β-alkynyl ketones can be easily converted into different heterocyclic compounds depending on the experimental conditions employed. Due to its potential, the acyl Sonogashira reaction has been deeply studied with particular attention to the nature of the catalysts and to the structures of both coupling compounds. Considering these two aspects, in this review, a detailed analysis of the literature data regarding the acyl Sonogashira reaction and its role in the synthesis of several heterocyclic derivatives is reported.
1. Introduction
The palladium-catalyzed Sonogashira reaction is probably the best method to create a new sp-sp2 chemical bond starting from aryl or alkenyl halides and terminal alkynes, with or without the presence of a copper(I) co-catalyst [1,2,3]. Due to its potentialities this process has been deeply investigated. In particular, the carbonylative version of this reaction has been developed [4,5,6,7,8], and nowadays is widely used for the synthesis of conjugated alkynyl ketones, biologically active molecules and important building blocks for the synthesis of natural products, pharmaceuticals, and organic materials. With respect to the Sonogashira reaction, its carbonylative version is often performed in the absence of CuI and under high pressure of carbon monoxide (CO). The severe toxicity of CO has led to the development of a Sonogashira type cross-coupling between an acid halide and a terminal alkyne, the so-called acyl Sonogashira reaction. This reaction has quickly become one of the most common methodology to create ynone derivatives, often used for their potential transformation into heterocyclic compounds [9,10,11,12,13]. Therefore the content of this mini-review will be divided into two main chapters: the first is dedicated to give a detailed overview on the acyl Sonogashira reactions (general methods, catalysts, substrates) and the second is centered on the synthesis of heterocycles compounds via multi-steps one-pot sequences which imply a fundamental Sonogashira step of synthesis of alkynyl ketones.
2. The Acyl Sonogashira Reaction
The story of the acyl Sonogashira reaction begun in 1977 when Sonogashira, Tohda and Hagihara reported the first examples of the synthesis of alkynyl ketones by means of a cross-coupling reaction between 1-alkynes and acyl halides promoted by a catalytic amount of PdCl2(PPh3)2 (0.1 mol%) and copper(I) iodide (0.5 mol %) [14]. The reactions were carried out under nitrogen at room temperature, although heating was required in certain cases. Triethylamine was used both as base and solvent. Phenylacetylene and 1-hexyne reacted readily with aromatic and aliphatic acid chlorides affording the expected ketones in good yields (61%–96%) (Scheme 1). On the contrary acetyl, propanoyl and butanoyl chloride underwent side reactions with triethylamine.
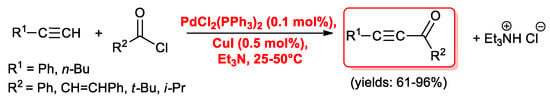
Scheme 1.
First examples of the acyl Sonogashira reaction.
Despite the potential of the method, the reaction remained almost forgotten until the early 2000s, but since then a large number of publications concerning the acyl Sonogashira reactions appeared in the literature. The use of different catalytic species, various experimental conditions and the application of the reaction to several 1-alkynes and acid chlorides, were deeply investigated.
2.1. PdCl2(PPh3)2 Catalyzed acyl Sonogashira Reactions
2.1.1. General Experimental Conditions for PdCl2(PPh3)2 Catalyzed acyl Sonogashira Reactions
Starting from Sonogashira’s data, Cox and co-workers reevaluated the experimental conditions of the reaction [15]. First, in order to limit the possible reactions of acid chlorides with Et3N, they reduced the base concentration and used 1.25 equivalent of amine in THF. Moreover, they increased the catalysts amount to 0.9 mol % for palladium and 3 mol % for copper species (Scheme 2). With the optimized conditions in hand, they tested the reactivity of benzoyl chloride with several acetylenes containing a wide range of functional groups such as alkyl, ester, silyl, silyloxy and protected amino groups (BOC and Ac). Reactions proceeded smoothly and the alkynyl ketones were obtained in high yields (70%–96%). The same experimental conditions were employed for the reactions between phenylacetylene and different acid chlorides. Both electron-withdrawing (NO2, Br) and electron-donating (OMe) groups were well tolerated.
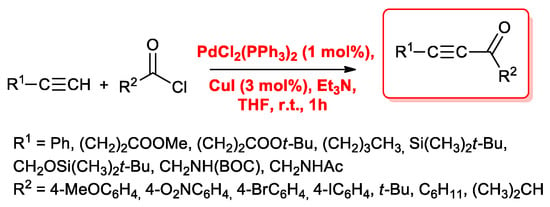
Scheme 2.
Cox’s acyl Sonogashira reactions.
In the case of p-iodobenzoyl chloride, both the acyl chloride and the iodo group reacted with the acetylene generating 3-phenyl-1-(4-(phenylethynyl)phenyl)prop-2-yn-1-one selectively (Scheme 3).
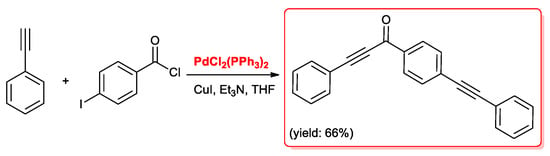
Scheme 3.
Acyl Sonogashira reaction between p-iodobenzoyl chloride and phenylacetylene.
As far as alkyl acid chlorides are concerned, pivaloyl-, 2-methylpropionyl- and cyclohexanecarbonyl- chlorides afforded the coupling products in high yield (86%–97%), while a linear substrate such as n-butanoyl chloride generated a mixture of the expected product together with the corresponding enol derivatives. Taking into account the mechanism generally accepted for Sonogashira reactions [1], Cox and co-workers proposed a sequence based on an initial oxidative addition of acid chlorides to Pd(0) (Scheme 4, I), a subsequent reaction of the obtained Pd complex with the alkyne activated by the CuI co-catalyst (Scheme 4, II), isomerization and reductive elimination (Scheme 4, III, IV) with the formation of alkynone and Pd(0)L2 which restarts the catalytic cycle.
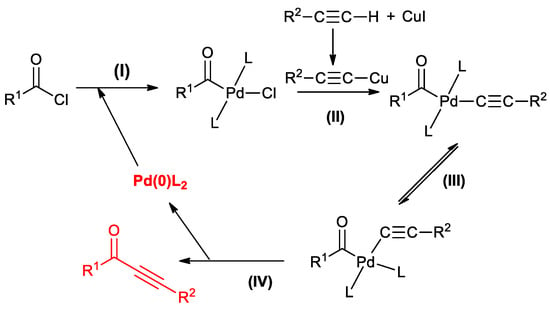
Scheme 4.
Cox’s proposed mechanism for the acyl Sonogashira reaction.
Cox’s experimental conditions were then employed by Prasad and colleagues [16] in the acyl Sonogashira reactions of benzoyl chloride with both aromatic and aliphatic 1-alkynes, yielding the alkynones selectively (72%–79%, Scheme 5).
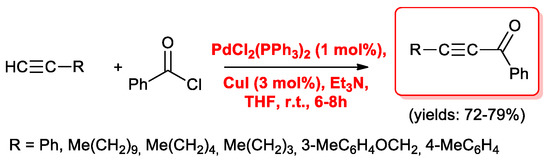
Scheme 5.
Cox’s conditions applied to the acyl Sonogashira reactions of phenylacetylene.
In 2018, Müller’s group [17] modified Cox’s conditions by reducing the amount of both catalysts to 0.25 mol % for PdCl2(PPh3)2 and 0.5 mol % for CuI, operating in 1,4-dioxane as solvent; nevertheless, the acyl Sonogashira coupling between aromatic (Ar = Ph, 4-tBuC6H4, 4-MeOC6H4, 4-MeC6H4, 4-FC6H4, 4-NCC6H4), or heteroaromatic (2-furyl, 2-thienyl) acid chlorides and aromatic acetylenes proceeded successfully (Scheme 6).
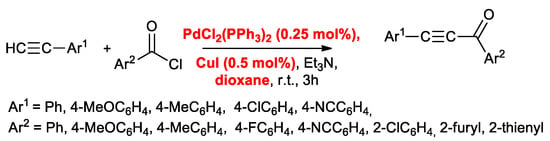
Scheme 6.
Acyl Sonogashira reactions according to Muller’s conditions.
Very recently, Aronica et al. instead proposed a copper-free acyl Sonogashira protocol for the synthesis of tris- and bis-[1-(thiophenyl)propynones], using 2 mol % of PdCl2(PPh3)2 and Et3N as both base and solvent [18,19]. Under these experimental conditions, new luminophores having different aryl nuclei and propynones moieties have been obtained via acyl Sonogashira reactions.
Thiophene-2-carbonyl chlorides possessing electron-withdrawing and electron-donating functional groups were successfully coupled with several diethynyl or triethinyl functionalized aromatic or heteroaromatic compounds.
In order to improve the greenness of the process, Li and co-workers investigated the possibility of carrying out the reaction in water [20]. Considering that acid chlorides can be easily hydrolyzed, the authors tested the reactivity of phenylacetylene and benzoyl chloride in the presence of PdCl2(PPh3)2/CuI as co-catalysts, K2CO3 as the base and a catalytic amount of sodium lauryl sulfate as the surfactant which should reduce the rate of hydrolysis. As a matter of fact, they obtained the desired product in a 98% isolated yield. The optimized experimental conditions were then extended to various acid chlorides and terminal acetylenes as depicted in Scheme 7.
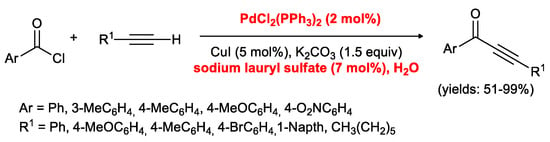
Scheme 7.
Acyl Sonogashira coupling of acid chlorides with 1-alkynes in water developed by Li and co-workers.
The same effort was made by Zhang et al. [21], who tested different ionic liquids (IL) as reaction media. Indeed, IL are considered to be a potent alternative to volatile organic solvents, and can be easily separated from the products. In particular, the authors tested different IL in the cross-coupling between 2-thiophenecarbonyl chloride and 1-hexyne, and found that 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIm]-PF6) was the most suitable ionic liquid. These experimental conditions were then applied to the Sonogashira reactions of (hetero)arenecarbonyl chloride with terminal acetylenes. Moreover, the catalyst obtained by solving PdCl2(PPh3)2/CuI in [BMIm]-PF6 could be easily separated from the products and reused five times with a small loss of activity (57% to 45%).
A copper-free acyl Sonogashira reaction catalyzed by PdCl2(PPh3)2 was investigated by Sonoda et al., where on the basis of previous studies, they examined the synthesis of alkynones using rigid trans-spinning ligands (Figure 1) [22].
Figure 1.
Rigid trans-spanning ligands used in Cu-free acyl Sonogashira protocol optimized by Sonoda et al.
Benzoyl chloride and 1-octyne were chosen as standard substrates and tested in the presence of different solvents, bases and ligands. PdCl2(PPh3)2 (2 mol %), triethylamine (3.5 equiv.), 40 °C, toluene and 2-((2-(thiophen-2-ylethynyl)phenyl)ethynyl)pyridine (I) (2 mol %) resulted to be the best experimental conditions which were then used in the acyl Sonogashira coupling of various acid chlorides and terminal acetylenes. Aromatic derivatives were coupled effectively, affording the alkynones in high yields (73%–99%). Cyclohexanecarbonyl and pivaloyl chlorides were reacted with 1-octyne to give the desired product (76%–77%), while acetyl chloride was found unsuitable for this reaction. Alkynes with nitrile, cyclohenyl, hydroxyl and triisopropylsilyl moieties were used too and yielded the expected coupling products (71%–99%).
2.1.2. PdCl2(PPh3)2 Catalyzed Acyl Sonogashira Reactions of Silylated Alkynes
The use of a silylated acetylene is particularly interesting, since the silyl group can be easily removed and the acetylene moiety can be submitted to several transformations. Indeed, Müller and co-workers reported the acyl Sonogashira reactions of trimethylsilylacetylene with (het)aroyl chlorides performed in the presence of PdCl2(PPh3)2 (2 mol %) and CuI (4 mol %) (Scheme 8) [23,24]. Both electron-donating and electron-withdrawing groups on the benzene ring were well tolerated, and the reaction between thiophene-2-carbonyl chloride and (TMS)-acetylene afforded the corresponding ynone in high yield.

Scheme 8.
Acyl Sonogashira coupling of acid chlorides with TMS-acetylene.
Almost the same results were described by Boons et al. [25] in the acyl Sonogashira coupling between equimolar amount of ethynyltrimethylsilane and ArCOCl (Ar = Ph, 4-MeOC6H4, 4-O2NC6H4, 4-FC6H4, 2-MeC6H4) promoted by Pd(PPh3)2Cl2 (5 mol %), copper(I) iodide (10 mol %) and triethylamine (1.0 equiv.) in THF. The obtained TMS-C≡C-CO-Ar compounds could be easily desilylated and contemporarily submitted to a cyclization process in the presence of a suitable azide yielding functionalized triazole derivatives.
Considering that triisopropylsilyl (TIPS)-protected ynones should be more stable than the corresponding TMS-derivatives because of the better protection effect of bulky TIPS on the C≡C moiety, Kim and co-workers compared the reactivity of (TIPS)-acetylene with that of TMS-acetylene in the cross-coupling reactions with different aroyl chlorides [26]. Indeed, when they employed TIPS derivatives under the same reaction conditions described by Müller et al. [23], they observed an increase of ynone yields up to 98% (Scheme 9).

Scheme 9.
Acyl Sonogashira coupling of acid chlorides with silylated acetylenes.
On the base of these results, Müller’s group [27] investigated the synthesis of pentadiynones via the Pd(PPh3)2Cl2-promoted Sonogashira reaction of TIPS-butadiyne with (hetero)aroyl chlorides (Scheme 10). The coupling products were obtained in good to excellent yields with both electron rich and electron poor acid chlorides.

Scheme 10.
Acyl Sonogashira coupling of acid chlorides with TIPS-butadiyne.
2.1.3. Synthesis of Polyfunctionalized Ynones via PdCl2(PPh3)2-Catalyzed Acyl Sonogashira Reactions
Very recently [28], Pd(PPh3)2Cl2 (1 mol %)/CuI (2 mol %) catalysts were used in the acyl Sonogashira reaction of 5′-ethynyllappaconitine with benzoyl chlorides possessing p-F, p-Br and p-OMe functional groups. The structural transformation of lappaconitine, a diterpenic aconitane alkaloid with interesting antiarrhythmic properties, was effected with the aim to reduce its side effects. Lappaconitine derivatives were effectively prepared using 5′-ethynyllappaconitine as the starting material. The reactions required benzene as the solvent, excess Et3N (4 equiv.) as the base, and were carried out at 65 °C for 8 h, affording alkynone derivatives in excellent yields (Scheme 11).
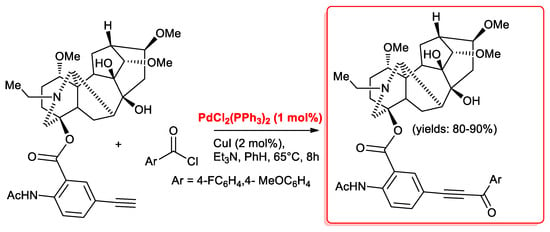
Scheme 11.
Acyl Sonogashira reaction of 5′-ethynyllappaconitine.
Other polyfunctionalized ynones were prepared by Osipov and co-workers [29] by means of an acyl Sonogashira reaction of allenynes with different aromatic and aliphatic acyl chlorides. The reactions were performed in THF at room temperature in the presence of 5 mol % of PdCl2(PPh3)2/CuI and a 1.5 equivalents of Et3N to furnish the corresponding allenynones in good isolated yields (Scheme 12).
