Computed Tomography Demonstration of the Production and Distribution of Oxygen Gas Following Intratumoral Injection of a New Radiosensitizer (KORTUC) for Patients with Breast Cancer—Is Intratumoral Injection Not an Ideal Approach to Solve the Major Problem of Tumor Hypoxia in Radiotherapy?
Abstract
:1. Introduction
2. Results
3. Discussion
4. Patients and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hall, E.J.; Giaccia, A.J. Physics and chemistry of radiation absorption. In Radiobiology for the Radiologist, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 3–11. [Google Scholar]
- Ogawa, Y. Raradigm shift in radiation biology/radiation oncology—Exploitation of the “H2O2 effect” for radiotherapy using low-LET (linear energy transfer) radiation such as X-rays and high-energy electrons. Cancers 2016. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Takahashi, T.; Kobayashi, T.; Kariya, S.; Nishioka, A.; Mizobuchi, H.; Noguchi, M.; Hamasato, S.; Tani, T.; Seguchi, H.; et al. Mechanism of apoptotic resistance of human osteosarcoma cell line, HS-Os-1, against irradiation. Int. J. Mol. Med. 2003, 12, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Takahashi, T.; Kobayashi, T.; Kariya, S.; Nishioka, A.; Ohnishi, T.; Saibara, T.; Hamasato, S.; Tani, T.; Seguchi, H.; et al. Apoptotic-resistance of the human osteosarcoma cell line HS-Os-1 to irradiation is converted to apoptotic-susceptibility by hydrogen peroxide: A potent role of