Influence of Impurities in a Methanol Solvent on the Epoxidation of Propylene with Hydrogen Peroxide over Titanium Silicalite-1
Abstract
1. Introduction
2. Results
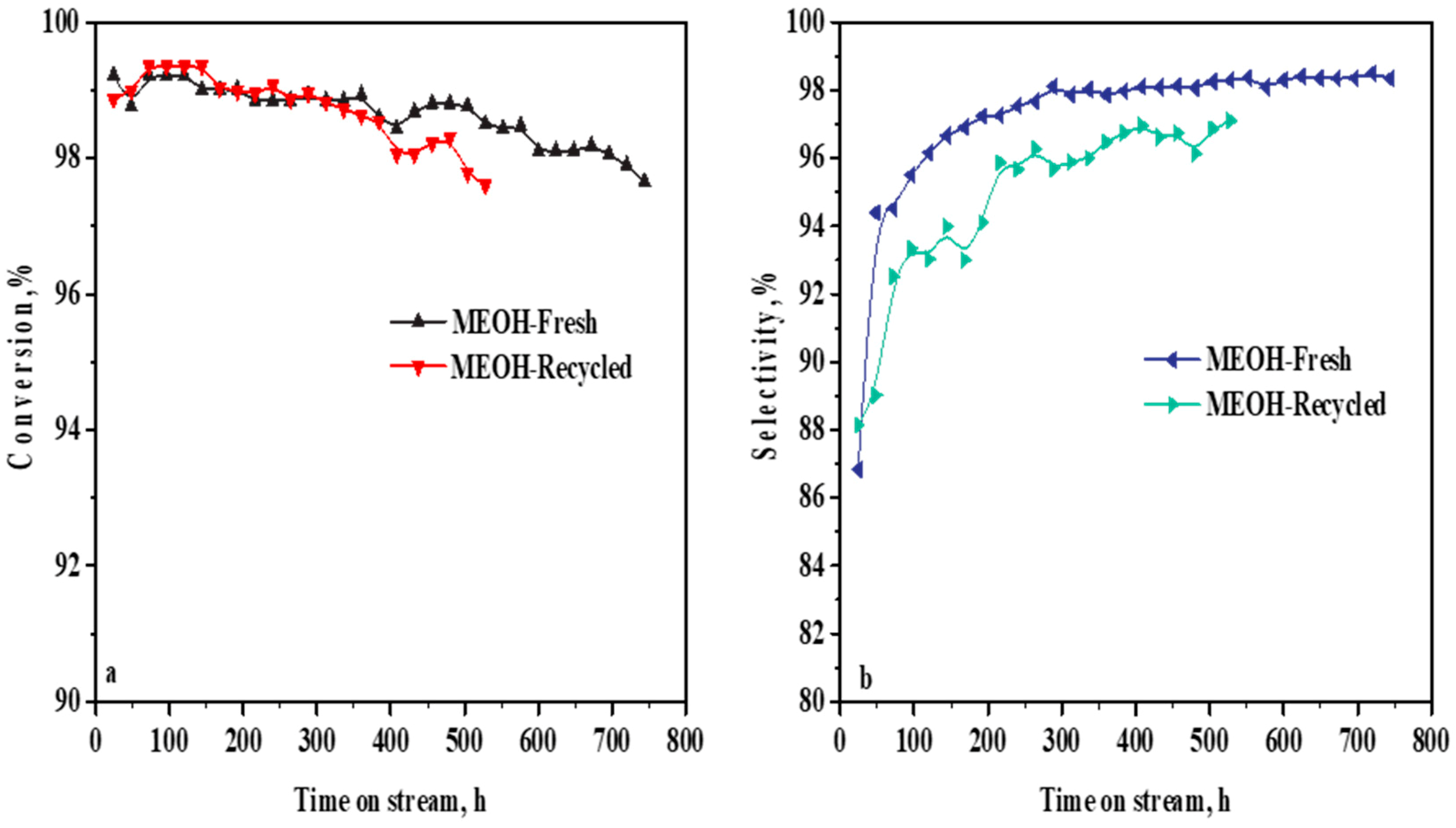
2.1. Liquid-Phase Epoxidation with Fresh and Recycled Methanol Solvents
2.2. Influence of Fusel Alcohol Content in a Methanol Solvent
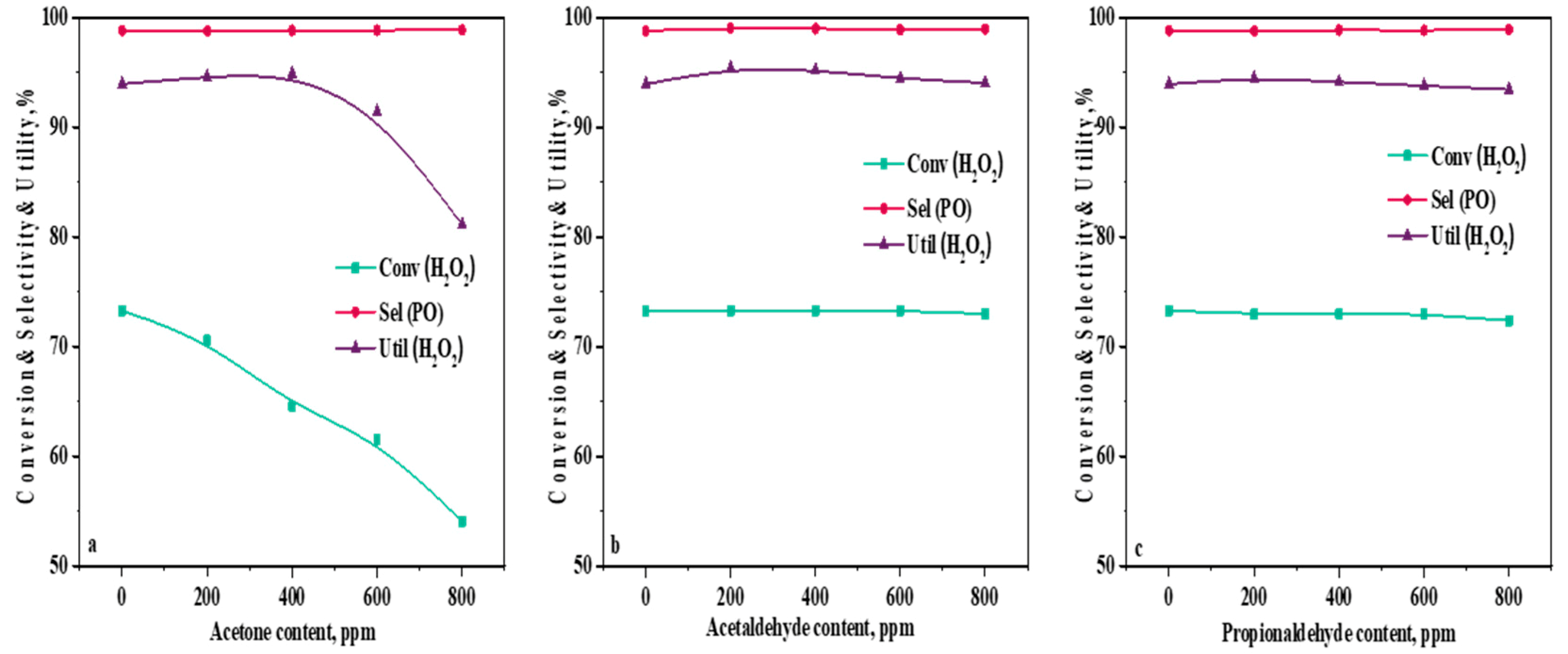
2.3. Influence of Ketone and Aldehyde Content in a Methanol Solvent
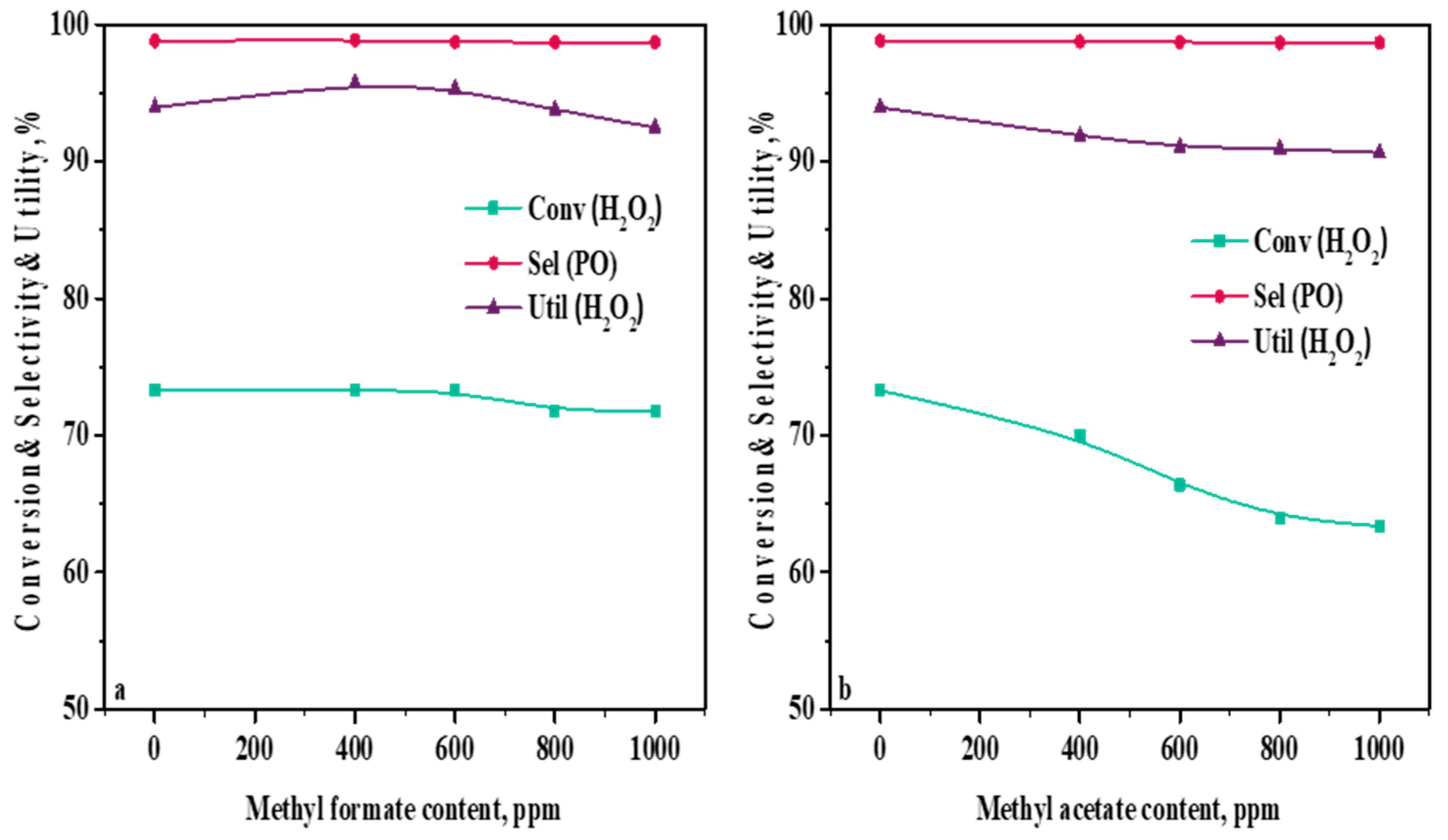
2.4. Influence of Ester Content in a Methanol Solvent
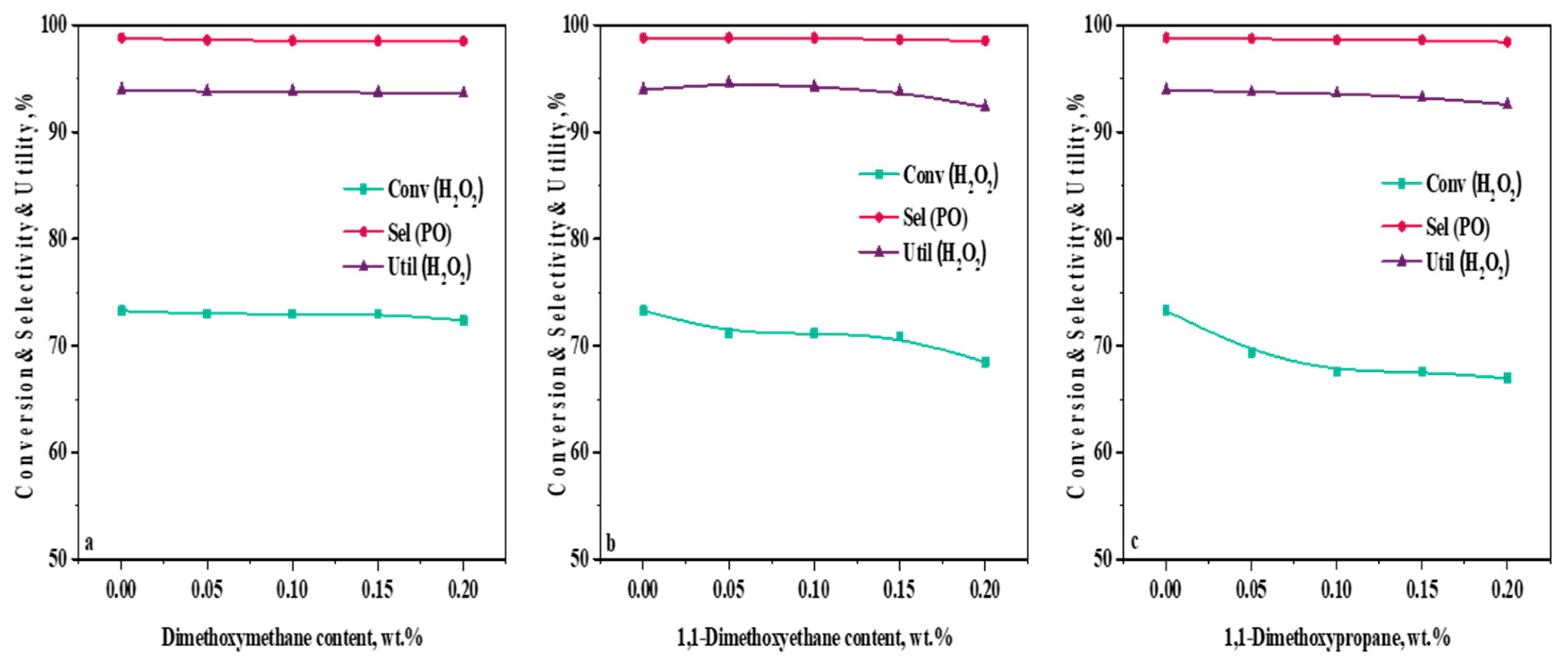
2.5. Influence of Acetal Content in a Methanol Solvent
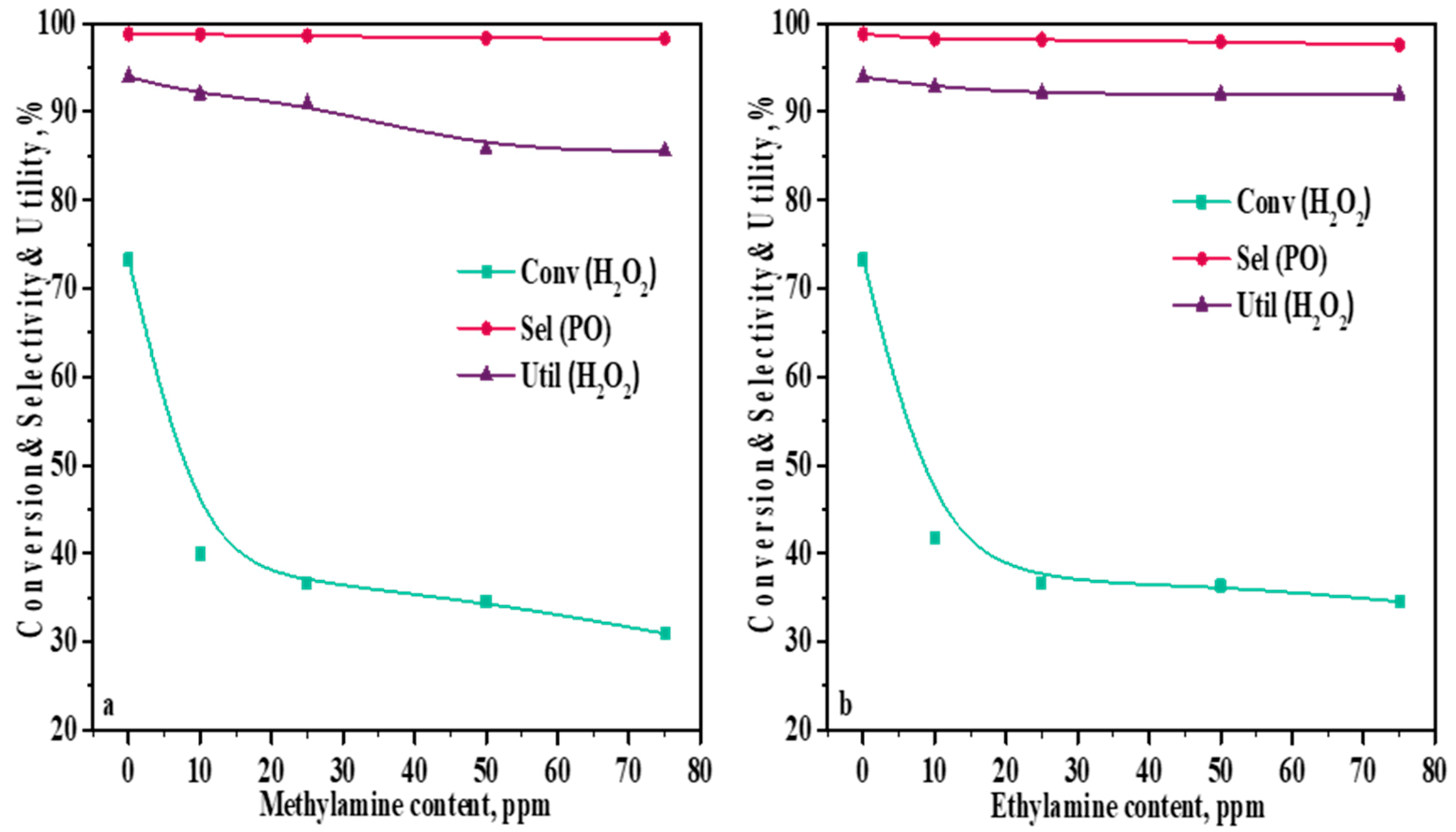
2.6. Influence of Amine Content in a Methanol Solvent
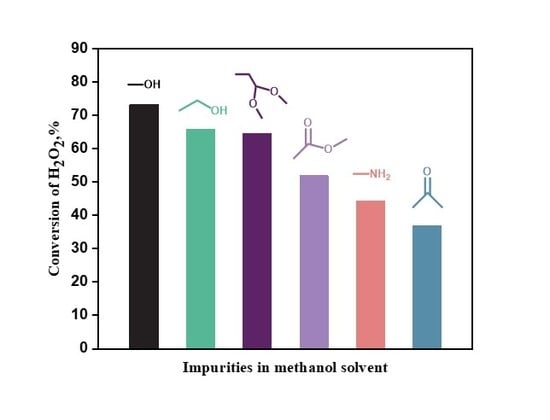
3. Discussion
4. Materials and Methods
4.1. Catalytic Reactions
4.1.1. Epoxidation with a Recycled Methanol Solvent
4.1.2. Epoxidation with Simulated Methanol Solvents
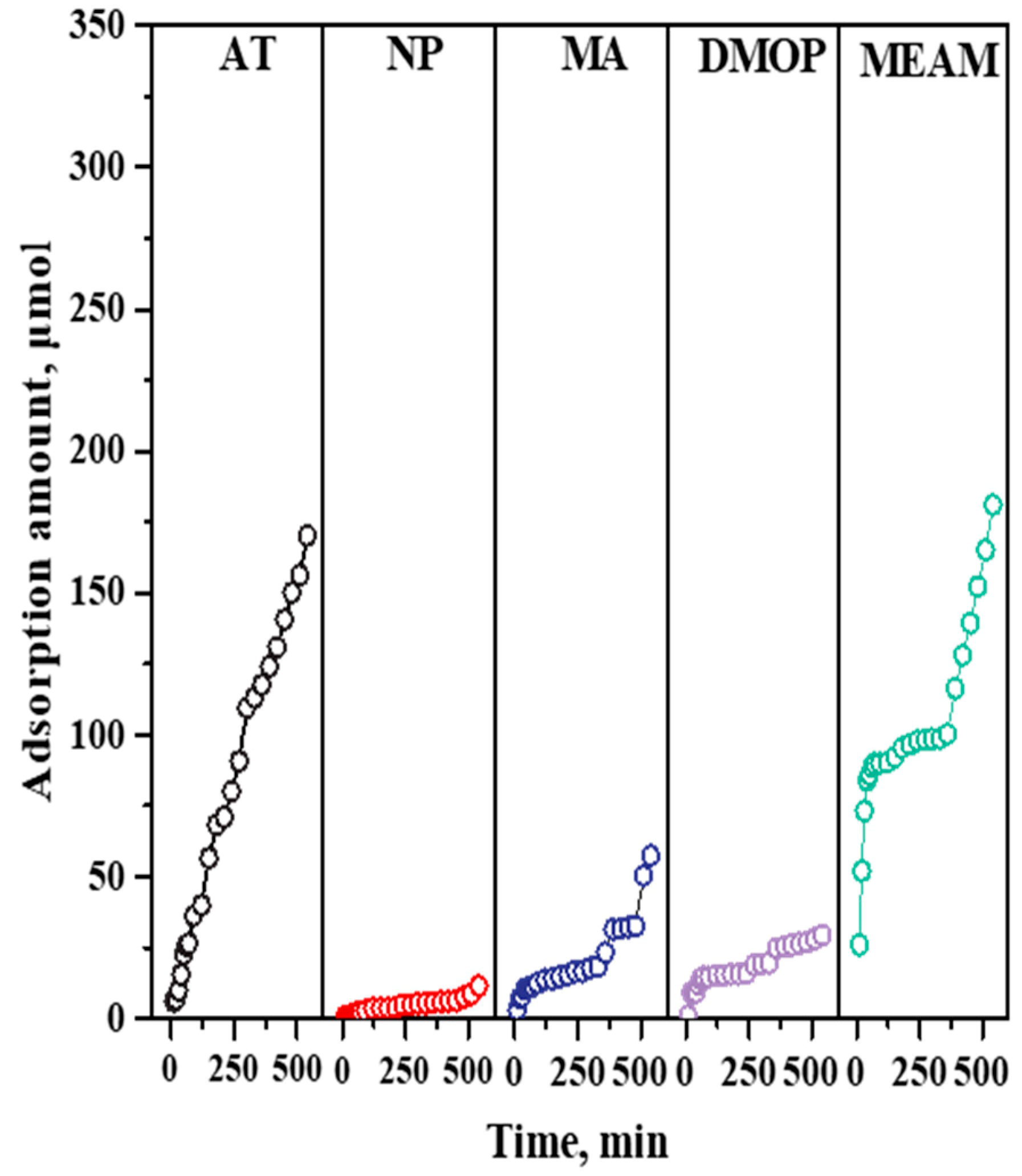
4.2. Competitive Adsorption and Desorption of Impurities on a TS-1 Catalyst
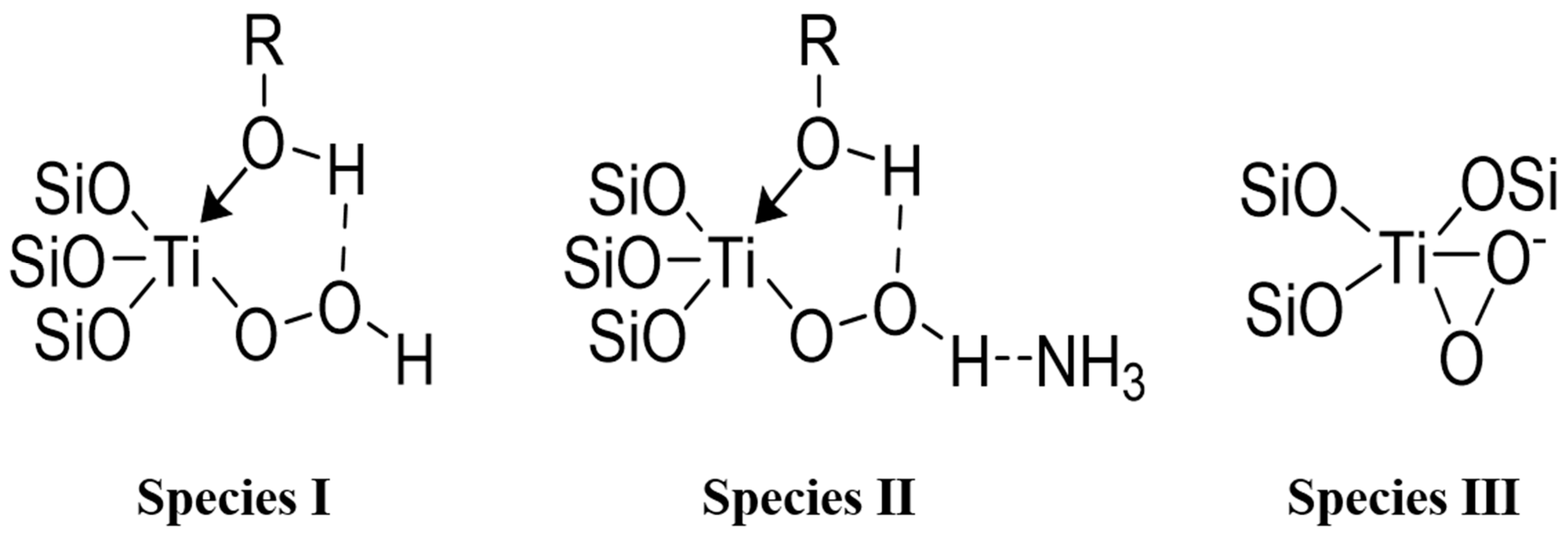
4.3. Characterizations of the Adsorption Site of Impurities on a TS-1 Catalyst
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Russo, V.; Tesser, R.; Santacesaria, E.; Serio, M.D. Chemical and Technical Aspects of Propene Oxide Production via Hydrogen Peroxide (HPPO Process). Ind. Eng. Chem. Res. 2013, 52, 1168–1178. [Google Scholar] [CrossRef]