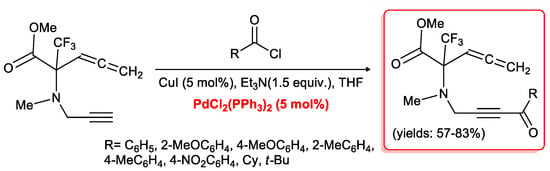
Scheme 12.
The acyl Sonogashira reaction of allenynes.
It is well known that molecules possessing extensive conjugated π-electron systems may exhibit large, nonlinear, optical properties. In particular, compounds like ferrocenylethene and ferrocenylethyne derivatives, may offer a variety of potential applications, such as photoactive semiconductors and liquid crystals. In this field, Chen et al. [30] described the synthesis of some new ethynyl ketones containing the ferrocenyl functional group via cross-coupling reactions of ferrocenylethyne with acyl chlorides catalyzed by PdCl2(PPh3)2 (10 mol %) and CuI (10 mol %) (Scheme 13). The reactions proceeded smoothly in Et3N at room temperature, although acyl chloride containing electron-withdrawing groups resulted in being faster than electron-donating functionalized substrates.
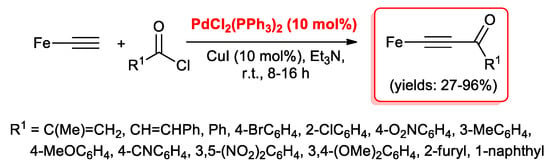
Scheme 13.
Synthesis of ferrocenyl ethynyl ketones via the acyl Sonogashira reaction.
The synthesis of indolyl alkynones, potential pro-apoptotic antitumor agents, was described by Westwell’s group [31], who applied the acyl Sonogashira reaction to functionalized (4-MeO, 3-MeO, 3-F) phenylacetylene and indole-2-carbonyl chlorides, under standard conditions [15], i.e., 0.9 mol % PdCl2(PPh3)2, 3 mol % CuI, room temperature, 1 h.
2.1.4. Synthesis of Ynones via PdCl2(PPh3)2 Catalyzed Acyl Sonogashira Reactions Starting from Different Substrates
Recently Müller and Götzinger [32] developed a PdCl2(PPh3)2 (5 mol %) catalytic system for a two steps, one-pot sequence for the synthesis of alkynylketones starting from aryl iodides. The corresponding terminal acetylenes were generated in situ by a palladium-catalyzed Kumada-type reaction of Ar-I with ethynyl magnesium bromide and subsequently coupled with different aroyl chlorides as depicted in Scheme 14.
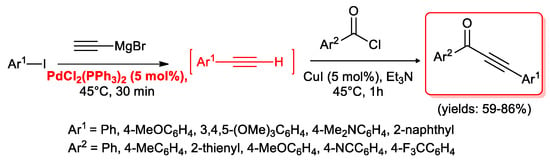
Scheme 14.
Synthesis of alkynones by Kumada/acyl Sonogashira reactions sequence.
Another one-pot, two steps synthesis of ynones was performed by Wu and co-workers [33], starting from functionalized benzoic acids which were converted in situ into the corresponding acid chlorides by means of oxalyl chloride and subsequently reacted with arylacetylenes. The reactions were performed under N2/H2 atmosphere in order to reduce acetylenes dimers resulting from a Glaser side reaction. Contemporary Müller et al. [34] developed an acylation–Sonogashira alkynylation sequence starting from heterocyclic carboxylic acids which were transformed into acid chlorides and then submitted to Sonogashira cross-coupling with terminal acetylenes (Scheme 15). The reactions were carried out in the presence of 2 mol % PdCl2(PPh3)2 and 4 mol % CuI as the catalytic system and furnished a broad variety of heteroaryl ynones in moderate to excellent yields. While the acylation step required 4 h at 50 °C, the Sonogashira coupling was finished after 1 h at room temperature.
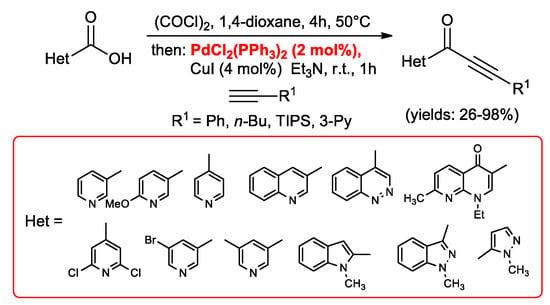
Scheme 15.
Synthesis of alkynones by the acylation/Sonogashira coupling sequence reported by Müller’s group.
Very recently Zeng and co-workers [35] described the first example of acyl Sonogashira reactions between a terminal acetylene and an amide as a coupling partner instead of classic acid chloride. The reactions required 1 mol % PdCl2(PPh3)2 and were performed under copper free conditions.
The authors tested electronically and sterically different amides, but only N-benzoylsaccarine was found to be suitable (Figure 2), affording alkynones in good to excellent yields (51%–98%).
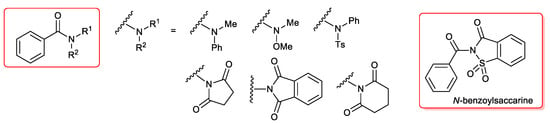
Figure 2.
Pd(PPh3)2Cl2-catalyzed Sonogashira coupling of amides.
2.2. Pd(OAc)2 Catalysed Acyl Sonogashira Reactions
Even if PdCl2(PPh3)2/CuI are the most employed catalysts for acyl Sonogashira reactions, some other palladium species have been tested in this coupling. In particular, Srinivasan’s group [36,37] developed a cross-coupling between acid chlorides and terminal acetylenes promoted by 0.2 mol % of Pd(OAc)2 and performed under solvent-free conditions at room temperature. After 10 min, quantitative conversion into the expected alkynones was observed in almost all cases. Only 1-hexyne required 3 h reaction time, and the corresponding product was isolated in a 40% yield. All other substrates generated the coupling products in good to excellent yields (68%–98%, Scheme 16).
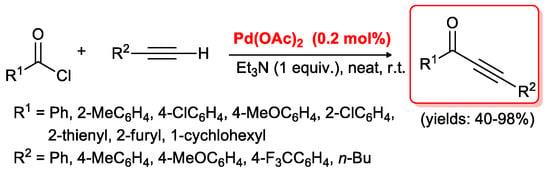
Scheme 16.
Acyl Sonogashira reaction promoted by Pd(OAc)2 developed by the Srinivasan’s group.
Analogously, Hoshi and coll. [38] employed 1 mol % Pd(OAc)2 and 2 mol % PPh3 in the coupling of alkenynes with aryl and heteroaryl acid chlorides. The corresponding alkynones were obtained in high yields (76%–90%) after 2 h at room temperature. The same catalyst was used by Ley et al. [39] in the acylation of terminal alkynes by means of a modular flow reactor. A mixture of Pd(OAc)2, (i-Pr)2NEt in CH2Cl2 was mixed with a solution of the acid chloride and terminal acetylene. The obtained mixture was heated to 100 °C through a convection flow coil for 30 min, then the flow stream was directed to a series of scavenger columns aimed to remove amine, ammonium salts and palladium contaminants. After removal of the solvent, the coupling products were isolated in excellent purity and moderate to high yields (41%–89%).
Najera and co-workers [40] compared the reactivity of Pd(OAc)2 with that of a palladacycle complex derived from 4,4′-dichlorobenzophenone (Figure 3) in the acyl Sonogashira reaction of several acid chlorides and terminal acetylenes.
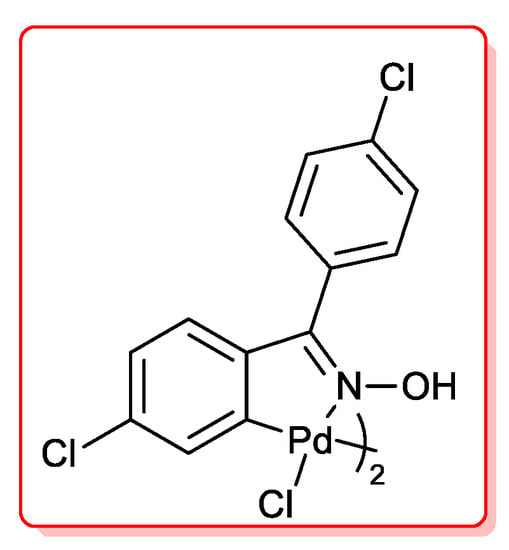
Figure 3.
Palladacycle catalysts for acylation of terminal alkynes.
The reactions were carried out in toluene as solvent, with three equivalents of Et3N, at 110 °C. A very low amount of both catalyst was employed (0.2 mol %), but the palladacycle species was slightly more active than Pd(OAc)2. Aromatic and heteroaromatic acyl chlorides were smoothly reacted with phenylacetylene affording the alkynones derivatives in good yields (50%–99%), while the coupling of benzoyl chloride and 1-octyne had to be performed in the presence of higher catalyst loading, i.e., 0.5 mol %.
2.3. Palladium(II) Complexes as Catalysts for Acyl Sonogashira Reactions
A few organometallic complexes containing a Palladium(II) core have been tested as catalysts in acyl Sonogashira reactions. For instance, Chauvib and Canac [41] prepared two different species starting from diphosphanes possessing imidazolyl and imidazolium substituents. They compared the reactivity of the two complexes in cross-coupling between benzoyl chloride and phenylacetylene (Scheme 17). Both catalysts were found to promote the reaction in the presence of CuI and Et3N, but with marked different reaction rate probably due to side reactions of imidazole nitrogen and benzoyl chloride.
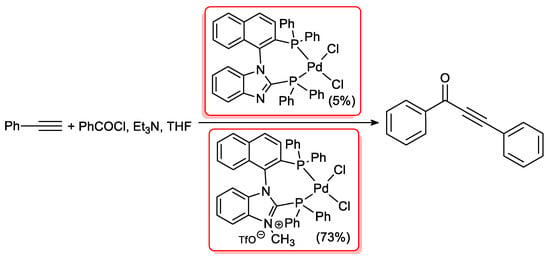
Scheme 17.
Diphosphane ligand Palladium(II) complex used by Chauvib and Canac in the acyl Sonogashira coupling of phenylacetylene.
Later on, Bakherad and co-workers [42] reported that an air stable Pd-salen complex efficiently catalyzed the copper- and solvent-free cross-coupling of several acid chlorides with terminal alkynes under aerobic conditions. The reactions proceeded smoothly with benzoyl chlorides featured by electron-donating and electron-withdrawing groups affording the corresponding ynones in high yields (69%–98%), even in the presence of aliphatic acetylenes (Scheme 18).
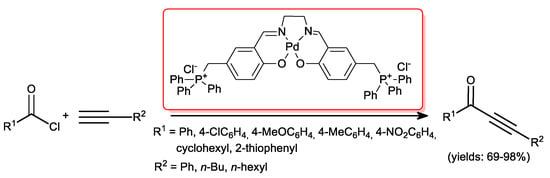
Scheme 18.
Palladium-salen complex as a catalyst for acyl Sonogashira reactions.
Vasilyev et al. [43] investigated the catalytic activity of four different palladium acyclic diaminocarbene complexes in the cross-coupling reactions of phenylacetylenes with aroyl chlorides. Even if the amount of palladium catalysts used was very low (0.04 mol % of Pd(II)), the reactions had to be performed in the presence of PPh3 (1 mol %) and CuI (2 mol %), in order to enhance the chemoselectivity towards the target 1,3-diarylpropinones.
Very recently, Cardenas [44] proposed an air stable phosphinito palladium(II) complex (Figure 4) as efficient catalyst for ynone synthesis via acyl Sonogashira reactions. Optimized reaction conditions required 5 mol % of the catalyst, 80 °C and Et3N for 18 h. When the reactions were carried out with microwave heating, similar high yields (81%–93%) were obtained in 1 h.
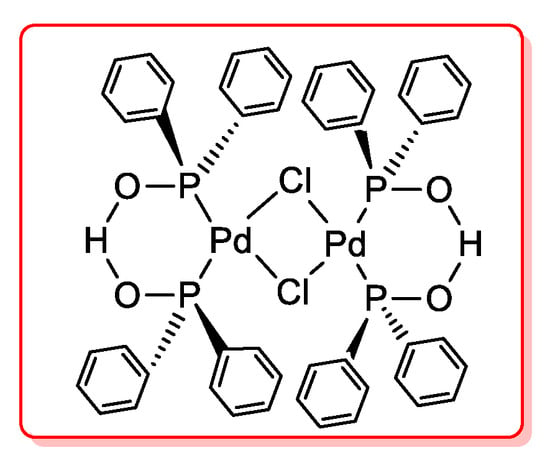
Figure 4.
Phosphinito palladium(II) complex as the catalyst for acyl Sonogashira coupling.
2.4. Palladium Supported Catalysts for Acyl Sonogashira Reactions
Interestingly, few examples of acyl Sonogashira reactions promoted by Pd-supported catalysts have been reported in the literature. Undoubtedly the most used palladium-supported specie is Pd/C for its availability, low cost and easy handling. Likhar and co-workers [45] investigated the activity of Pd/C with 10% loading in a copper-free Sonogashira coupling of acid chlorides with terminal acetylenes. Initially, they studied the reaction between benzoyl chloride and ethynylbenzene under different experimental conditions. The highest amount of the alkynyl ketone was obtained working with 1 mol % of Pd/C at 110 °C, in dry toluene as solvent and with triethylamine as base. These optimized conditions were extended to the reactions of different acid chlorides and acetylenes, obtaining the expected products in good to excellent yields (60%–95%). Unfortunately, when the Likhar group tested the reusability of their catalyst, they observed a marked reduction of the reagents’ conversion after six reaction cycles (i.e., from 96% to 67%), together with 15% leaching of the palladium into the solution. Later on, Kundu’s [46] and Rocha’s [47] groups compared the activity of Pd/C with that of classical catalysts PdCl2(PPh3)2/CuI. In all cases, homogeneous species resulted in being much more efficient than Pd/C in promoting the acyl cross-coupling reactions.
A different supported catalyst was developed by Bakherad et al. [48] They used a high surface area diatomite as their matrix for the deposition of a palladium(II) salophen complex which was then tested in copper- and solvent-free acyl Sonogashira reactions. The reactions were performed at room temperature, with diisopropylethylamine (DIEA) as base and in the presence of 1 mol % of catalyst. Acid chloride featured by electron-withdrawing and electron-donating groups reacted quantitatively with both aryl and alkyl alkynes (91%–99% yields). The investigation on recyclability of the diatomite-supported Pd(II) salophen complex showed only a slight decrease of activity (85%) after four reaction cycles. The same group [49] prepared the polystyrene-supported Pd(0) complex depicted in Figure 5.
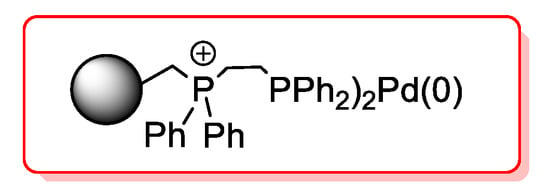
Figure 5.
[PS-dpp-Pd(0)] as efficient catalyst for acyl Sonogashira reaction developed by Bakherad et al.
This supported species resulted to be active in the acyl Sonogashira reaction of p-chlorobenzoyl chloride with Ph-C≡CH used as a reference substrate. The optimized conditions (i.e., 0.5 mol % of catalyst, Et3N as base, r.t. for 15 min) were then extended to several aroyl chlorides and RC≡CH with excellent results (72%–92% yields). The stability of [PS-dpp-Pd(0)] was studied in recycling reactions which indicated that the catalyst could be reused ten times without loss of activity.