hydrogen peroxide as a new radiosensitizer. Int. J. Mol. Med. 2003, 12, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Takahashi, T.; Kobayashi, T.; Kariya, S.; Nishioka, A.; Hamasato, S.; Moriki, T.; Seguchi, H.; Yoshida, S.; Sonobe, H. Immunocytochemical characteristics of human osteosarcoma cell line HS-Os-1: Possible implication in apoptotic resistance against irradiation. Int. J. Mol. Med. 2004, 14, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kariya, S.; Sawada, K.; Kobayashi, T.; Karashima, T.; Shuin, T.; Nishioka, A.; Ogawa, Y. Combination treatment of hydrogen peroxide and X-rays induces apoptosis in human prostate cancer PC-3 cells. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Ue, H.; Tsuzuki, K.; Tadokoro, M.; Miyatake, K.; Sasaki, T.; Yokota, N.; Hamada, N.; Kariya, S.; Hitomi, J.; et al. New radiosensitization treatment (KORTUC I) using hydrogen peroxide solution-soaked gauze bolus for unresectable and superficially exposed neoplasms. Oncol. Rep. 2008, 19, 1389–1394. [Google Scholar] [PubMed]
- Tokuhiro, S.; Ogawa, Y.; Tsuzuki, A.; Akima, R.; Ue, H.; Kariya, S.; Nishioka, A. Development of a novel enzyme-targeting radiosensitizer (KORTUC) containing hydrogen peroxide for intratumoral injection for patients with low linear energy transfer-radioresistant neoplasms. Oncol. Lett. 2010, 1, 1025–1028. [Google Scholar] [PubMed]