- Schmidt, F.; Bernhard, M.; Pascaly, M. HPPO Process Technology a Novel Route to Propylene Oxide without Coproducts. Chim. Oggi 2014, 32, 31–35. [Google Scholar]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent 4410501, 18 October 1983. [Google Scholar]
- Perera, A.S.; Coppens, M.O. Titano-Silicates: Highlights on Development, Evolution and Application in Oxidative Catalysis. Catalysis 2016, 28, 119–143. [Google Scholar]
- Xin, H.C.; Zhao, J.; Xu, S.T.; Li, J.P.; Zhang, W.P.; Guo, X.W.; Hensen, E.J.M.; Yang, Q.H.; Li, C. Enhanced Catalytic Oxidation by Hierarchically Structured TS-1 Zeolite. J. Phys. Chem. C 2010, 114, 6553–6559. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, X.Y.; Tian, G.; Li, Y.; Rooke, J.C.; Zhu, G.S.; Qiu, S.L.; Yang, X.Y.; Su, B.L. Highly Stable and Reusable Multimodal Zeolite TS-1 Based Catalysts with Hierarchically Interconnected Three-Level Micro-Meso-Macroporous Structure. Angew. Chem. Int. Ed. 2011, 50, 11156–11161. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, X.S.; Guo, X.W. Synthesis of Titanium Silicalite-1 with Small Crystal Size by Using Mother Liquid of Titanium Silicalite-1 as Seed. Ind. Eng. Chem. Res. 2011, 50, 8485–8491. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Ahn, W.S.; Ryoo, R. MFI Titanosilicate Nanosheets with Single-Unit-Cell Thickness as an Oxidation Catalyst Using Peroxides. ACS Catal. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Y.M.; Xie, W.; Wu, H.H.; Li, X.H.; He, M.; Wu, P. Improving the Hydrophobicity and Oxidation Activity of Ti-MWW by Reversible Structural Rearrangement. J. Phys. Chem. C 2008, 112, 6132–6138. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Meng, X.J.; Zhang, W.P.; Zhang, J.; Pan, S.X.; Shen, Z.; Xiao, F.S. A Significant Enhancement of Catalytic Activities in Oxidation with H2O2 over the TS-1 Zeolite by Adjusting the Catalyst Wettability. Chem. Commun. 2014, 50, 2012–2014. [Google Scholar] [CrossRef]
- Wu, L.Z.; Zhao, S.F.; Lin, L.F.; Fang, X.Q.; Liu, Y.M.; He, M.Y. In-depth Understanding of Acid Catalysis of Solvolysis of Propene Oxide over Titanosilicates and Titanosilicate/H2O2 Systems. J. Catal. 2016, 337, 248–259. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.S.; Yan, H.S.; Chen, Y.Y.; Su, Q.S. Effect of Sodium Ions on Propylene Epoxidation Catalyzed by Titanium Silicalite. Appl. Catal. A Gen. 2001, 218, 31–38. [Google Scholar] [CrossRef]
- Arca, V.; Furlan, P.; Buzzoni, R. Process for the Preparation of Olefinic Epoxides. Eur. Patent 0930308B1, 3 March 2004. [Google Scholar]
- Paparatto, G.; Forlin, A.; De Alberti, G.; D’Aloisio, R.; Tegon, P. Integrated Process for the Preparation of Olefin Oxides. U.S. Patent 6888013 B2, 3 May 2005. [Google Scholar]
- Signorile, M.; Crocellà, V.; Damin, A.; Rossi, B.; Lamberti, C.; Bonino, F.; Bordiga, S. Effect of Ti Speciation on Catalytic Performance of TS-1 in the Hydrogen Peroxide to Propylene Oxide Reaction. J. Phys. Chem. C 2018, 122, 9021–9034. [Google Scholar] [CrossRef]
- Wu, L.Z.; Deng, X.J.; Zhao, S.F.; Yin, H.M.; Zhuo, Z.X.; Fang, X.Q.; Liu, Y.M.; He, M.Y. Synthesis of a Highly Active Oxidation Catalyst with Improved Distribution of Titanium Coordination States. Chem. Commun. 2016, 52, 8679–8682. [Google Scholar] [CrossRef] [PubMed]