Another anchored catalyst (Figure 6) was prepared by Tsai’s group [50] and used in the formation of ynones via Sonogashira reaction of aroyl, heteroaroyl and alkyl acyl chlorides and terminal alkynes. A bipyridyl palladium complex was linked to a mesoporous silica material MCM-41 and employed in very low amount, 0.02 mol %; nevertheless, optimized reaction conditions required Et3N as the base and the solvent, CuI and triphenylphosphine. The catalyst could be reused for four cycles but with a progressive low reduction of activity (98% to 90%).
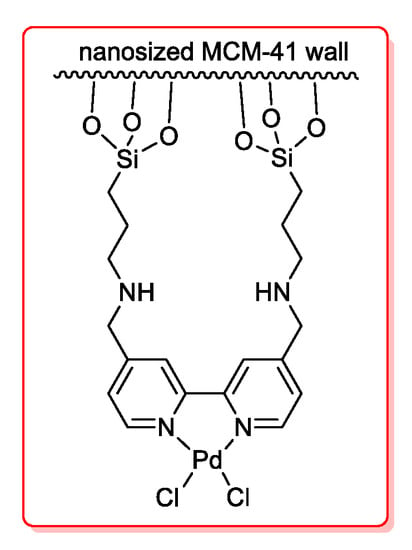
Figure 6.
Nanosized MCM-41-Pd as catalyst for acyl Sonogashira reactions.
More recently, Sharma and coll. [51] developed a new Pd(II) catalyst anchored to functionalized iron oxide nanoparticles (Pd-Sc@ASMNPs, Figure 7): its synthesis required a complex sequence starting from magnetic nanoparticles (MNPs) which were treated with silica affording silica-coated MNPs (SMNPs). Their surface was functionalized with (3-aminopropyl)triethoxysilane and then with salicylaldehyde generating an imine group which acted as ligand for PdCl2.
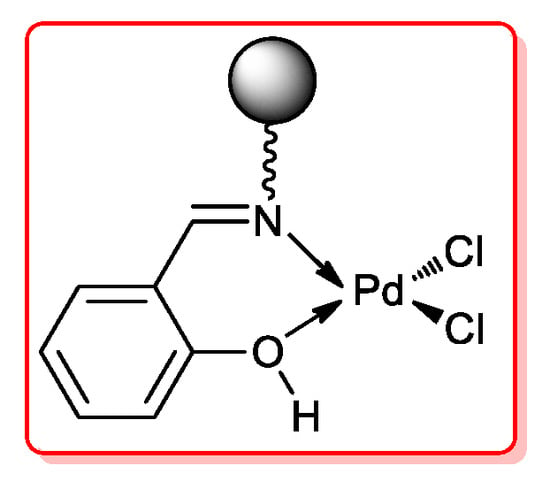
Figure 7.
Pd-Sc@ASMNPs catalyst for acyl Sonogashira reactions developed by Sharma and coll.
Pd-Sc@ASMNPs was used in the synthesis of ynones via acyl Sonogashira reactions performed at room temperature with Et3N as the base and a mixture of H2O/CH3CN as a solvent. The reaction proceeded quantitatively, and recycling and hot-filtration tests seemed to indicate a truly heterogeneous nature of the magnetic nanocatalyst.
A different behavior was observed in cross-coupling reactions of RCOCl and RC≡CH promoted by 1.8 mol % of Pd(II) catalyst anchored to silica gel functionalized with 3-mercaptopropyltrimethoxysilane (Pd-SH-SILICA), proposed by Jin’s group [52]. In this case, thiol moiety can recapture palladium at the end of the reaction according to a “release and catch” mechanism [53]. The same behavior was hypothesized by Aronica et al. [54] in the acyl Sonogashira reactions promoted by palladium(0) nanoparticles (NP) deposited on metal scavengers. Among the tested catalysts, Pd supported on polyvinylpyridine (Pd/PVPy) resulted as especially effective in the synthesis of alkynyl ketones with different stereoelectronic properties (70%–92% yields) under copper-free conditions (Scheme 19).
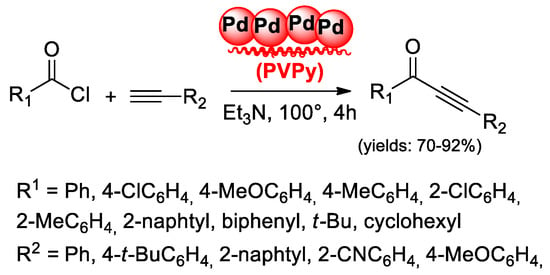
Scheme 19.
Pd(0)NP supported on PVPy as the catalyst for acyl Sonogashira reactions.
The reactions were performed with a low amount of catalyst (0.2 mol% Pd) with Et3N as the solvent and the base. Pd/PVPy seemed to act as a reservoir of Pd nanoparticles which are partially leached during the reaction and then recaptured at the end of the process. Moreover, the same sample of catalyst could be reused at least four times without loss of catalytic activity and selectivity.
Mandal and co-workers [55] developed the synthesis of Pd(0) nanoparticles embedded into poly(p-phenylene sulfide) (Pd(0)NPs-PPS) and tested their activity in the cross-coupling reactions of aryl/aliphatic acid chlorides with different aromatic/aliphatic terminal alkynes. The corresponding ynones were obtained in good yields (55%–98%), but recycling tests indicated a relevant leaching of the palladium during the reaction. In order to obtain a recyclable catalyst, the same authors [56] prepared palladium(0) NPs anchored onto the surface of single-walled carbon nanotubes (SWNT) functionalized with COOH groups. The strong interaction between PdNPs and the carboxylic acid moieties was responsible for very low leaching observed in the acyl Sonogashira reactions of various RCOCl and RC≡CH. The catalyst was reused up to seven times without losing its catalytic property.
Recently Sureshbabu’s group [57] developed a new supported catalyst based on Pd nanoparticles deposited on montmorillonite K-10 clay. The obtained species was applied to the Sonogashira between phenyl acetylene and aroyl chlorides, but 2.4 mol % of palladium was necessary to achieve the products with good yields (87%–96%).
2.5. Copper-Based Catalysts for Acyl Sonogashira Reactions
A few examples of palladium-free acylation of terminal alkynes are described in the literature. In 1996 Kundu and co-workers [58] reported the first case of a coupling of terminal alkynes with acid chlorides performed in the presence of CuI without the need for any palladium species. The reactions were carried out in Et3N for 30 h at room temperature, but 5 mol % of copper iodide was necessary for the coupling process to take place in good yields (61%–83%) This method was applied [59] to several substrates, and in particular to the synthesis of a uracil derivative containing an ynone moiety as depicted in Scheme 20.
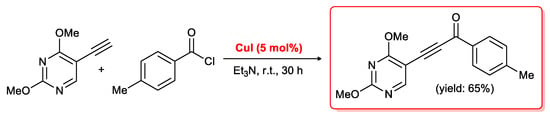
Scheme 20.
CuI-promoted acyl Sonogashira coupling for the synthesis of an alkynone-functionalized uracil derivative.
Similar experimental conditions (CuI 5 mol %, Et3N) were employed by Wang and co-workers [60], who reduced the reaction time to 10 min. by performing the reactions under microwave (MW) irradiation. Tuning the power and the time of MW irradiation, it was possible to achieve alkynyl ketones from cross-coupling reactions of phenylacetylene and aroyl chlorides almost quantitatively.
In order to reduce the amount of copper(I) iodide catalyst, He and coll. [61] developed a new CuI/N,N,N′,N′-tetramethylethylenediamine (TMEDA) catalytic system for the coupling reaction of acid chlorides with terminal alkynes. Ynones possessing electron donating and electron withdrawing groups on the aryl ring of both reactives were obtained in high yields (82%–96%) performing the reaction with 2 mol % of CuI, 5 mol % of TMEDA, at room temperature for 1 h.
Contemporary, a solvent- and palladium-free acylation of terminal alkynes was achieved by Movassgh’s research group [62] using a Cu(I) complex prepared by reacting CuI with a commercially-available azacrown ether (1,4,10,13-tetraoxa-7,16-diazacyclooctadecane, C22). The high air-stability of the obtained species CuI/C22 was probably due to the chelating effect of its N- and O-containing ligand (Figure 8).
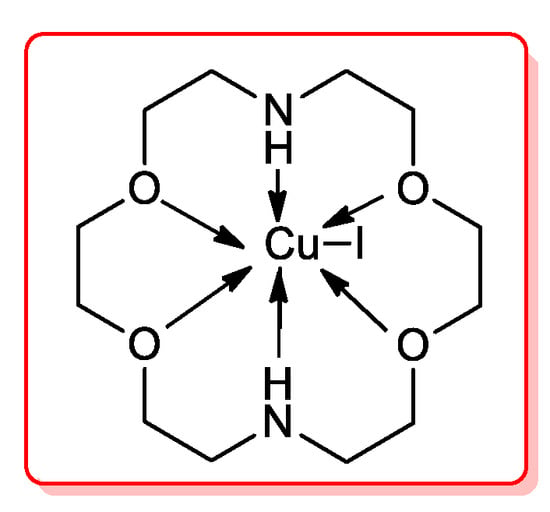
Figure 8.
CuI/C22 catalyst for acylation of terminal alkynes.
This homogeneous catalyst was tested in the cross-coupling reaction of RCOCl and RC≡CH which yielded the corresponding alkynones almost quantitatively when aryl alkyne were used, while a lower amount of products were detected with 1-hexyne and 1-octyne.
The first example of supported copper nanoparticles as the catalytic system for the synthesis of ynones by the coupling of acyl chlorides and terminal alkynes was reported by Yao and co-workers in 2014 [63]. They investigated the effect of two different supports (γ-Al2O3 and silica gel) on the nanoparticles’ catalytic efficiency: Cu (NPs)/SiO2 gave the best results and 1 mol % of catalyst was enough to generate high yields (71%–98%) of the desired products. Analogously, Bhanage’s group [64] described the preparation of Cu/CuO2 nanoparticles via microwave irradiation and their excellent applicability to acyl Sonogashira reactions of substrates with different functional groups (NH2, OH, Cl, OCH3, CH3). More recently, a mesoporous polymer-supported Cu NPs catalyst was developed [65] and applied to palladium- and solvent-free coupling of acid chloride and aryl alkynes. Similar experimental conditions were used in the acylation of aryl acetylenes with benzoyl chloride promoted by a nanosized Cu2O/SiO2 egg-shell catalyst [66]. Finally, Moghaddam and coworkers [67] reported the application of cobalt–copper ferrite nanoparticles (CoCuFe2O4), obtained by the co-precipitation method, to the synthesis of alkynyl ketones by acyl Sonogashira reactions. The catalyst could be recovered using an external magnet, and then could be reused in up to nine reaction cycles, even though with a substantial reduction of its catalytic activity (from 90% to 70%) was observed.
3. Application of Acyl Sonogashira Reaction to the Synthesis of Heterocyclic Compounds
Alkynones, easily obtained via acyl Sonogashira reactions between terminal alkynes and acyl chlorides, represent powerful and versatile bifunctional building blocks in organic synthesis. However, the typically mild experimental conditions of the acyl Sonogashira reaction may also allow synthetic routes to heterocycles by sequential one-pot transformations, where alkynylketones are synthesized by a coupling of 1-alkynes with acid chlorides and then used without isolation in a following cyclization step (in particular, [3+2] cycloaddition with 1,3-dipoles or Michael addition-cyclocondensation with bifunctional nucleophiles). Although a couple of reviews on this topic have been previously reported by Müller and co-workers [9,12], mainly covering two different time frames, the recent advances in acyl Sonogashira coupling-based strategies requires a more complete and updated overview on these transformations. Therefore, in the second part of this mini-review, we shall give a comprehensive and detailed overview on the synthesis of heterocyclic compounds via one-pot acyl Sonogashira reaction–cyclization sequences: in particular, we shall follow a systematic approach, organizing literature on the heteroatoms (N-heterocycles, N,O-heterocycles, N,S-heterocycles, O-heterocycles and S-heterocycles), ring size (five-membered, six- membered and seven-membered) and number of cycles (monocyclic, bicyclic and polycyclic compounds).
3.1. Synthesis of N-Heterocycles by Acyl Sonogashira Reactions
Nitrogen-containing heterocycles are the largest class of compounds which can be synthesized by the one-pot acyl Sonogashira reaction–cyclization sequence. In particular, we shall consider five-membered monocyclic (pyrroles, pyrazoles, triazoles), six-membered monocyclic (pyridines, pyridones, pyrimidines), bicyclic (indoles, quinolines, quinoxalines, benzodiazepines, indolizines) and polycyclic (α-carbolines, tetrahydro-β-carbolines, pyrrolo(iso)quinolines, pyrimidinoindoles) systems.
3.1.1. Synthesis of Five-Membered N-Containing Monocyclic Compounds
In 2009, Müller and coll. reported a fascinating three-component synthesis of N-Boc-4-iodopyrroles, where acid chlorides are coupled with N-Boc propargylamide by an acyl Sonogashira reaction to give the corresponding alkynones, which are then converted into Boc-protected 2-substituted 4-iodopyrroles via one-pot Michael addition-cyclocondensation in the presence of sodium iodide [68]. The reaction, performed with PdCl2(PPh3)2 (2 mol %) as catalyst, and CuI (4 mol %) as co-catalyst under mild conditions, gave good yields with aromatic acid chlorides bearing electroneutral, electron-withdrawing or electron-donating substituents, as well as with heteroaryl, alkenyl and selected alkyl (i.e., cyclopropyl and adamantyl) acyl chlorides (Scheme 21). Interestingly, by the addition of another alkyne to the reaction mixture, iodopyrroles can give one-pot a further Sonogashira coupling, obtaining the corresponding N-Boc-2-substituted-4- alkynylpyrroles in good yields.
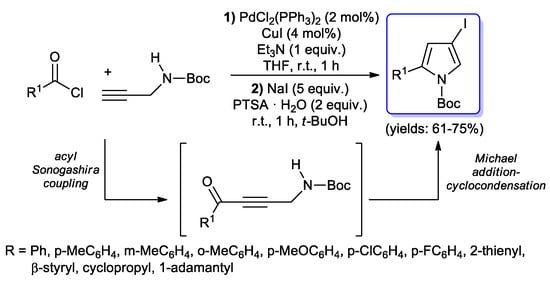
Scheme 21.
Three-component synthesis of N-Boc-4-iodopyrroles.
In a following paper, a protocol for the synthesis of 2-substituted 3-acylpyrroles was described by Müller and co-workers: 2-chlorobenzoyl chloride and phenylacetylene were treated under copper-free acyl Sonogashira conditions (1 mol % of PdCl2 and 2 mol % of di(1-adamantyl)-benzyl-phosphonium bromide as the catalyst, 1.1 equiv. of Et3N as base and CH2Cl2 as solvent) at room temperature, giving the alkynone within 2 h; aminoacetaldehyde diethylacetal was then added to the reaction mixture, obtaining the corresponding β-enaminone by Michael addition, followed by a methanesulfonic acid-promoted cyclocondensation to final pyrrole (Scheme 22) [69]. This one-pot sequence was successfully extended to several aryl acid chlorides using both aromatic and aliphatic alkynes.
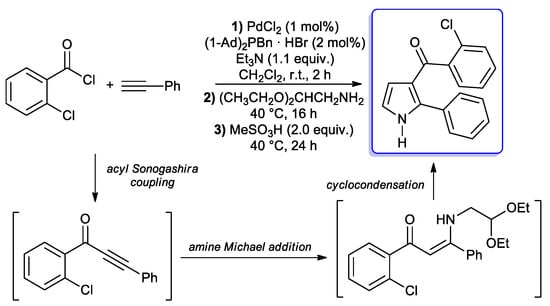
Scheme 22.