- Morita-Tokuhiro, S.; Ogawa, Y.; Yokota, N.; Tsuzuki, A.; Oda, H.; Ishida, N.; Aoyama, N.; Nishioka, A. Development of a novel enzyme-targeting radiosensitizer (New KORTUC) using a gelatin-based hydrogel instead of a sodium hyaluronate. Cancers 2016. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kubota, K.; Ue, H.; Kataoka, Y.; Tadokoro, M.; Miyatake, K.; Tsuzuki, K.; Yamanishi, T.; Itoh, S.; Hitomi, J.; et al. Phase I study of a new radiosensitizer containing hydrogen peroxide and sodium hyaluronate for topical tumor injection: A new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II). Int. J. Oncol. 2009, 34, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, K.; Kubota, K.; Ogawa, Y.; Hamada, N.; Murata, Y.; Nishioka, A. Non-surgical care for locally advanced breast cancer: Radiologically assessed therapeutic outcome of a new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II) with systemic chemotherapy. Oncol. Rep. 2010, 34, 1161–1168. [Google Scholar]
- Hitomi, J.; Kubota, K.; Ogawa, Y.; Hamada, N.; Murata, Y.; Nishioka, A. Non-surgical therapy and radiologic assessment of stage I breast cancer treatment with novel enzyme-targeting radiosensitization: Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, type II (KORTUC II). Exp. Ther. Med. 2010, 1, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kubota, K.; Ue, H.; Tadokoro, M.; Matsui, R.; Yamanishi, T.; Hamada, N.; Kariya, S.; Nishioka, A.; Nakajima, H.; et al. Safety and effectiveness of a new enzyme-targeting radiosensitization treatment (KORTUC II) for intratumoral injection for low-LET radioresistant tumors. Int. J. Oncol. 