- Notari, B. Titanium Silicalites. Catal. Today 1993, 18, 163–172. [Google Scholar] [CrossRef]
- Lin, M.; Xia, C.J.; Zhu, B.; Li, H.; Shu, X.T. Green and Efficient Epoxidation of Propylene with Hydrogen Peroxide (HPPO Process) Catalyzed by Hollow TS-1 Zeolite: A 1.0 kt/a Pilot-scale Study. Chem. Eng. J. 2016, 295, 370–375. [Google Scholar] [CrossRef]
- Clerici, M.G.; Kholdeeva, O.A. Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 474–494. [Google Scholar]
- Kraïem, J.; Ghedira, D.; Ollevier, T. Hydrogen Peroxide/Dimethyl Carbonate: A Green System for Epoxidation of N-alkylimines and N-sulfonylimines. One-pot Synthesis of N-alkyloxaziridines from N-alkylamines and (hetero) Aromatic Aldehydes. Green Chem. 2016, 18, 4859–4864. [Google Scholar] [CrossRef]
- Grigoropoulou, G.; Clark, J.H.; Elings, J.A. Recent Developments on the Epoxidation of Alkenes Using Hydrogen Peroxide as an Oxidant. Green Chem. 2003, 5, 1–7. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oguma, T.; Katsuki, T. Highly Enantioselective Epoxidation of Styrenes Catalyzed by Proline-Derived C1-Symmetric Titanium (Salan) Complexes. Angew. Chem. Int. Ed. 2009, 48, 7432–7435. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Gangemi, C.M.A.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Sfrazzetto, G.T. (Salen) Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol. Int. J. Mol. Sci. 2016, 17, 1112. [Google Scholar] [CrossRef]