Model reaction for the optimization of one-pot synthesis of 2-substituted 3-acylpyrroles.
The preparation of pyrazoles through one-pot protocols involving acyl Sonogashira reactions has been more intensively investigated. The first example was reported in 2008 by Jiang et al., wherein (hetero)aryl or cyclohexyl acid chlorides were coupled with some terminal alkynes under standard acyl Sonogashira conditions (1 mol % of PdCl2(PPh3)2, 3 mol % of CuI, 2 equiv. of Et3N and THF) to give α,β-unsaturated ynones, which were then converted into final 3,5-disubstituted-1H-pyrazoles by the addition of hydrazine to the reaction mixture (Scheme 23) [70].
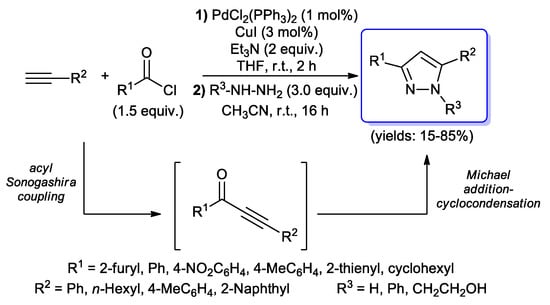
Scheme 23.
First example of 1,3,5-trisubstituted-1H-pyrazoles synthesis via acyl Sonogashira–Michael addition–cyclocondensation sequence.
However, a more extensive investigation was performed by the Müller’s group. First of all, they explored [71] a possible extension of the Jiang protocol to substituted hydrazines (in particular methylhydrazine or arylhydrazines), in order to obtain 1,3,5-trisubstituted-1H-pyrazoles; interestingly, despite the possible formation of two different regioisomers in the Michael addition–cyclocondensation step, in each case only one of them was obtained, depending on the nature of the hydrazine substituent (Scheme 24). Final pyrazoles were obtained in good to excellent yields (53%–95%), also thanks to the microwave heating adopted for the cyclization step.
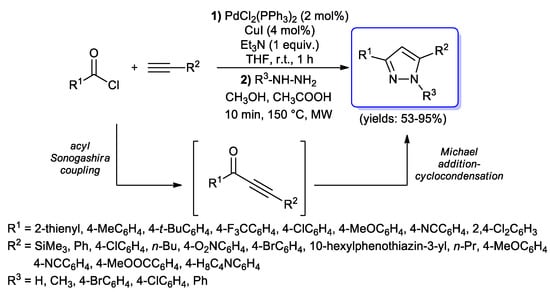
Scheme 24.
One pot acyl Sonogashira–Michael addition–cyclocondensation sequence for the synthesis of 1,3,5-trisubstituted-1H-pyrazoles.
As a further development of the above described synthetic method, more recently they reported a one-pot, four-step sequence for the preparation of 1,3,4,5-tetrasubstituted-1H-pyrazoles. Upon the reaction of acid chlorides with terminal alkynes under the same acyl Sonogashira conditions, (with PdCl2(PPh3)2 as the catalyst and CuI as the co-catalyst, Et3N as the base and THF as the solvent), and being followed by microwaves-assisted Michael addition–cyclocondensation with methyl hydrazine and bromination with N-bromosuccinimide, with the final Suzuki- Miyaura step with alkyl or aryl boronic acids in the presence of K2CO3 and water, catalytic amounts of PPh3 and microwave heating gave tetrasubstituted pyrazoles in excellent regioselectivity (about 95:5) and in moderate to good yields (22–61%) as light yellow solids (Scheme 25) [72].
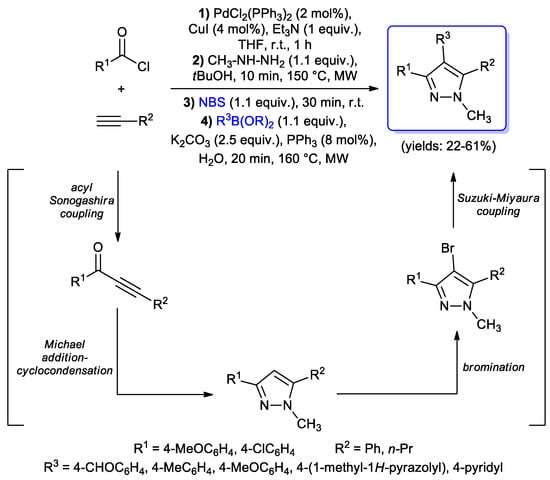
Scheme 25.
Application of the acyl Sonogashira reaction in a one-pot four-step sequence for the synthesis of 1,3,4,5-tetrasubstituted-1H-pyrazoles.
Along the lines of the last two mentioned works, in 2015 a four-component preparation of 1-, 3- or 5-biaryl-substituted-1H-pyrazoles was reported by Müller et al., following a sequential acyl Sonogashira coupling/Michael addition–cyclocondensation/Suzuki-Miyaura coupling [73]. However, very recently they also developed a more interesting one-pot synthesis of 5-triazolyl-substituted-1H-pyrazoles; in this case, the acyl Sonogashira step was performed between acyl chlorides and triisopropylsilyl (TIPS)-protected butadiyne, giving the corresponding TIPS-protected pentadiynones; upon cyclocondensation with methyl or benzyl hydrazine, 5-TIPS-ethynyl-1H-pyrazoles were generated, which gave final compounds after tetra-n-butylammonium fluoride (TBAF) promoted desilylation, followed by copper(I)-catalyzed alkyne− azide cycloaddition (CuAAC) with benzyl, homobenzyl or (hetero)aryl azides (Scheme 26) [27].
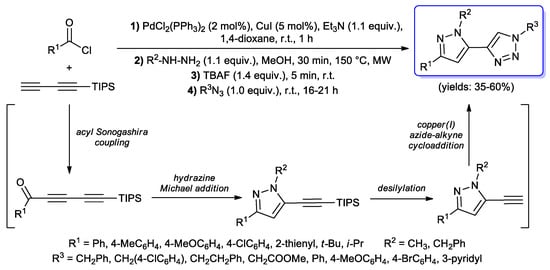
Scheme 26.
Müller’s one-pot synthesis of 5-triazolyl-substituted-1H-pyrazoles: sequential acyl Sonogashira coupling/Michael addition–cyclocondensation/desilylation/CuAAC.
In all the above described synthetic routes to pyrazoles, the acyl Sonogashira step has been performed by using PdCl2(PPh3)2 as catalyst and CuI as the co-catalyst. However, several other catalytic systems were successfully tested for the same transformation. In 2016, Cai and co-workers developed an interesting protocol based on 3-(2-aminoethylamino)propyl-functionalized MCM-41-immobilized palladium(II) acetate complex [MCM-41-2N-Pd(OAc)2], wherein the acyl Sonogashira coupling of (hetero)aryl or cyclohexyl acid chlorides and aryl or alkyl terminal alkynes, performed in Et3N with MCM-41-2N-Pd(OAc)2 (0.5 mol %) and CuI (1.0 mol %), gave the corresponding alkynones, which were then converted in situ into final 1H-pyrazoles (yield 25%–88%) via Michael addition–cyclocondensation of hydrazines, performed at room temperature with acetonitrile as co-solvent (Scheme 27) [74].
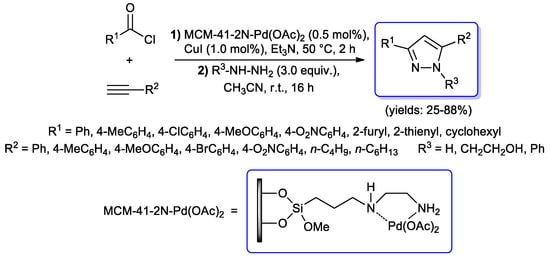
Scheme 27.
Synthesis of 1H-pyrazoles via a one-pot protocol starting with a MCM-41-2N-Pd(OAc)2 catalyzed acyl Sonogashira coupling.
Jin et al. instead proposed a copper free method, where a thiol groups-functionalized silica gel-supported palladium catalyst (Pd-SH-SILICA) was used in the acyl Sonogashira alkynylation step [52]. Very recently, a further one-pot regioselective synthesis of 3,5-disubstituted and 3,4,5-trisubstituted pyrazoles via a Cu-free protocol was developed, exploiting in situ generated Pd-nanoparticles as the catalyst in an environmentally benign PEG-400/H2O medium [75].
Keivanloo [76] reported a palladium- and copper-free procedure for the synthesis of 3,5-disubstituted- 1H-pyrazoles from various acid chlorides, alkynes and hydrazine, based on an acyl Sonogashira-type alkynylation performed in the presence of silica-supported-zinc bromide as catalyst.
Some one-pot procedures for the synthesis of 1,2,3-triazoles starting with an acyl Sonogashira coupling have also been described in the literature. In 2009, Chen and coll. [77] developed an interesting protocol for the preparation of 4,5-disubstituted-2H-1,2,3-triazoles; acyl chlorides and alkynes were treated under common acyl Sonogashira conditions (1 mol % of PdCl2(PPh3)2, 2 mol % of CuI, 3 equiv. of Et3N) at room temperature in an ultrasonic bath, followed by one-pot 1,3-dipolar cycloaddition of the resulting ynones with NaN3 (Scheme 28). The substrate scope of the protocol was quite general, and final 2H- 1,2,3-triazoles were always obtained in excellent yields.
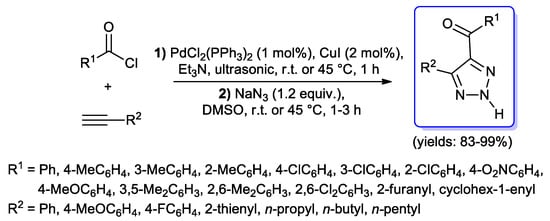
Scheme 28.
Preparation of 4,5-disubstituted-2H-1,2,3-triazoles.
Boons et al. instead described a one-pot three-steps sequence for the synthesis of 4-acyl-1H-1,2,3- triazoles: First, an acyl Sonogashira coupling of aroyl chlorides with trimethylsilylacetylene, giving trimethylsilyl (TMS)-protected propynones; second, TBAF-promoted desilylation of the TMS protecting group; third, CuAAC of desilylated propynones with benzyl azides, affording final 4-aroyl-1H-1,2,3- triazoles [25]. However, a more extended investigation on a similar synthetic strategy was then performed by Kim and co-workers, in which (hetero)aryl acid chlorides were treated with TIPS-acetylene under standard acyl Sonogashira alkynylation conditions (2 mol % of PdCl2(PPh3)2, 5 mol % of CuI, 3 equiv. of Et3N and THF), followed by an in situ TIPS group deprotection/CuAAC reaction with several benzyl, aryl or alkyl azides under two different protocols (method A: 1.5 equiv. of azide, 0.5 equiv. of CuI and 1.5 equiv. of AgF in THF at 60 °C; method B: 1.5 equiv. of azide, 0.1 equiv. of CuI, 1.5 equiv. of AgF and 0.02 equiv. of 1,10-phenanthroline in THF at room temperature), both providing 1H-1,2,3-triazole products in satisfactory yields (Scheme 29) [26].
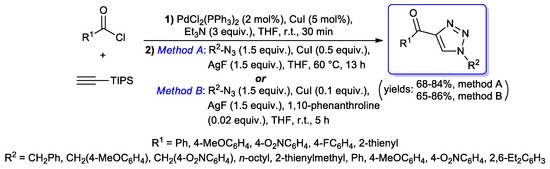
Scheme 29.
One-pot synthesis of 4-acyl-1H-1,2,3-triazoles involving the acyl Sonogashira alkynylation step described by Kim and co-workers.
3.1.2. Synthesis of Six-Membered N-Containing Monocyclic Compounds
Moving on six-membered monocyclic compounds, in 2016 Müller’s group developed a powerful synthesis of tetra-substituted pyridines, where, after an acyl Sonogashira reaction step (2 mol % of PdCl2(PPh3)2 as catalyst, 4 mol % of CuI as co-catalyst, 1 equiv. of Et3N as base, THF as solvent, at room temperature for 2 h), resulting ynones were treated without isolation with ethyl-3-aminocrotonate via a Bagley–Bohlmann–Rahtz coupling (i.e., enamine Michael addition followed by high temperature cyclocondensation) to give after purification 4,6-disubstituted-2-methyl-3-ethoxycarbonyl-pyridines (yields 19%–52%) (Scheme 30) [78].
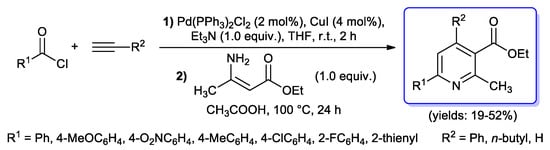
Scheme 30.
One-pot synthesis of tetra-substituted pyridines reported in 2016 by Müller’s group.
Some acyl Sonogashira-promoted protocols for the synthesis of pyridin-2(1H)ones have also been developed. An interesting one-pot, consecutive, four-component synthesis of 5-acyl-3,4-dihydropyridin- 2(1H)ones was described in 2013 by Müller and coll.: in this case, intermediate alkynones were generated by a copper-free acyl Sonogashira methodology, using only PdCl2 as catalytic precursor in the presence of Beller’s ligand (i.e., di(1-adamantyl)-benzyl-phosphonium bromide); upon addition of a benzylamine, corresponding β-enaminones were obtained, which were then directly treated with acryloyl chloride to give the final lactam through aza-annulation reaction (Scheme 31) [79].
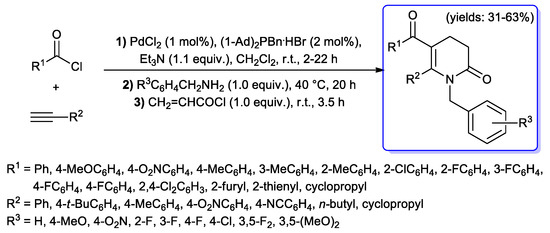
Scheme 31.
One-pot four-component synthesis of 5-acyl-3,4-dihydropyridin- 2(1H)ones.
In a following work, the same protocol (except for small variations in the experimental conditions) was extended to variously substituted anilines, used instead of benzylamines [80]. Very recently, in a work mainly focused on the sequential synthesis of α-pyrones (described below on Section 3.4.1), they also developed an alkynylation–Michael addition–cyclocondensation–ammonolysis protocol for the preparation of 4,6-diphenylpyridin-2(1H)ones, based on the ammonolysis of α-pyrones in the presence of 25% aqueous ammonia solution to give the corresponding α-pyridones [17].
Pyrimidines are one of the most investigated N-heterocyclic compounds which can be obtained by a sequential protocol involving an acyl Sonogashira alkynylation step. In 2003, Müller et al. reported the first example of 2,4-disubstituted pyrimidines via Sonogashira coupling of p-anisoyl or 2-thiophenecarbonyl chloride with trimethylsilylacetylene, followed by treatment with amidinium or guanidinium salts and 2.5–3.0 equiv of Na2CO3 ·10 H2O in a one-pot process; although performed only on a few examples, final compounds were obtained in modest to good yields (30%–81%) (Scheme 32) [23].
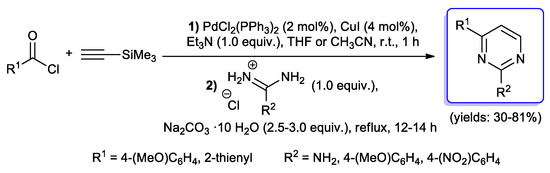
Scheme 32.
First example of 2,4-disubstituted pyrimidines synthesis via one-pot process involving the acyl Sonogashira reaction.