2011, 39, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, A.; Ogawa, Y.; Kubota, K.; Tokuhiro, S.; Akima, R.; Yaogawa, S.; Itoh, K.; Yamada, Y.; Sasaki, T.; Onogawa, M.; et al. Evaluation of changes in tumor shadows and microcalcifications on mammography following KORTUC II, a new radiosensitization treatment without any surgical procedure for elderly patients with stage I and II breast cancer. Cancers 2011, 3, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, N.; Ogawa, Y.; Kubota, K.; Ohgi, K.; Kataoka, Y.; Miyatake, K.; Tadokoro, M.; Yamanishi, T.; Ohnishi, T.; Hamada, N.; et al. Therapeutic response to a new enzyme-targeting radiosensitization treatment (KORTUC-SC) for patients with chemotherapy-resistant supraclavicular lymph node metastasis. J. Cancer Res. Ther. 2013, 1, 215–219. [Google Scholar]
- Ogawa, Y.; Kubota, K.; Aoyama, N.; Yamanishi, T.; Kariya, S.; Hamada, N.; Nogami, M.; Nishioka, A.; Onogawa, M.; Miyamura, M. Non-surgical breast-conserving treatment (KORTUC-BCT) using a new radiosensitization method (KORTUC II) for patients with stage I or II breast cancer. Cancers 2015, 7, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, N.; Ogawa, Y.; Yasuoka, M.; Takahashi, M.; Iwasa, H.; Miyatake, K.; Yamanishi, T.; Hamada, N.; Tamura, T.; Nishioka, A.; et al. Therapeutic response to a novel enzyme-targeting radiosensitization treatment (Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas) in patients with recurrent breast cancer. Oncol. Lett. 2016, in press. [Google Scholar]