- Zahedi-Niaki, M.H.; Kapoor, M.P.; Kaliaguine, S. H2O2 Oxidation and Epoxidation of Hydrocarbons and Alcohols over Titanium Aluminophosphates TAPO-5, TAPO-11, and TAPO-36. J. Catal. 1998, 177, 231–239. [Google Scholar] [CrossRef]
- Rueter, M.A. Propylene Oxide Purification. PCT. Patent WO 99/07690, 18 February 1999. [Google Scholar]
- Göbbel, H.G.; Bassler, P.; Teles, J.H.; Rudolf, P.; Müller, U.; Forlin, A.; Schulz, M.; Weidenbach, M. A Process for Epoxidizing Propene. PCT. Patent WO 2007/074101 A1, 5 July 2007. [Google Scholar]
- Berges, J.; Brasse, C.; Eickhoff, H.; Haas, T.; Hofen, W.; Kampeis, P.; Moroff, G.; Pohl, W.; Stochniol, G.; Thiele, G.; et al. Process for the Epoxidation of Olefins. PCT. Patent WO 03/093255 A1, 13 November 2003. [Google Scholar]
- Teles, J.H.; Rehfinger, A.; Bassler, P.; Wenzel, A.; Rieber, N.; Rudolf, P. Method for the Production of Propylene Oxide. U.S. Patent 2003/0146080 A1, 7 August 2003. [Google Scholar]
- Haas, T.; Hofen, W.; Wöll, W.; Brasse, C.; Stochniol, G.; Ullrich, N. Process for the Epoxidation of Olefins. PCT. Patent WO 2004/048354 A1, 10 June 2004. [Google Scholar]
- Wang, Z.J.; Pascaly, M.; Bernhard, M. Process for the Epoxidation of Propene. PCT. Patent WO 2018/205244 A1, 15 November 2018. [Google Scholar]
- Hofen, W.; Thiele, G.; Moller, A. Process for the Epoxidation of Olefins. U.S. Patent 2003/0114694 A1, 19 June 2003. [Google Scholar]
- Rueter, M.A.; Jubin, J.C., Jr. Separation of Methanol and Propylene Oxide from a Reaction Mixture. U.S. Patent 5849938, 15 December 1998. [Google Scholar]