A more extended investigation was then described by the same authors for the preparation of 2,4,6- trisubstituted pyrimidines: several aryl, heteroaryl and tert-butyl acid chlorides were treated with terminal alkynes bearing alkyl or aryl groups under typical acyl Sonogashira conditions (2 mol % of PdCl2(PPh3)2, 4 mol % of CuI, 1 equiv. of Et3N, THF or CH3CN, room temperature, 1 h) to give the corresponding alkynones, which were then treated with amidinium salts in the presence of sodium carbonate as base under reflux for 12–14 h, giving pyrimidines with yields of 26%–84% (Scheme 33) [81].
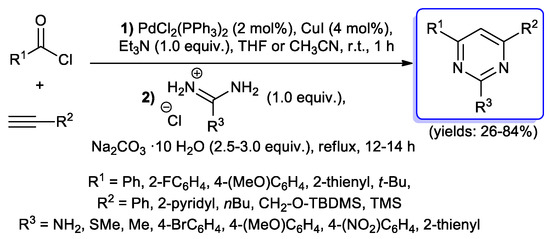
Scheme 33.
Synthesis of 2,4,6-trisubstituted pyrimidines reported in 2003 by Müller’s group.
The applicability of the present protocol was also tested on the synthesis of a dipyrimidyl pyridine (DIPYRIMPY) derivative starting from pyridine-2,6-dicarbonyl dichloride [24,81]. A slightly different protocol, including an in situ generation of acyl chlorides from the corresponding carboxylic acids by treatment with oxalyl chloride, was developed in 2011 by the same research group (Scheme 34) [34].
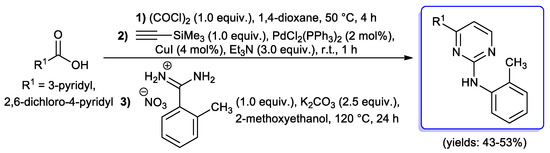
Scheme 34.
One-pot three-component synthesis of 4-pyridyl-2-o-tolylaminopyrimidines.
More recently, Mandal and co-workers reported the synthesis of 4-substituted pyrimidin-2-amines through a copper-free acyl Sonogashira step of (hetero)aryl, tert-butyl and adamantyl acid chlorides with trimethylsilylacetylene, performed using palladium(0) NPs anchored onto single walled carbon nanotubes (SWNT-PdNPs) as their catalyst, followed by treatment with guanidine hydrochloride in the presence of Na2CO3 as the base; in all cases, N-heterocycles were obtained in satisfactory yields (Scheme 35) [55,56]. In 2018, Shults et al. [28] proposed instead the synthesis of diterpene alkaloid lappaconitine derivatives with pyrimidine rings by one-pot acyl Sonogashira/guanidinium Michael addition/cyclocondensation.
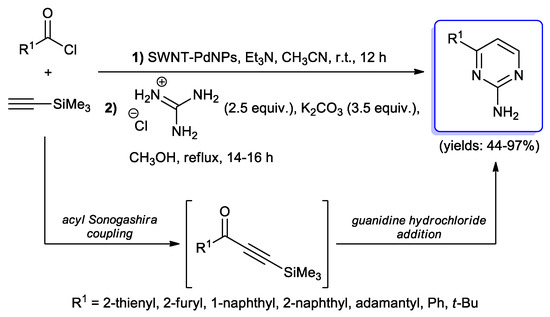
Scheme 35.
Synthesis of 4-substituted pyrimidin-2-amines via one-pot sequence involving a single-walled carbon nanotubes-phenyl di-n-pentylphosphinate nanoparticles (SWNT-PdNPs) catalyzed acyl Sonogashira step.
3.1.3. Synthesis of N-Containing Bicyclic Compounds
Synthetic pathways starting with an acyl Sonogashira alkynylation represent a very elegant way for the preparation of several classes of benzofused N-bicyclic compounds, including quinolines, quinoxalines and benzodiazepines.
In 2010, Liang and coll. reported the synthesis of 2-aryl-4-aroylquinolines though a domino reaction, consisting in an acyl Sonogashira coupling of benzimidoyl chlorides—here used instead of acyl chlorides—with 1,6-enynes performed under standard conditions (PdCl2(PPh3)2, CuI, Et3N, 80 °C, 7 h), followed by one-pot cyclization to form quinolines in good yields (62%–90%) [82]. In particular, authors proposed the following mechanism: After formation of the alkynyl imine intermediate via an acyl Sonogashira coupling of benzimidoyl chlorides and 1,6-enynes, an allenyl-isomerization takes place, followed by a 6π-electrocyclization step generating the quinoline ring, which is then converted into the final product by Pd-promoted removal of an allyl moiety (Scheme 36).
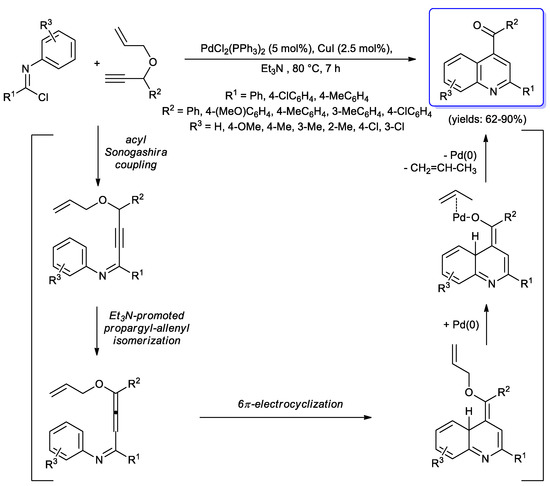
Scheme 36.
Liang’s synthesis of 2-aryl-4-aroylquinolines via one-pot acyl Sonogashira-cyclization of benzimidoyl chlorides with 1,6-enynes.
Interestingly, 2,4-disubstituted quinolines were also synthesized by Müller’s research group through a quite different approach, that is, (hetero)aroyl chlorides, terminal alkynes, and 2-aminothiophenols could give a fascinating, one-pot microwave-assisted Sonogashira coupling–Michael addition–cyclocondensation –sulfur extrusion sequence [83]. A very crucial point of this protocol is obtaining a quantitative terminal sulfur extrusion, or otherwise, mixtures of final quinolines and preceding benzothiazepines were obtained, very difficult to separate by column chromatography due to their similar polarity.
A very interesting one-pot glyoxylation–alkynylation–cyclization sequence for the synthesis of 2-(hetero)aryl-3-ethynylquinoxalines was described in 2014: electron-rich π-nucleophiles (N-methylindole, N-substituted pyrroles, 2-methoxythiophene and azulene) were first treated with oxalyl chloride in THF to give the corresponding glyoxylyl chlorides, which were then alkynylated under palladium-free conditions, by adding 1.0 equiv. of alkyne, 5 mol % of CuI and 3.0 equiv. of Et3N to the reaction mixture; the resulting alkynediones were finally treated without isolation with 1,2-diaminobenzene, affording final quinoxalines in most cases with satisfactory yields (28%–87%) (Scheme 37) [84].
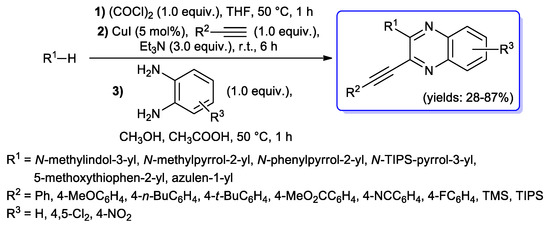
Scheme 37.
One-pot glyoxylation–alkynylation–cyclization sequence to 2-substituted 3-ethynylquinoxalines developed by Müller’s research group.
In 2007, Srinivasan et al. reported the synthesis of 2,4-disubstituted-3H-benzo[b][1,4]diazepines via a one-pot, three-component sequence. The methodology involved a first acyl Sonogashira coupling step between (hetero)aroyl chlorides and aryl alkynes, performed under copper-, ligand- and solvent-free conditions using Pd(OAc)2 (0.2 mol %) as catalyst and Et3N (1.0 equiv.) as base in only 10 min at room temperature, followed by Michael addition and cyclocondensation of ortho-phenylenediamines added to the reaction mixture using water as a solvent (Scheme 38) [85].
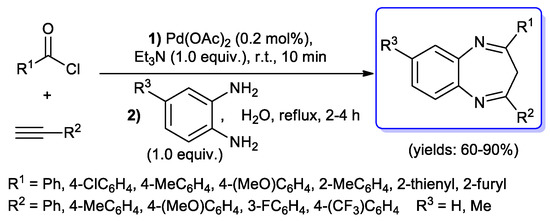
Scheme 38.
Synthesis of 2,4-disubstituted-3H-benzo[b][1,4]diazepines via one-pot acyl Sonogashira–Michael addition–cyclocondensation sequence developed by Srinivasan et al.
Different experimental conditions were adopted by Müller and coll. for the preparation of the same systems: the acyl Sonogashira step of acid chlorides and alkynes was easily carried out in THF at room temperature with PdCl2(PPh3)2 as their catalyst, CuI as the co-catalyst and Et3N as the base; in the following Michael addition–cyclocondensation step, alkynones were treated one-pot with o-phenylenediamines and acetic acid under microwaves heating, affording in only 1 h final benzodiazepines in modest to good yields (40%–88%) [86].
The synthesis of other N-bicyclic compounds via acyl Sonogashira coupling has been described in the literature. In 2005, Müller and co-workers reported the preparation of highly fluorescent indolizines and biindolizines in moderate yields by a consecutive acyl Sonogashira/1,3-dipolar cycloaddition sequence: (hetero)aroyl chlorides and terminal alkynes were treated under standard coupling conditions (2 mol % of PdCl2(PPh3)2, 4 mol % of CuI, 20 equiv. of Et3N, THF, room temperature, 2 h) giving the corresponding ynones, which were then treated in a one-pot procedure with suiTable 1-(2-oxoethyl)pyridin-1-ium or 1,1′- bis(2-oxoethyl)-4,4′-bipyridine-1,1′-diium bromides in a following 1,3-dipolar cycloaddition step to afford the target heterocyclic products (Scheme 39) [87].
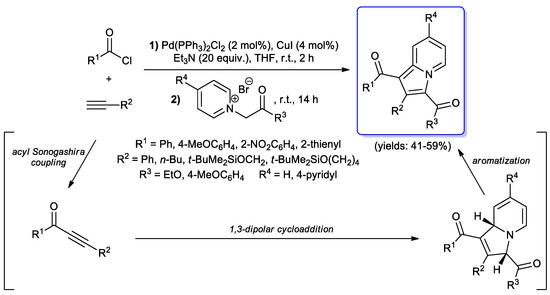
Scheme 39.
Synthesis of indolizines via one-pot acyl Sonogashira/1,3-dipolar cycloaddition/aromatization sequence reported in 2005 by Müller and co-workers.
3.1.4. Synthesis of N-Containing Polycyclic Compounds
Multicomponent, one-pot protocols starting with an acyl Sonogashira coupling were found very useful for the preparation of several nitrogen-based polycyclic compounds.
Kundu et al. reported an interesting synthesis of α-carboline derivatives involving aryl or tert-butyl acid chlorides, terminal alkynes and 2-aminoindole hydrochlorides via sequential acyl Sonogashira/[3+3] cyclocondensation protocol. Interestingly, the authors found that the presence of water in the reaction medium may facilitate the cyclization step, while non-aqueous conditions furnished final products only in poor yields (Scheme 40) [88].
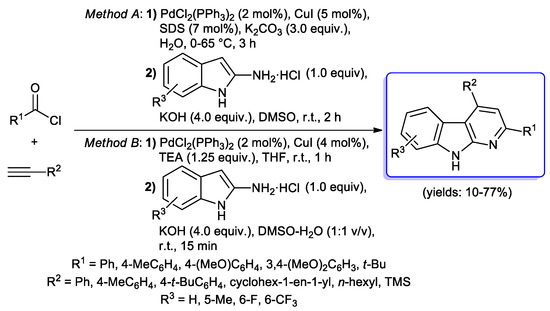
Scheme 40.
Synthesis of α-carbolines via sequential acyl Sonogashira/[3+3] cyclocondensation protocol reported by Kundu et al.
Müller’s group developed instead a fascinating route to tetrahydro-β-carbolines via domino acyl Sonogashira coupling/amine Michael addition/aza-annulation/Pictet-Spengler sequence of acid chlorides, terminal alkynes, tryptamine derivatives and acryloyl chlorides, creating five new σ-bonds and four new stereocenters in a one-pot fashion. In the present protocol, (hetero)aryl acid chlorides having electronically different substituents and both aliphatic and aromatic alkynes were first treated in THF with PdCl2(PPh3)2 (2 mol %), CuI (4 mol %) and Et3N (1.0 equiv.) at room temperature; to the reaction mixture were then added tryptamines (70 °C, 10 h), giving Michael addition to the in situ-generated ynones, and acryloyl chlorides (70 °C, 3 h), affording aza-annulation and Pictet–Spengler steps to final tetrahydro-β-carbolines with reasonable yields (Scheme 41) [89,90].
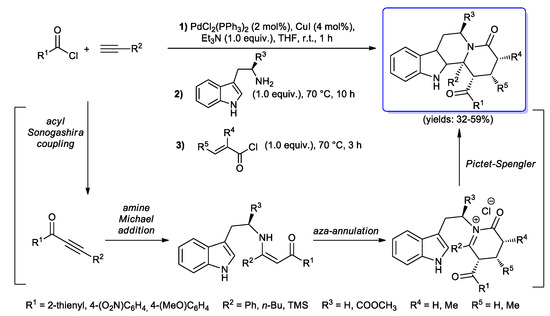
Scheme 41.
One-pot acyl Sonogashira coupling/amine Michael addition/aza-annulation/Pictet-Spengler sequence for the synthesis of tetrahydro-β-carbolines.
Interestingly, more recently Mandal and co-workers reported a similar sequence to tetrahydro-β-carbolines, although starting with a copper-free acyl Sonogashira step, performed with palladium(0) NPs anchored onto single-walled carbon nanotubes as the catalyst [55].
An acyl Sonogashira coupling/1,3-dipolar cycloaddition sequence of (hetero)aryl acid chlorides, terminal alkynes and suitable quinolinium or isoquinolinium bromides was developed by Zhang et al. for the synthesis of pyrroloquinolines or pyrroloisoquinolines, respectively; the protocol was carried out in ionic liquids (1-butyl-3-methylimidazolium hexafluorophosphate showed the best performance), with PdCl2(PPh3)2 as the catalyst, CuI as the co-catalyst and Et3N as the base, affording final heterocycles in moderate yields (49%–68%) [21].
Kundu and coll. described instead the synthesis of highly diversified pyrimido[1,2-a]indoles via one-pot acyl Sonogashira coupling of aroyl chlorides and terminal alkynes followed by [3+3] cyclocondensation of the resulting alkynones with ethyl 2-amino-1H-indole-3-carboxylates, performed in the presence of Cs2CO3 as the base in refluxing acetonitrile for 6 h (Scheme 42) [46].
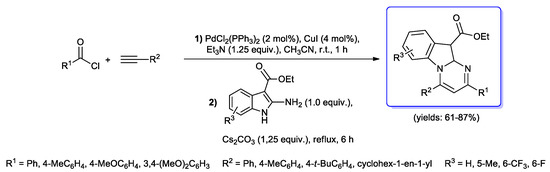
Scheme 42.
Kundu’s synthesis of pyrimido[1,2-a]indoles via one-pot acyl Sonogashira step followed by [3+3] cyclocondensation with ethyl 2-amino-1H-indole-3-carboxylates.
3.2. Synthesis of N,O-Heterocycles by Acyl Sonogashira Reactions
The oxazole and isoxazole moieties are common heterocyclic motifs identified in many natural products and biologically active compounds. An efficient three steps synthesis of 2,5-polyfunctionalized oxazole derivatives was developed by Müller’s group [91,92] starting from acyl chlorides and propargyl amine. The first reaction consisted in an amidation of the acid chloride promoted by Et3N, followed by in situ Sonogashira cross-coupling of the generated alkynyl amide with another equivalent of RCOCl. Subsequent rapid cycloisomerization of the ynone derivative generated the oxazole ring (Scheme 43). The consecutive amidation–coupling–isomerization (ACCI) reactions proceeded with a large number of electronically and sterically diverse acid chlorides, under mild experimental conditions, affording the target compounds with good yields (49%–75%).
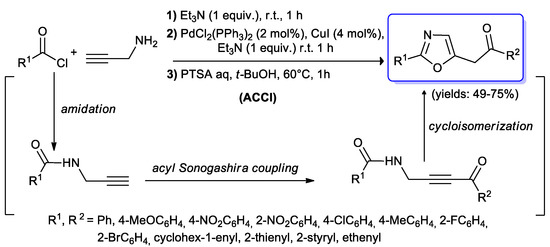
Scheme 43.
Synthesis of oxazole via an ACCI sequence involving an acyl Sonogashira coupling developed by Müller’s group.
The same research group extended [93] this methodology to a one-pot preparation of indolyl-oxazoles, potentially useful organic chromophores. In this case N-propynylacetamides were initially coupled with acid chlorides, and the acyl Sonogashira reaction was accelerated by using MW irradiation, while the amount of the catalytic system remained unchanged (2 mol % of PdCl2(PPh3)2 and 4 mol % of CuI). Alkynones were then cycloisomerized by an addition of PTSA and then oxazole intermediates were treated with hydrazine hydrochlorides, affording the desired products in moderate to good yields (20–58%).
Isoxazoles, constitutional isomers of oxazole derivatives, were obtained through a simple synthetic sequence described by Liu and co-workers [94]. The reactions were carried out in the same reaction vessel, and begun with a Sonogashira coupling of (hetero)aroyl chlorides with terminal aryl alkynes, performed in the presence of PdCl2(PPh3)2 (1 mol %) and CuI (3 mol %). After one hour at ambient temperature, the reaction mixture was treated with hydroxylamine and NaOAc, affording the expected isoxazoles (58%–76%) (Scheme 44).
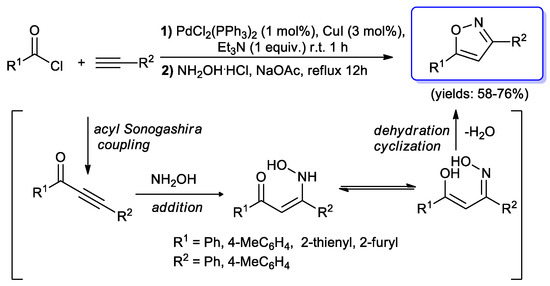
Scheme 44.
Synthesis of isoxazole by an acyl Sonogashira coupling–cyclocondensation sequence.
A similar approach was used by Müller and co-workers [95] who substituted the hydroxylamine addition step with the treatment of alkynyl ketones with hydroximino chloride. Hence, the optimized sequence involved an initial acyl Sonogashira reaction of variously substituted acid chlorides and terminal acetylenes catalyzed by PdCl2(PPh3)2/CuI (2–4 mol %), followed by an addition of N-hydroxy-4- methoxybenzimidoyl chloride to the obtained ynones in the same reaction vessel. Finally, after heating for 30 min under microwaves irradiation, the isoxazole was obtained in moderate to excellent yields (Scheme 34). Moreover, when ferrocenyl moieties were introduced on the acid chloride or on the acetylenic substrate (i.e., R1 and R2, Scheme 45), functionalized substituted redox active isoxazoles were generated [96].
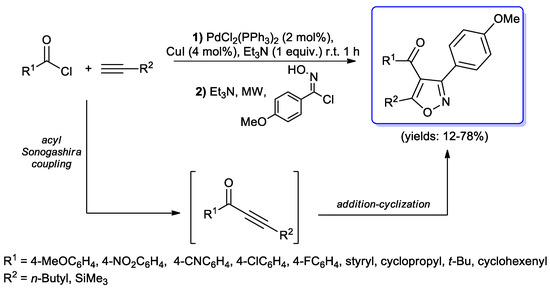
Scheme 45.
Synthesis of isoxazole via coupling–addition–cyclocondensation sequence.
Finally, Müller and Görgen reported the preparation of 3,5-disubstituted isoxazoles [97] through a coupling-azidation–cyclisation sequence (Scheme 46). The first step of the Sonogashira cross-coupling reaction between RCOCl and RC≡CH was initially performed with a classic PdCl2(PPh3)2/CuI catalytic system. Under these experimental conditions the azidation step afforded an enaminone instead of the expected isoxazole, probably due to the presence of Cu(I) in the reaction vessel. To overcome this problem, the authors decided to test PdCl2 as catalytic precursor in the presence of Beller’s ligand (di(1-adamantyl)-benzyl-phosphonium bromide). As a result, aryl–substituted isoxazole was exclusively obtained (10%–71%).
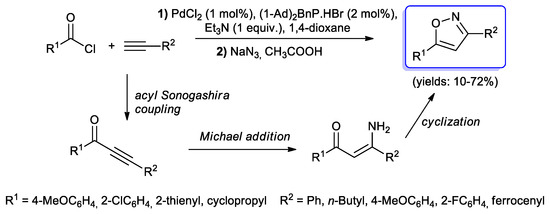
Scheme 46.
Synthesis of isoxazole via coupling–azidation–cyclocondensation one-pot reactions.
3.3. Synthesis of N,S-Heterocycles by Acyl Sonogashira Reactions
To our knowledge, only one example of benzothiazepines synthesis via a coupling–addition– cyclocondensation sequence has been described in the literature. Müller and Willy optimized [98] a one-pot protocol involving an initial acyl Sonogashira reaction performed under typical experimental conditions (PdCl2(PPh3)2/CuI, 2–4 mol %, 1 equiv. Et3N) which afforded the expected alkynones quantitatively.
The second step required a Michael addition of 2-amino-thiophenol which was performed under microwave heating and followed by a cyclocondensation reaction to give various 2,4-disubstituted benzo[b][1,5]thiazepines in isolated yields of 45%–77% (Scheme 47).
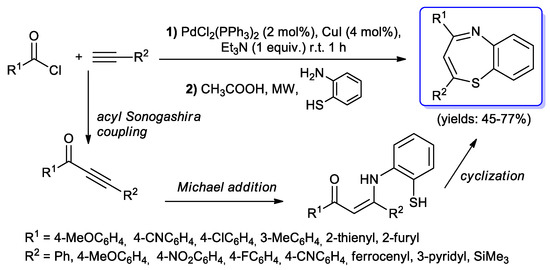
Scheme 47.
Synthesis of benzothiazepines by a three-steps process starting with an acyl Sonogashira coupling.
3.4. Synthesis of O-Heterocycles by Acyl Sonogashira Reactions
3.4.1. Synthesis of Five- and Six-Membered O-Containing Monocyclic Compounds
Polyfunctionalized furans were prepared by Müller and coll. by a three-steps, one-pot synthesis [99]. The sequence begun with an acyl Sonogashira reaction of RCOCl and THP-protected propynols, performed with a stoichiometric equivalent of Et3N, 2 mol % of PdCl2(PPh3)2 and CuI (4 mol %) as co-catalyst. The almost neutral experimental conditions allowed the treatment of the obtained ynones with para-tolylsulfonic acid in order to remove the protecting group, followed by an acid-assisted cyclocondensation of the 3-hydroxy alkynones with the formation of 3-halofurans (Scheme 48).
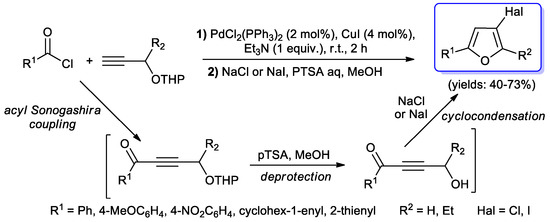
Scheme 48.
Synthesis of 3-halofurans via a one-pot protocol involving an acyl Sonogashira coupling.
Since the expected products were obtained with moderate yields, Müller’s group investigated [100] a three-steps sequence in order to improve the results. THF resulted to be the best solvent for all the processes which were performed at room temperature when NaI was used, while 50 °C for 20 h were requested when the cyclocondensation step was carried out with NaCl. Moreover, treatment of alkynol with a mixture of ICl/NaCl afforded 3-chloro-4-iodofurans in moderate to good yields (31–64%) (Scheme 49).
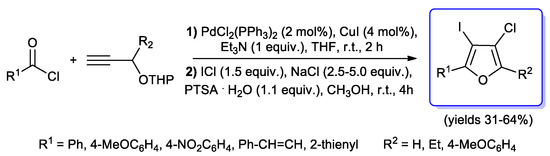
Scheme 49.
One-pot synthesis of 3-chloro-4-iodofurans starting with an acyl Sonogashira step reported in 2006 by Müller et al.
Recently Müller and Breuer [17] applied a similar multi-component sequence to the synthesis of α-pyrones, six-membered unsaturated lactones possessing a wide range of biological properties. The three steps synthesis started with a Sonogashira coupling of (hetero)aroyl chlorides and terminal alkynes, performed with a low amount of PdCl2(PPh3)2/CuI catalytic system (0.25%–0.5%), in 1,4-dioxane at room temperature. The obtained alkynones were treated in situ with two equivalents of malonates in the presence of Na2CO3·10H2O with the aim to induce a Michael addition–cyclocondensation sequence (Scheme 50). Electron-withdrawing and electron-donating groups could be introduced at the 4- and 6-position of the pyrone ring which was obtained in moderate to very good yields (29%–80%).
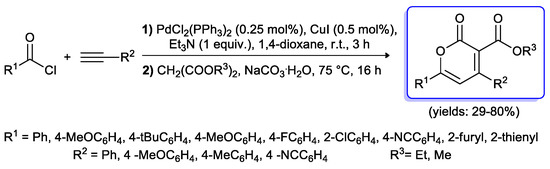
Scheme 50.
Synthesis of α-pyrones via Sonogashira acylation–Michael addition–cyclocondensation sequence developed by Müller and Breuer.
3.5. Synthesis of S-Heterocycles by Acyl Sonogashira Reactions
3.5.1. Synthesis of Five-Membered S-Heterocyclic Compounds
Thiophenes represent important building blocks for the synthesis of pharmaceutically-active molecules and π-conjugated fluorophores. In this field Müller and co-workers [101] developed a three-component process involving acid chlorides, terminal alkynes and ethyl mercapto-acetate to give 2,4-disubstituted thiophenes. The first step of the one-pot sequence was based on the acyl Sonogashira reaction promoted by of PdCl2(PPh3)2 (2 mol %) and CuI (4 mol %) in THF at room temperature. Subsequently the obtained alkynones were submitted to a Fiesselmann cyclocondensation with excess ethyl mercapto-acetate and DBU, affording the expected thiophenes in moderate to excellent yields (Scheme 51).
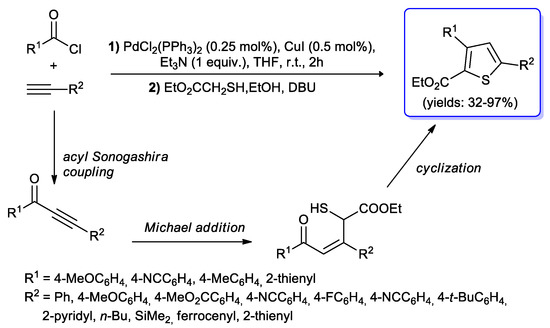
Scheme 51.
Synthesis of 2,4-disubstituted thiophenes by the Sonogashira–Fiesselmann cyclocondensation sequence.
This methodology was extended [102] to the preparation of polycyclic π-conjugated oligomers with benzene or thiophene as the central core. In these cases, terephthaloylchloride or thiophene 2,5-dicarbonyl chloride were reacted with different RC≡CH and the double ynones were treated in situ with EtO2CCH2SH furnishing the dumbbells structures depicted in Figure 9a; analogously, 1,4-diethynylbenzene and 2,5-diethynylthiophene were acylated and cyclocondensated to give the p-phenylene and oligothiophene symmetrical molecules represented in Figure 9b. In both cases, the Sonogashira step required large amount of PdCl2(PPh3)2 (8 mol %) and CuI (16 mol %).
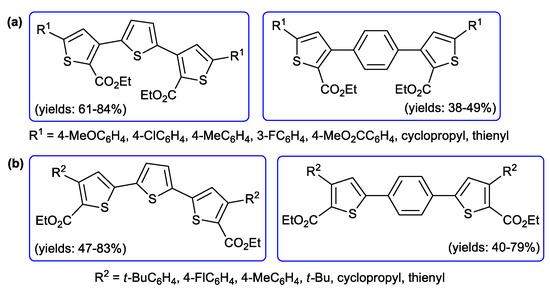
Figure 9.
Polycyclic π-conjugated oligomers containing benzene or thiophene as the central core synthesized by Müller and co-workers.
The synthetic route optimized by Müller et al. was used by Tang’s group [103] to generate linear polyarylene thiophenylenes by acyl Sonogashira cross-coupling of terephthaloylchloride with diyne derivatives of 1,1,2,2-tetraphenylethylene. Polyinones were not isolated, but directly reacted with EtO2CCH2SH, undergoing Fiesselmann cyclocondensation in excellent yields (86%–96%).
A completely different approach to the synthesis of thiophenes was developed by Zhou and co-workers [104]. Their strategy was based on an initial acyl Sonogashira coupling between acid chlorides and suitable alkynylallylsulfurs catalyzed by the PdCl2(PPh3)2/CuI system, followed by a 1,3-H shift to form an electron-deficient allene which underwent intramolecular nucleophilic attack by the sulfur and final sigmatropic rearrangement. Thus several thiophenyl and benzothiophenyl ketones were obtained in good yields (64%–81%) (Scheme 52).
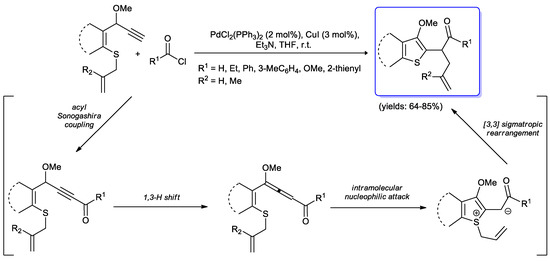
Scheme 52.
Synthesis of thiophenyl and benzothiophenyl derivatives by an initial acyl Sonogashira coupling developed by Zhou and co-workers.
3.5.2. Synthesis of Six-Membered S-Heterocyclic Compounds
A multi-steps synthesis of annelated 4H-thiopyranones was achieved by the Müller’s group [105,106] starting from 2-halobenzoyl chlorides and terminal alkynes under typical acyl Sonogashira conditions (i.e., PdCl2(PPh3)2 2 mol % and CuI 4 mol %). The alkynones thus formed were reacted in situ with sodium sulfide nonahydrate and ethanol, at 90 °C under MW irradiation. Michael addition of Na2S to the ynones followed by intramolecular SNAr reactions generated a thiopyranone core fused to benzene, pyridine, thiophene and benzothiophene rings (Scheme 53). Both aromatic and aliphatic acetylenes could be successfully employed, and fluorine was found to be a better leaving group instead of chlorine.