- Clerici, M.G.; Ingallina, P. Epoxidation of Lower Olefins with Hydrogen Peroxide and Titanium Silicalite. J. Catal. 1993, 140, 71–83. [Google Scholar] [CrossRef]
- Li, G.; Meng, J.W.; Wang, X.S.; Guo, X.W. Effect of Solvents on Propene Epoxidation Catalyzed by Titanium Silicalite. React. Kinet. Catal. Lett. 2004, 82, 73–80. [Google Scholar] [CrossRef]
- Liu, X.W.; Wang, X.S.; Guo, X.W.; Li, G. Effect of Solvent on the Propylene Epoxidation Over TS-1 Catalyst. Catal. Today 2004, 93, 505–509. [Google Scholar] [CrossRef]
- Li, C.; Xiong, G.; Xin, Q.; Liu, J.K.; Ying, P.L.; Feng, Z.C.; Li, J.; Yang, W.B.; Wang, Y.Z.; Wang, G.R.; et al. UV Resonance Raman Spectroscopic Identification of Titanium Atoms in the Framework of TS-1 Zeolite. Angew. Chem. Int. Ed. 1999, 38, 2220–2222. [Google Scholar] [CrossRef]
- Bolis, V.; Bordiga, S.; Lamberti, C.; Zecchina, A.; Carati, A.; Rivetti, F.; Spanò, G.; Petrini, G. A Calorimetric, IR, XANES and EXAFS Study of the Adsorption of NH3 on Ti-Silicalite as a Function of the Sample Pre-treatment. Microporous Mesoporous Mater. 1999, 30, 67–76. [Google Scholar] [CrossRef]
- Bordiga, S.; Coluccia, S.; Lamberti, C.; Marchese, L.; Zecchina, A. XAFS Study of Ti-Silicalite: Structure of Framework Ti (IV) in the Presence and Absence of Reactive Molecules (H2O, NH3) and Comparison with Ultraviolet-Visible and IR Results. J. Phys. Chem. 1994, 98, 4125–4132. [Google Scholar] [CrossRef]
- Scarano, D.; Zecchina, A.; Bordiga, S.; Geobaldo, F.; Spoto, G. Fourier-transform Infrared and Raman Spectra of Pure and Al-, B-, Ti- and Fe-substituted Silicalites: Stretching-mode Region. J. Chem. Soc. Faraday Trans. 1993, 89, 4123–4130. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009; pp. 287–335. [Google Scholar]
- Field, L.D.; Sternhell, S.; Kalman, J.R. Organic Structures from Spectra; John Wiley & Sons, Ltd.: West Sussex, WI, USA, 2013; pp. 15–17. [Google Scholar]
- Bellussi, G.; Carati, A.; Clerici, M.G.; Maddinelli, G.; Millini, R. Reactions of Titanium Silicalite with Protic Molecules and Hydrogen Peroxide. J. Catal. 1992, 133, 220–230. [Google Scholar] [CrossRef]
- Clerici, M.G.; Ingallina, P. Oxidation Reactions with in Situ Generated Oxidants. Catal. Today 1998, 41, 351–364. [Google Scholar] [CrossRef]
- Deng, X.J.; Zhang, S.; Wang, B.S.; Wang, Y.N.; Wu, H.H.; Liu, Y.M.; He, M.Y. Enhanced Catalytic Activity of Titanosilicates Controlled by Hydrogen-Bonding Interactions. Chem. Commun. 2013, 49, 7504–7506. [Google Scholar] [CrossRef]
- Sever, R.R.; Root, T.W. DFT Study of Solvent Coordination Effects on Titanium-Based Epoxidation Catalysts. Part One: Formation of the Titanium Hydroperoxo Intermediate. J. Phys. Chem. B 2003, 107, 4080–4089. [Google Scholar] [CrossRef]
- Song, W.C.; Xiong, G.; Long, H.Y.; Jin, F.Y.; Liu, L.P.; Wang, X.S. Effect of Treatment with Different Bases on the Catalytic Properties of TS-1/SiO2 Extrudates in Propylene Epoxidation. Microporous Mesoporous Mater. 2015, 212, 48–55. [Google Scholar] [CrossRef]

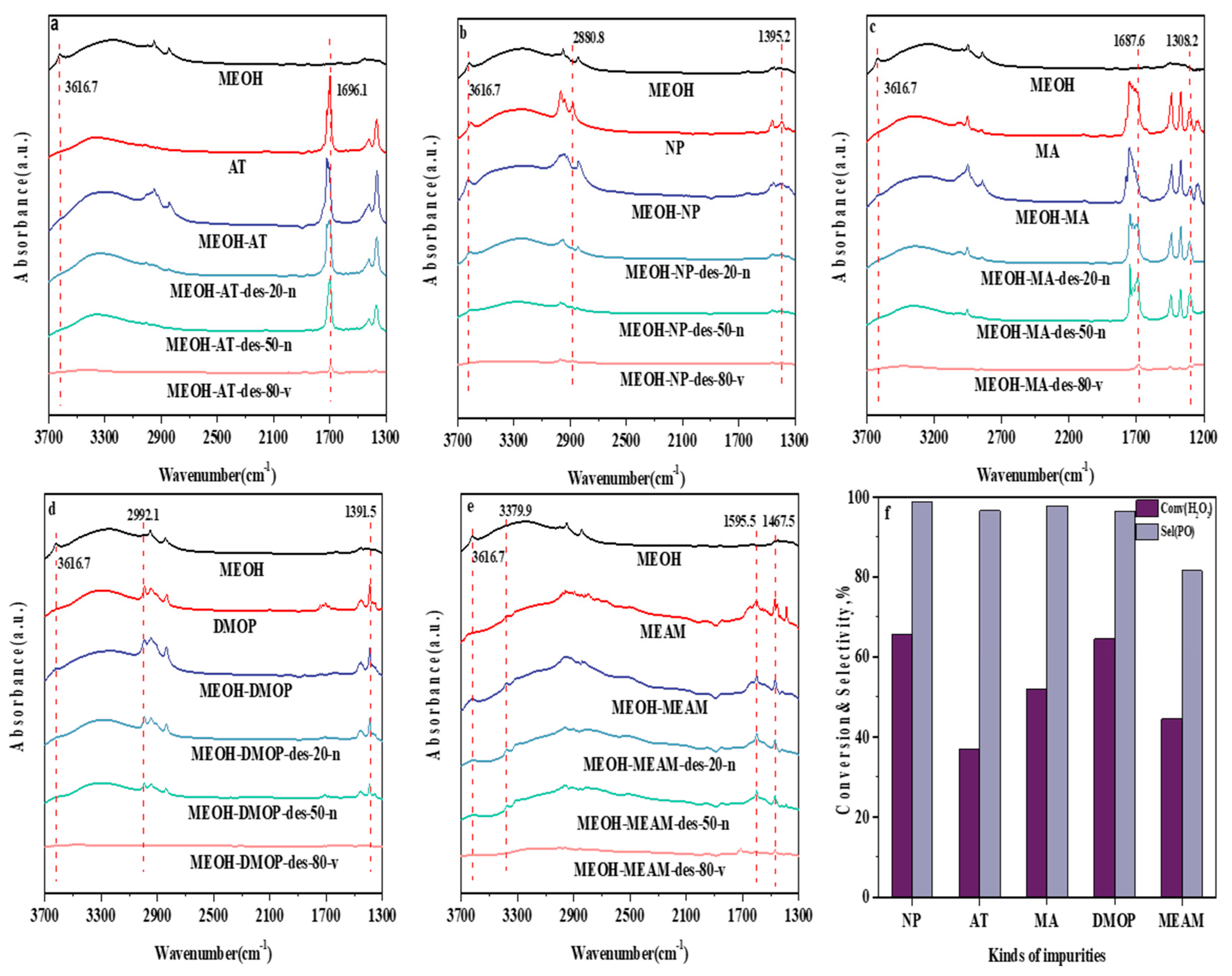


| Impurity | Band, cm−1 | Interpretation [40,41] | Vibration Mode |