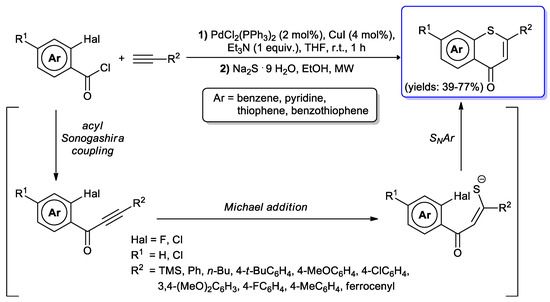
Scheme 53.
Consecutive acid Sonogashira coupling-addition-SNAr synthesis of thiopyranones.
4. Conclusions
Metal-catalyzed C–C bond formation between an acid chloride and a terminal alkyne is the basis of the acyl Sonogashira reaction. Different palladium- and/or copper-based catalytic systems have been developed to enhance the efficiency of this method. This process tolerates the presence of many functional groups (–OR, –COOR, –NO2, –CN, –NHAc, –F, –Cl, –Br, –SiMe3), and results in structurally modulated products, depending on the choice of two coupling partners whereas the obtained α,β-alkynyl ketones can be easily transformed into different heterocyclic compounds.
Considering all these important features, we hope that this review will stimulate further research in the field of the acyl Sonogashira reactions and its application to the formation of new biologically relevant heterocycles.
Author Contributions
Manuscript outline, L.A.A.; bibliographic material selection, G.A. and L.A.A.; manuscript writing—original draft preparation, review and editing, G.A. and L.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Doucet, H.; Hierso, J.-C. Palladium-Based Catalytic Systems for the Synthesis of Conjugated Enynes by Sonogashira Reactions and Related Alkynylations. Angew. Chem. Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. A General and Convenient Palladium-Catalyzed Carbonylative Sonogashira Coupling of Aryl Bromides. Chem. Eur. J. 2010, 16, 12104–12107. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009. [Google Scholar] [CrossRef] [PubMed]
- Genelot, M.; Dufaud, V.; Djakovitch, L. Carbonylative Sonogashira Coupling in the Synthesis of Ynones: A Study of “Boomerang” Phenomena. Adv. Synth. Catal. 2013, 355, 2604–2616. [Google Scholar] [CrossRef]
- Gadge, S.T.; Bhanage, B.M. Recent developments in palladium catalysed carbonylation reactions. RSC Adv. 2014, 4, 10367–10389. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. Consecutive multi-component syntheses of heterocycles via palladium-copper catalyzed generation of alkynones. ARKIVOC 2008, 2008, 195–208. [Google Scholar]
- Willy, B.; Muller, T.J.J. Multi-component Heterocycle Syntheses via Catalytic Generation of Alkynones. Curr. Org. Chem. 2009, 13, 1777–1790. [Google Scholar] [CrossRef]
- Whittaker, R.E.; Dermenci, A.; Dong, G. Synthesis of Ynones and Recent Application in Transition-Metal-Catalyzed Reactions. Synthesis 2016, 48, 161–183. [Google Scholar]
- Gers-Panther, C.F.; Müller, T.J.J. Multicomponent Syntheses of Heterocycles Initiated by Catalytic Generation of Ynones and Ynediones. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 120, pp. 67–98. [Google Scholar]
- Nájera, C.; Sydnes, L.K.; Yus, M. Conjugated Ynones in Organic Synthesis. Chem. Rev. 2019, 119, 11110–11244. [Google Scholar] [CrossRef] [PubMed]
- Tohda, Y.; Sonogashira, K.; Hagihara, N. A Convenient Synthesis of 1-Alkynyl Ketones and 2-Alkynamides. Synthesis 1977, 1977, 777–778. [Google Scholar] [CrossRef]
- Cox, R.J.; Ritson, D.J.; Dane, T.A.; Berge, J.; Charmant, J.P.H.; Kantacha, A. Room temperature palladium catalysed coupling of acyl chlorides with terminal alkynes. Chem. Commun. 2005, 1037–1039. [Google Scholar] [CrossRef]
- Kumar, B.; Maity, J.; Kumar, A.; Khatri, V.; Shankar, B.; Prasad, A.K. Synthesis of novel 3-[(1-glycosyl-1H-1,2,3-triazol-4-yl)- methylamino]ket-2-en-1-ones. Chem. Heterocycl. Compd. 2018, 54, 362–368. [Google Scholar] [CrossRef]
- Breuer, N.; Müller, T.J.J. Consecutive Alkynylation–Michael Addition–Cyclocondensation (AMAC) Multicomponent Syntheses of α-Pyrones and α-Pyridones. Synthesis 2018, 50, 2741–2752. [Google Scholar]
- Albano, G.; Aronica, L.A.; Biver, T.; Detti, R.; Pucci, A. Tris-Ethynylphenyl-amine Fluorophores: Synthesis, Characterisation and Test of Performances in Luminescent Solar Concentrators. Chem. Sel. 2018, 3, 1749–1754. [Google Scholar] [CrossRef]
- Albano, G.; Colli, T.; Nucci, L.; Charaf, R.; Biver, T.; Pucci, A.; Aronica, L.A. Synthesis of new bis[1-(thiophenyl)propynones] as potential organic dyes for colorless luminescent solar concentrators (LSCs). Dyes Pigm. 2019. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.-J. A Remarkably Efficient Coupling of Acid Chlorides with Alkynes in Water. Org. Lett. 2004, 6, 3151–3153. [Google Scholar] [CrossRef]
- Ma, C.; Ren, Y.; Zhang, Q.; Ding, K.; Zhao, J.; Zhang, D. A Novel One-pot Synthesis of Pyrroloquinoline and Pyrroloisoquinoline Derivatives in Ionic Liquid. Chem. Lett. 2007, 36, 1152–1153. [Google Scholar] [CrossRef]
- Atobe, S.; Masuno, H.; Sonoda, M.; Suzuki, Y.; Shinohara, H.; Shibata, S.; Ogawa, A. Pd-catalyzed coupling reaction of acid chlorides with terminal alkynes using 1-(2-pyridylethynyl)-2-(2-thienylethynyl)benzene ligand. Tetrahedron Lett. 2012, 53, 1764–1767. [Google Scholar] [CrossRef]
- Karpov, A.S.; Müller, T.J.J. New Entry to a Three-Component Pyrimidine Synthesis by TMS−Ynones via Sonogashira Coupling. Org. Lett. 2003, 5, 3451–3454. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.M.; Müller, T.J.J. Catalytic alkynone generation by Sonogashira reaction and its application in three-component pyrimidine synthesis. Nat. Protoc. 2008, 3, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Friscourt, F.; Boons, G.-J. One-Pot Three-Step Synthesis of 1,2,3-Triazoles by Copper-Catalyzed Cycloaddition of Azides with Alkynes formed by a Sonogashira Cross-Coupling and Desilylation. Org. Lett. 2010, 12, 4936–4939. [Google Scholar] [CrossRef]
- Hwang, S.; Bae, H.; Kim, S.; Kim, S. An efficient and high-yielding one-pot synthesis of 4-acyl-1,2,3-triazoles via triisopropylsilyl-protected ynones. Tetrahedron 2012, 68, 1460–1465. [Google Scholar] [CrossRef]
- Niesobski, P.; Klukas, F.; Berens, H.; Makhloufi, G.; Janiak, C.; Müller, T.J.J. De Novo Ring-Forming Consecutive Four-Component Syntheses of 4-Pyrazolyl-1,2,3-triazoles from (Triisopropylsilyl)butadiyne as a C4 Building Block. J. Org. Chem. 2018, 83, 4851–4858. [Google Scholar] [CrossRef]
- Cheremnykh, K.P.; Savel’ev, V.A.; Shkurko, O.P.; Shults, E.E. Synthesis of hybrid molecules containing pyrimidine and diterpene alkaloid lappaconitine fragments. Chem. Heterocycl. Compd. 2018, 54, 1131–1138. [Google Scholar] [CrossRef]
- Mailyan, A.K.; Peregudov, A.S.; Dixneuf, P.H.; Bruneau, C.; Osipov, S.N. Cyclobutene Ring-Opening of Bicyclo[4.2.0]octa-1,6-dienes: Access to CF3-Substituted 5,6,7,8-Tetrahydro-1,7-naphthyridines. J. Org. Chem. 2012, 77, 8518–8526. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Liang, Y.; Wu, X.; Chen, B.; Ma, Y. Synthesis of Ferrocenylethynyl Ketones by Coupling of Ferrocenylethyne with Acyl Chlorides. Synthesis 2004, 2004, 331–333. [Google Scholar] [CrossRef]
- Md Tohid, S.F.; Ziedan, N.I.; Stefanelli, F.; Fogli, S.; Westwell, A.D. Synthesis and evaluation of indole-containing 3,5-diarylisoxazoles as potential pro-apoptotic antitumour agents. Eur. J. Med. Chem. 2012, 56, 263–270. [Google Scholar] [CrossRef]
- Götzinger, A.C.; Müller, T.J.J. Rapid access to unsymmetrical tolanes and alkynones by sequentially palladium-catalyzed one-pot processes. Org. Biomol. Chem. 2016, 14, 3498–3500. [Google Scholar] [CrossRef] [PubMed]
- Chuang, D.-W.; El-Shazly, M.; Balaji, D.B.; Chung, Y.-M.; Chang, F.-R.; Wu, Y.-C. Synthesis of Flavones and γ-Benzopyranones Using Mild Sonogashira Coupling and 18-Crown-6 Ether Mediated 6-endo Cyclization. Eur. J. Org. Chem. 2012, 2012, 4533–4540. [Google Scholar] [CrossRef]
- Boersch, C.; Merkul, E.; Müller, T.J.J. Catalytic Syntheses of N-Heterocyclic Ynones and Ynediones by In Situ Activation of Carboxylic Acids with Oxalyl Chloride. Angew. Chem. Int. Ed. 2011, 50, 10448–10452. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wu, H.; Jian, J.; Wang, H.; Liu, C.; Daniel, S.; Zeng, Z. Palladium-catalyzed Sonogashira coupling of amides: Access to ynones via C–N bond cleavage. Chem. Commun. 2016, 52, 12076–12079. [Google Scholar] [CrossRef]
- Palimkar, S.S.; Kumar, P.H.; Jogdand, N.R.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Copper-, ligand- and solvent-free synthesis of ynones by coupling acid chlorides with terminal alkynes. Tetrahedron Lett. 2006, 47, 5527–5530. [Google Scholar] [CrossRef]
- Palimkar, S.S.; More, V.S.; Srinivasan, K.V. Simple and Efficient One-Pot, Three-Component, Solvent-Free Synthesis of β-Enaminones via Sonogashira Coupling–Michael Addition Sequences. Synth. Commun. 2008, 38, 1456–1469. [Google Scholar] [CrossRef]
- Hoshi, M.; Yamazaki, H.; Okimoto, M. One-Pot Synthesis of Conjugated (E)-Enynones via Two Types of Cross-Coupling Reaction. Synlett 2010, 2010, 2461–2464. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Schou, S.C.; Sedelmeier, J.; Ley, S.V. Multi-Step Synthesis by Using Modular Flow Reactors: The Preparation of Yne Ones and Their Use in Heterocycle Synthesis. Chem. Eur. J. 2010, 16, 89–94. [Google Scholar] [CrossRef]
- Alonso, D.A.; Nájera, C.; Pacheco, M.C. Synthesis of Ynones by Palladium-Catalyzed Acylation of Terminal Alkynes with Acid Chlorides. J. Org. Chem. 2004, 69, 1615–1619. [Google Scholar] [CrossRef]
- Debono, N.; Canac, Y.; Duhayon, C.; Chauvin, R. An Atropo-Stereogenic Diphosphane Ligand with a Proximal Cationic Charge: Specific Catalytic Properties of a Palladium Complex Thereof. Eur. J. Inorg. Chem. 2008, 2008, 2991–2999. [Google Scholar] [CrossRef]
- Bakherad, M.; Amin, A.H.; Keivanloo, A.; Bahramian, B.; Raessi, M. Using Pd–salen complex as an efficient catalyst for the copper- and solvent-free coupling of acyl chlorides with terminal alkynes under aerobic conditions. Chin. Chem. Lett. 2010, 21, 656–660. [Google Scholar] [CrossRef]
- Ryabukhin, D.S.; Sorokoumov, V.N.; Savicheva, E.A.; Boyarskiy, V.P.; Balova, I.A.; Vasilyev, A.V. Catalytic activity of palladium acyclic diaminocarbene complexes in the synthesis of 1,3-diarylpropynones via Sonogashira reaction: Cross- versus homo-coupling. Tetrahedron Lett. 2013, 54, 2369–2372. [Google Scholar] [CrossRef]
- Islas, R.E.; Cárdenas, J.; Gaviño, R.; García-Ríos, E.; Lomas-Romero, L.; Morales-Serna, J.A. Phosphinito palladium(ii) complexes as catalysts for the synthesis of 1,3-enynes, aromatic alkynes and ynones. RSC Adv. 2017, 7, 9780–9789. [Google Scholar] [CrossRef]
- Likhar, P.R.; Subhas, M.S.; Roy, M.; Roy, S.; Kantam, M.L. Copper-Free Sonogashira Coupling of Acid Chlorides with Terminal Alkynes in the Presence of a Reusable Palladium Catalyst: An Improved Synthesis of 3-Iodochromenones (=3-Iodo-4H-1-benzopyran-4-ones). Helv. Chim. Acta 2008, 91, 259–264. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.K.; Mandadapu, A.K.; Gauniyal, H.M.; Kundu, B. Three-component tandem reaction involving acid chlorides, terminal alkynes, and ethyl 2-amino-1H-indole-3-carboxylates: Synthesis of highly diversified pyrimido[1,2-a]indoles via sequential Sonogashira and [3 + 3] cyclocondensation reactions. Tetrahedron Lett. 2011, 52, 4288–4291. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Synthesis of 4,5-disubstituted-1H-1,2,3-triazoles. Mon. Chem. 2019, 150, 1479–1486. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Bahramian, B.; Kalantar, Z.; Ashrafi, N.F. Coupling Reaction of Acid Chlorides with Terminal Alkynes Catalyzed by Diatomite-Supported Palladium(II) Salophen Complex. Lett. Org. Chem. 2011, 8, 364–367. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Bahramian, B.; Rajaie, M. A copper- and solvent-free coupling of acid chlorides with terminal alkynes catalyzed by a polystyrene-supported palladium(0) complex under aerobic conditions. Tetrahedron Lett. 2010, 51, 33–35. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Lin, T.-C.; Chen, S.-C.; Chen, A.-J.; Mou, C.-Y.; Tsai, F.-Y. Highly-efficient and recyclable nanosized MCM-41 anchored palladium bipyridyl complex-catalyzed coupling of acyl chlorides and terminal alkynes for the formation of ynones. Tetrahedron 2009, 65, 10134–10141. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, M.; Gaur, R.; Gupta, R.; Adholeya, A.; Gawande, M.B. Synthesis of Iron Oxide Palladium Nanoparticles and Their Catalytic Applications for Direct Coupling of Acyl Chlorides with Alkynes. ChemPlusChem 2016, 81, 1312–1319. [Google Scholar] [CrossRef]
- Hossain, S.; Park, J.-H.; Park, M.-K.; Jin, M.-J. Silica gel-Supported Palladium Catalyst for the Acyl Sonogashira Reaction. J. Korean Chem. Soc. 2013, 57, 411–415. [Google Scholar] [CrossRef]
- Zhao, F.; Shirai, M.; Ikushima, Y.; Arai, M. The leaching and re-deposition of metal species from and onto conventional supported palladium catalysts in the Heck reaction of iodobenzene and methyl acrylate in N-methylpyrrolidone. J. Mol. Catal. A Chem. 2002, 180, 211–219. [Google Scholar] [CrossRef]