|---|---|---|---|
| methanol | 3616.7 | O-H | stretching |
| 1-propanol | 2880.8 | CH2 | stretching |
| 1395.2 | CH3 | symmetric bending | |
| acetone | 1696.1 | C=O | stretching |
| methyl acetate | 1687.6 | C=O | stretching |
| 1308.2 | C-O-C | stretching | |
| 1,1-dimethoxypropane | 2992.1 | unknown | -- |
| 1391.5 | CH3 | bending | |
| methylamine | 3379.9 | NH2 | stretching |
| 1595.5 | NH2 | bending | |
| 1467.5 | CH3 | asymmetric bending |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Li, Y.; Zhu, Q.; Li, G.; Zhang, C.; Guo, H. Influence of Impurities in a Methanol Solvent on the Epoxidation of Propylene with Hydrogen Peroxide over Titanium Silicalite-1. Catalysts 2020, 10, 15. https://doi.org/10.3390/catal10010015
Wang G, Li Y, Zhu Q, Li G, Zhang C, Guo H. Influence of Impurities in a Methanol Solvent on the Epoxidation of Propylene with Hydrogen Peroxide over Titanium Silicalite-1. Catalysts. 2020; 10(1):15. https://doi.org/10.3390/catal10010015
Chicago/Turabian StyleWang, Gang, Yue Li, Quanren Zhu, Gang Li, Chao Zhang, and Hongchen Guo. 2020. "Influence of Impurities in a Methanol Solvent on the Epoxidation of Propylene with Hydrogen Peroxide over Titanium Silicalite-1" Catalysts 10, no. 1: 15. https://doi.org/10.3390/catal10010015
APA StyleWang, G., Li, Y., Zhu, Q., Li, G., Zhang, C., & Guo, H. (2020). Influence of Impurities in a Methanol Solvent on the Epoxidation of Propylene with Hydrogen Peroxide over Titanium Silicalite-1. Catalysts, 10(1), 15. https://doi.org/10.3390/catal10010015