- Albano, G.; Interlandi, S.; Evangelisti, C.; Aronica, L.A. Polyvinylpyridine-Supported Palladium Nanoparticles: A Valuable Catalyst for the Synthesis of Alkynyl Ketones via Acyl Sonogashira Reactions. Catal. Lett. 2019. [Google Scholar] [CrossRef]
- Santra, S.; Dhara, K.; Ranjan, P.; Bera, P.; Dash, J.; Mandal, S.K. A supported palladium nanocatalyst for copper free acyl Sonogashira reactions: One-pot multicomponent synthesis of N-containing heterocycles. Green Chem. 2011, 13, 3238–3247. [Google Scholar] [CrossRef]
- Santra, S.; Ranjan, P.; Bera, P.; Ghosh, P.; Mandal, S.K. Anchored palladium nanoparticles onto single walled carbon nanotubes: Efficient recyclable catalyst for N-containing heterocycles. RSC Adv. 2012, 2, 7523–7533. [Google Scholar] [CrossRef]
- Loganathan, R.K.; Ramachandra, S.N.; Shekharappa; Sureshbabu, V.V. Montmorillonite K-10-Supported Palladium Nanoparticles for Copper-Free Acyl Sonogashira Reaction. Chem. Sel. 2017, 2, 8059–8062. [Google Scholar] [CrossRef]
- Chowdhury, C.; Kundu, N.G. Copper(I)-catalysed acylation of terminal alkynes. Tetrahedron Lett. 1996, 37, 7323–7324. [Google Scholar] [CrossRef]
- Chowdhury, C.; Kundu, N.G. Studies on copper(I) catalysed cross-coupling reactions: A convenient and facile method for the synthesis of diversely substituted α,β-acetylenic ketones. Tetrahedron 1999, 55, 7011–7016. [Google Scholar] [CrossRef]
- Wang, J.-X.; Wei, B.; Hu, Y.; Liu, Z.; Fu, Y. Microwave-assisted coupling reaction by copper(I)-catalyzed of terminal alkynes with aroyl chlorides. Synth. Commun. 2001, 31, 3527–3532. [Google Scholar] [CrossRef]
- Yin, W.; He, H.; Zhang, Y.; Luo, D.; He, H. A Highly Active CuI/TMEDA Catalytic System for the Coupling Reaction of Acid Chlorides with Terminal Alkynes under Solvent-Free Conditions. Synthesis 2014, 46, 2617–2621. [Google Scholar] [CrossRef][Green Version]
- Mohammadi, E.; Movassagh, B.; Navidi, M. Palladium- and Solvent-Free Synthesis of Ynones by Copper(I)-Catalyzed Acylation of Terminal Alkynes with Acyl Chlorides under Aerobic Conditions. Helv. Chim. Acta 2014, 97, 70–75. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Wu, X.; Yao, X. Palladium-, ligand-, and solvent-free synthesis of ynones by the coupling of acyl chlorides and terminal alkynes in the presence of a reusable copper nanoparticle catalyst. Green Chem. 2013, 15, 2356–2360. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Sasaki, T.; Bhanage, B.M. A facile and rapid route for the synthesis of Cu/Cu2O nanoparticles and their application in the Sonogashira coupling reaction of acyl chlorides with terminal alkynes. Catal. Sci. Technol. 2014, 4, 4274–4280. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Zhao, W.; Cao, L.; Sun, Z.; Zhang, F. A facile synthesis of copper nanoparticles supported on an ordered mesoporous polymer as an efficient and stable catalyst for solvent-free sonogashira coupling Reactions. Green Chem. 2017, 19, 1949–1957. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.W.; Kim, A.; Yusuf, M.; Park, J.C.; Park, K.H. A highly efficient nano-sized Cu2O/SiO2 egg-shell catalyst for C–C coupling reactions. RSC Adv. 2018, 8, 6200–6205. [Google Scholar] [CrossRef]
- Matloubi Moghaddam, F.; Pourkaveh, R.; Ahangarpour, M. Nano CoCuFe2O4 catalyzed coupling reaction of acid chlorides with terminal alkynes: A powerful toolbox for palladium-free ynone synthesis. Catal. Commun. 2017, 102, 71–75. [Google Scholar] [CrossRef]
- Merkul, E.; Boersch, C.; Frank, W.; Müller, T.J.J. Three-Component Synthesis of N-Boc-4-iodopyrroles and Sequential One-Pot Alkynylation. Org. Lett. 2009, 11, 2269–2272. [Google Scholar] [CrossRef]
- Nordmann, J.; Müller, T.J.J. A one-pot coupling–addition–cyclocondensation sequence (CACS) to 2-substituted 3-acylpyrroles initiated by a copper-free alkynylation. Org. Biomol. Chem. 2013, 11, 6556–6561. [Google Scholar] [CrossRef]
- Liu, H.-L.; Jiang, H.-F.; Zhang, M.; Yao, W.-J.; Zhu, Q.-H.; Tang, Z. One-pot three-component synthesis of pyrazoles through a tandem coupling-cyclocondensation sequence. Tetrahedron Lett. 2008, 49, 3805–3809. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. Regioselective Three-Component Synthesis of Highly Fluorescent 1,3,5-Trisubstituted Pyrazoles. Eur. J. Org. Chem. 2008, 2008, 4157–4168. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. Rapid One-Pot, Four-Step Synthesis of Highly Fluorescent 1,3,4,5-Tetrasubstituted Pyrazoles. Org. Lett. 2011, 13, 2082–2085. [Google Scholar] [CrossRef] [PubMed]
- Denißen, M.; Nordmann, J.; Dziambor, J.; Mayer, B.; Frank, W.; Müller, T.J.J. Sequential palladium catalyzed coupling–cyclocondensation–coupling (C3) four-component synthesis of intensively blue luminescent biarylsubstituted pyrazoles. RSC Adv. 2015, 5, 33838–33854. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, F.; Yin, L.; Cai, M. A highly efficient heterogeneous palladium-catalyzed cascade three-component reaction of acid chlorides, terminal alkynes and hydrazines leading to pyrazoles. J. Organomet. Chem. 2016, 804, 108–113. [Google Scholar] [CrossRef]
- Thirukovela, N.S.; Balaboina, R.; Botla, V.; Vadde, R.; Jonnalagadda, S.B.; Vasam, C.S. One-pot regioselective synthesis of substituted pyrazoles and isoxazoles in PEG-400/water medium by Cu-free nano-Pd catalyzed sequential acyl Sonogashira coupling–intramolecular cyclization. Catal. Sci. Technol. 2019, 9, 6471–6481. [Google Scholar] [CrossRef]
- Keivanloo, A.; Bakherad, M.; Samangooei, S. Synthesis of 3,5-Disubstituted-1H-Pyrazoles from Acid Chlorides, Alkynes, and Hydrazine in the Presence of Silica-Supported-Zinc Bromide. J. Chem. Res. 2015, 39, 484–486. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Zhang, Y.; Li, J.; Chen, B. Facile One-Pot Synthesis of 4,5-Disubstituted 1,2,3-(NH)-Triazoles through Sonogashira Coupling/1,3-Dipolar Cycloaddition of Acid Chlorides, Terminal Acetylenes, and Sodium Azide. Org. Lett. 2009, 11, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Dohe, J.; Müller, T.J.J. Consecutive three- and four-component coupling-Bagley-Bohlmann-Rahtz syntheses of tri- and tetrasubstituted pyridines. Z. Naturforsch. B Chem. Sci. 2016, 71, 705–718. [Google Scholar] [CrossRef]
- Nordmann, J.; Breuer, N.; Müller, T.J.J. Efficient Consecutive Four-Component Synthesis of 5-Acylpyrid-2-ones Initiated by Copper-Free Alkynylation. Eur. J. Org. Chem. 2013, 2013, 4303–4310. [Google Scholar] [CrossRef]
- Nordmann, J.; Müller, T.J.J. Anilines as Substrates in Consecutive Four-Component Synthesis of Novel 1-Aryl-5-benzoyl-6-phenyl-3,4-dihydropyridin-2(1H)-ones. Synthesis 2014, 46, 522–530. [Google Scholar] [CrossRef]
- Karpov, A.S.; Müller, T.J.J. Straightforward Novel One-Pot Enaminone and Pyrimidine Syntheses by Coupling-Addition-Cyclocondensation Sequences. Synthesis 2003, 2003, 2815–2826. [Google Scholar] [CrossRef]
- Gao, G.-L.; Niu, Y.-N.; Yan, Z.-Y.; Wang, H.-L.; Wang, G.-W.; Shaukat, A.; Liang, Y.-M. Unexpected Domino Reaction via Pd-Catalyzed Sonogashira Coupling of Benzimidoyl Chlorides with 1,6-Enynes and Cyclization To Synthesize Quinoline Derivatives. J. Org. Chem. 2010, 75, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Rotzoll, S.; Willy, B.; Schönhaber, J.; Rominger, F.; Müller, T.J.J. Regiospecific Three-Component Access to Fluorescent 2,4-Disubstituted Quinolines via One-Pot Coupling-Addition-Cyclocondensation-Sulfur Extrusion Sequence. Eur. J. Org. Chem. 2010, 2010, 3516–3524. [Google Scholar] [CrossRef]
- Gers, C.F.; Nordmann, J.; Kumru, C.; Frank, W.; Müller, T.J.J. Solvatochromic Fluorescent 2-Substituted 3-Ethynyl Quinoxalines: Four-Component Synthesis, Photophysical Properties, and Electronic Structure. J. Org. Chem. 2014, 79, 3296–3310. [Google Scholar] [CrossRef] [PubMed]
- Palimkar, S.S.; Lahoti, R.J.; Srinivasan, K.V. A novel one-pot three-component synthesis of 2,4-disubstituted-3 H-benzo[b][1,4]diazepines in water. Green Chem. 2007, 9, 146–152. [Google Scholar] [CrossRef]
- Willy, B.; Dallos, T.; Rominger, F.; Schönhaber, J.; Müller, T.J.J. Three-Component Synthesis of Cryofluorescent 2,4-Disubstituted 3H-1,5-Benzodiazepines—Conformational Control of -Emission Properties. Eur. J. Org. Chem. 2008, 2008, 4796–4805. [Google Scholar] [CrossRef]
- Rotaru, A.V.; Druta, I.D.; Oeser, T.; Müller, T.J.J. A Novel Coupling 1,3-Dipolar Cycloaddition Sequence as a Three-Component Approach to Highly Fluorescent Indolizines. Helv. Chim. Acta 2005, 88, 1798–1812. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, B.; Kundu, B. Three-Component Tandem Reaction Involving Acid Chlorides, Terminal Alkynes, and 2-Aminoindole Hydrochlorides: Synthesis of α-Carboline Derivatives in Aqueous Conditions via Regioselective [3 + 3] Cyclocondensation. J. Org. Chem. 2011, 76, 10154–10162. [Google Scholar] [CrossRef]
- Karpov, A.S.; Oeser, T.; Müller, T.J.J. A novel one-pot four-component access to tetrahydro-β-carbolines by a coupling-amination-aza-annulation-Pictet–Spengler sequence (CAAPS). Chem. Commun. 2004, 35, 1502–1503. [Google Scholar] [CrossRef]
- Karpov, A.S.; Rominger, F.; Müller, T.J.J. A diversity oriented four-component approach to tetrahydro-β-carbolines initiated by Sonogashira coupling. Org. Biomol. Chem. 2005, 3, 4382–4391. [Google Scholar] [CrossRef]
- Merkul, E.; Müller, T.J.J. A new consecutive three-component oxazole synthesis by an amidation–coupling–cycloisomerization (ACCI) sequence. Chem. Commun. 2006, 4817–4819. [Google Scholar] [CrossRef]
- Merkul, E.; Grotkopp, O.; Müller, T.J.J. 2-Oxazol-5-ylethanones by Consecutive Three-Component Amidation-Coupling-Cycloisomerization (ACCI) Sequence. Synthesis 2009, 2009, 502–507. [Google Scholar] [CrossRef]
- Grotkopp, O.; Ahmad, A.; Frank, W.; Müller, T.J.J. Blue-luminescent 5-(3-indolyl)oxazoles via microwave-assisted three-component coupling–cycloisomerization–Fischer indole synthesis. Org. Biomol. Chem. 2011, 9, 8130–8140. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Geng, Z.-F.; Zhang, S.-Y.; Han, J. One-Pot Three-Component Synthesis of 3,5-Disubstituted Isoxazoles by a Coupling-Cyclocondensation Sequence. Heterocycles 2014, 89, 1221–1227. [Google Scholar] [CrossRef]
- Willy, B.; Rominger, F.; Müller, T.J.J. Novel Microwave-Assisted One-Pot Synthesis of Isoxazoles by a Three-Component Coupling-Cycloaddition Sequence. Synthesis 2008, 2008, 293–303. [Google Scholar] [CrossRef]
- Willy, B.; Frank, W.; Rominger, F.; Müller, T.J.J. One-pot three-component synthesis, structure and redox properties of ferrocenyl isoxazoles. J. Organomet. Chem. 2009, 694, 942–949. [Google Scholar] [CrossRef]
- Görgen, C.; Müller, T.J.J. Facile consecutive three-component synthesis of 3,5-disubstituted isoxazoles. Chem. Heterocycl. Compd. 2017, 53, 422–429. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. Three-component synthesis of benzo[b][1,5]thiazepines via coupling–addition–cyclocondensation sequence. Mol. Divers. 2010, 14, 443–453. [Google Scholar] [CrossRef]
- Karpov, A.S.; Merkul, E.; Oeser, T.; Müller, T.J.J. A novel one-pot three-component synthesis of 3-halofurans and sequential Suzuki coupling. Chem. Commun. 2005, 2581–2583. [Google Scholar] [CrossRef]
- Karpov, A.S.; Merkul, E.; Oeser, T.; Müller, T.J.J. One-Pot Three-Component Synthesis of 3-Halofurans and 3-Chloro-4-iodofurans. Eur. J. Org. Chem. 2006, 2006, 2991–3000. [Google Scholar] [CrossRef]
- Teiber, M.; Müller, T.J.J. Rapid consecutive three-component coupling-Fiesselmann synthesis of luminescent 2,4-disubstituted thiophenes and oligothiophenes. Chem. Commun. 2012, 48, 2080–2082. [Google Scholar] [CrossRef]
- Teiber, M.; Giebeler, S.; Lessing, T.; Müller, T.J.J. Efficient pseudo-five-component coupling-Fiesselmann synthesis of luminescent oligothiophenes and their modification. Org. Biomol. Chem. 2013, 11, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Hu, R.; Zhao, E.; Chan, C.Y.K.; Lam, J.W.Y.; Tang, B.Z. One-Pot Three-Component Tandem Polymerization Toward Functional Poly(arylene thiophenylene) with Aggregation-Enhanced Emission Characteristics. Macromolecules 2014, 47, 4920–4929. [Google Scholar] [CrossRef]
- Chen, D.; Xing, G.; Yao, J.; Zhou, H. Applicable β-sulfonium carbanions: Facile construction of thiophene derivatives. Org. Chem. Front. 2017, 4, 1042–1045. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. A Novel Consecutive Three-Component Coupling-Addition-SNAr (CASNAR) Synthesis of 4H-Thiochromen-4-ones. Synlett 2009, 2009, 1255–1260. [Google Scholar] [CrossRef]
- Willy, B.; Frank, W.; Müller, T.J.J. Microwave-assisted three-component coupling-addition-SNAr (CASNAR) sequences to annelated 4H-thiopyran-4-ones. Org. Biomol. Chem. 2010, 8, 90–95. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).