- Nishioka, A.; Ogawa, Y.; Mityatake, K.; Tadokoro, M.; Nogami, M.; Hamada, N.; Kubota, K.; Kariya, S.; Kohsaki, T.; Saibara, T.; et al. Safety and efficacy of image-guided enzyme targeting radiosensitization and intraoperative radiotherapy for locally advanced unresectable pancreatic cancer. Oncol. Lett. 2014, 8, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Yaogawa, S.; Ogawa, Y.; Morita-Tokuhiro, S.; Tsuzuki, A.; Akima, R.; Itoh, K.; Morio, K.; Yasunami, H.; Onogawa, M.; Kariya, S.; et al. Serial assessment of therapeutic response to a new radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II) in patients with stage I/II breast cancer using breast contrast-enhanced magnetic resonance imaging. Cancers 2016, 8, 1–11. [Google Scholar]
- Overgaard, J. Hypoxic radiosensitization: Adored and ignored. J. Clin. Oncol. 2007, 25, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Nishioka, A.; Inomata, T.; Ohnishi, T.; Kariya, S.; Terashima, M.; Yoshida, S.; Tohchika, N.; Tanaka, Y.; Kumon, M. Conservation treatment intensified with an anti-estrogen agent and CAF chemotherapy for stage I and II breast cancer. Oncol. Rep. 2000, 7, 479–489. [Google Scholar] [CrossRef] [PubMed]
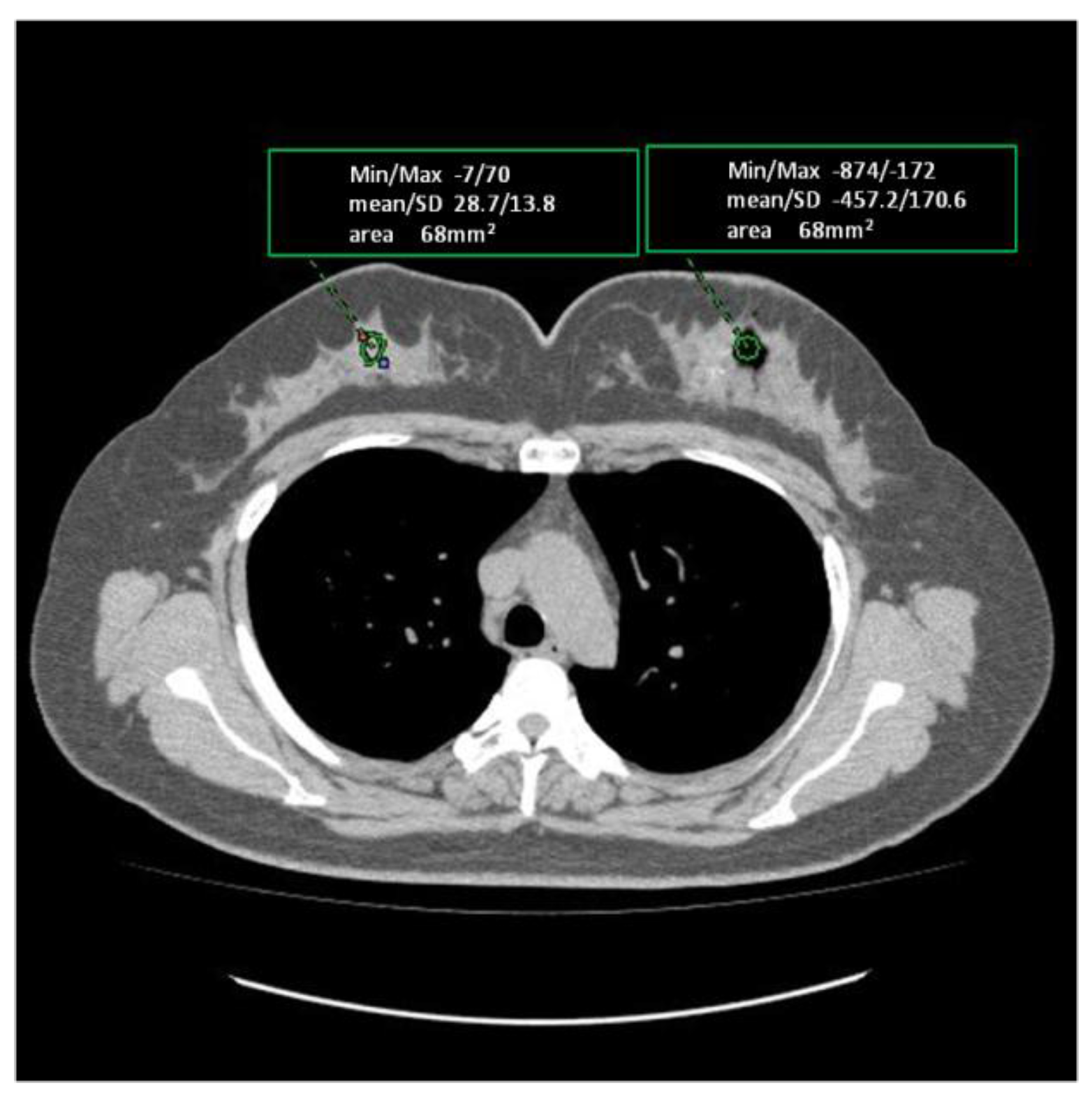

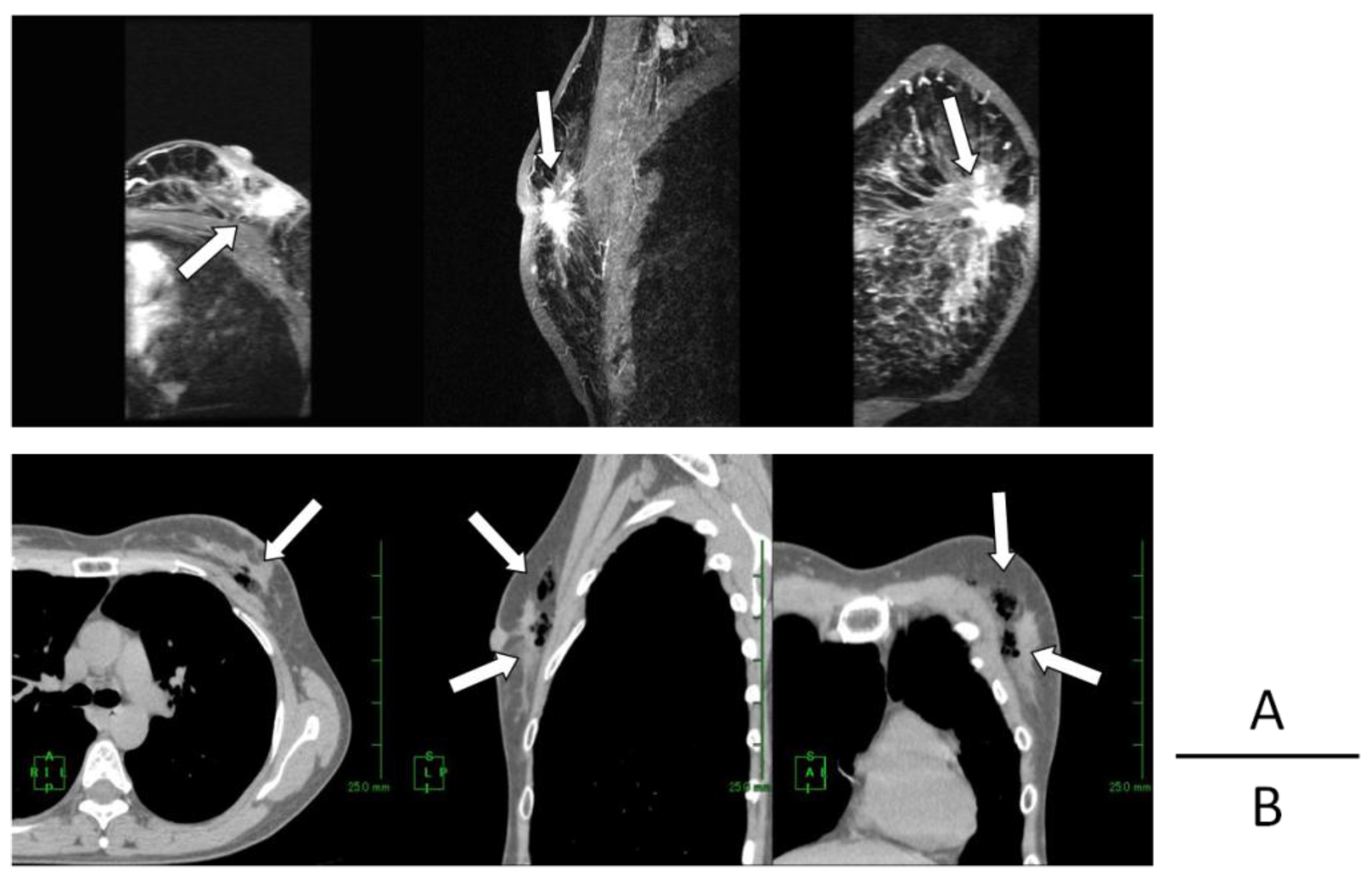
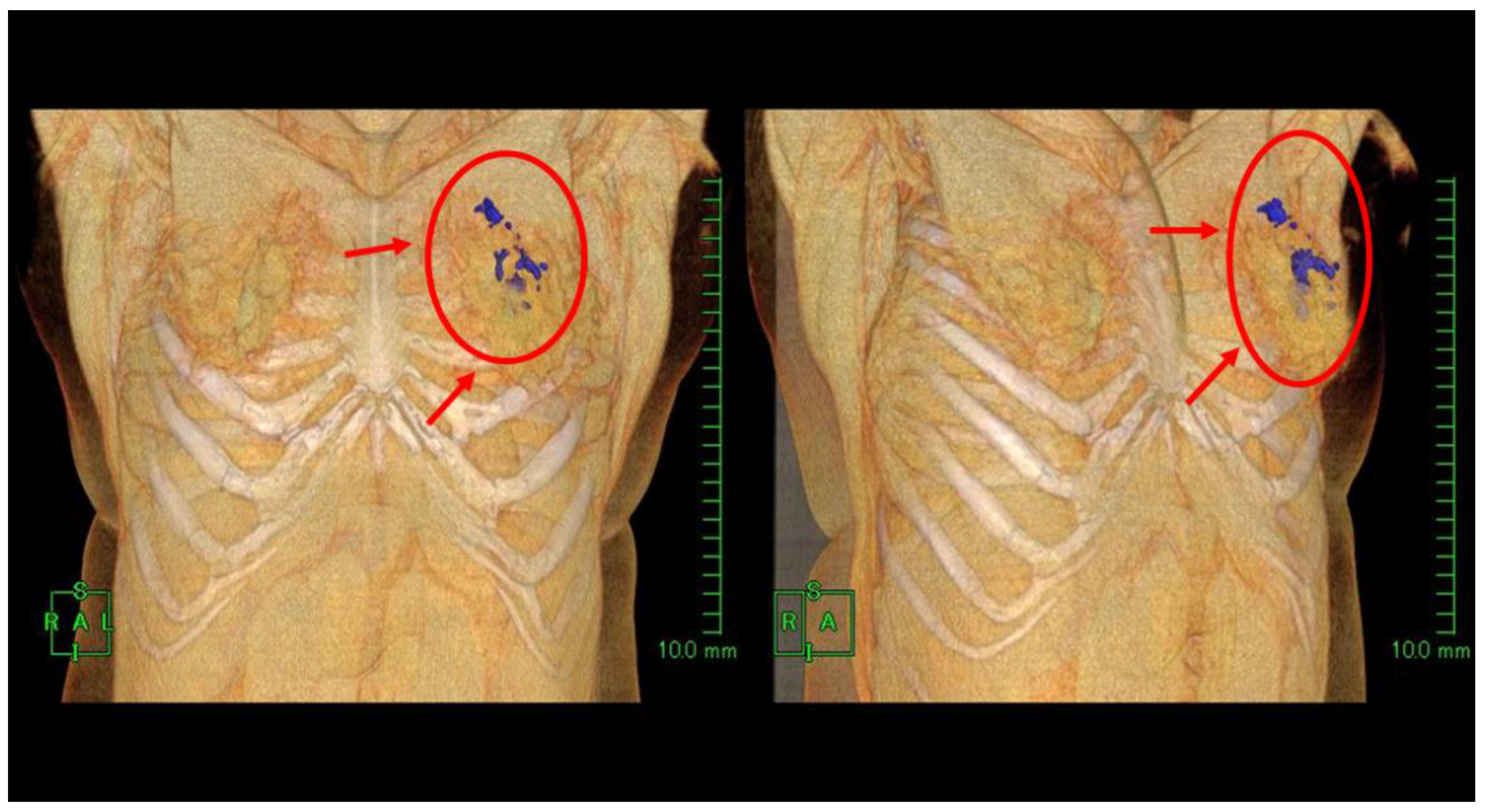
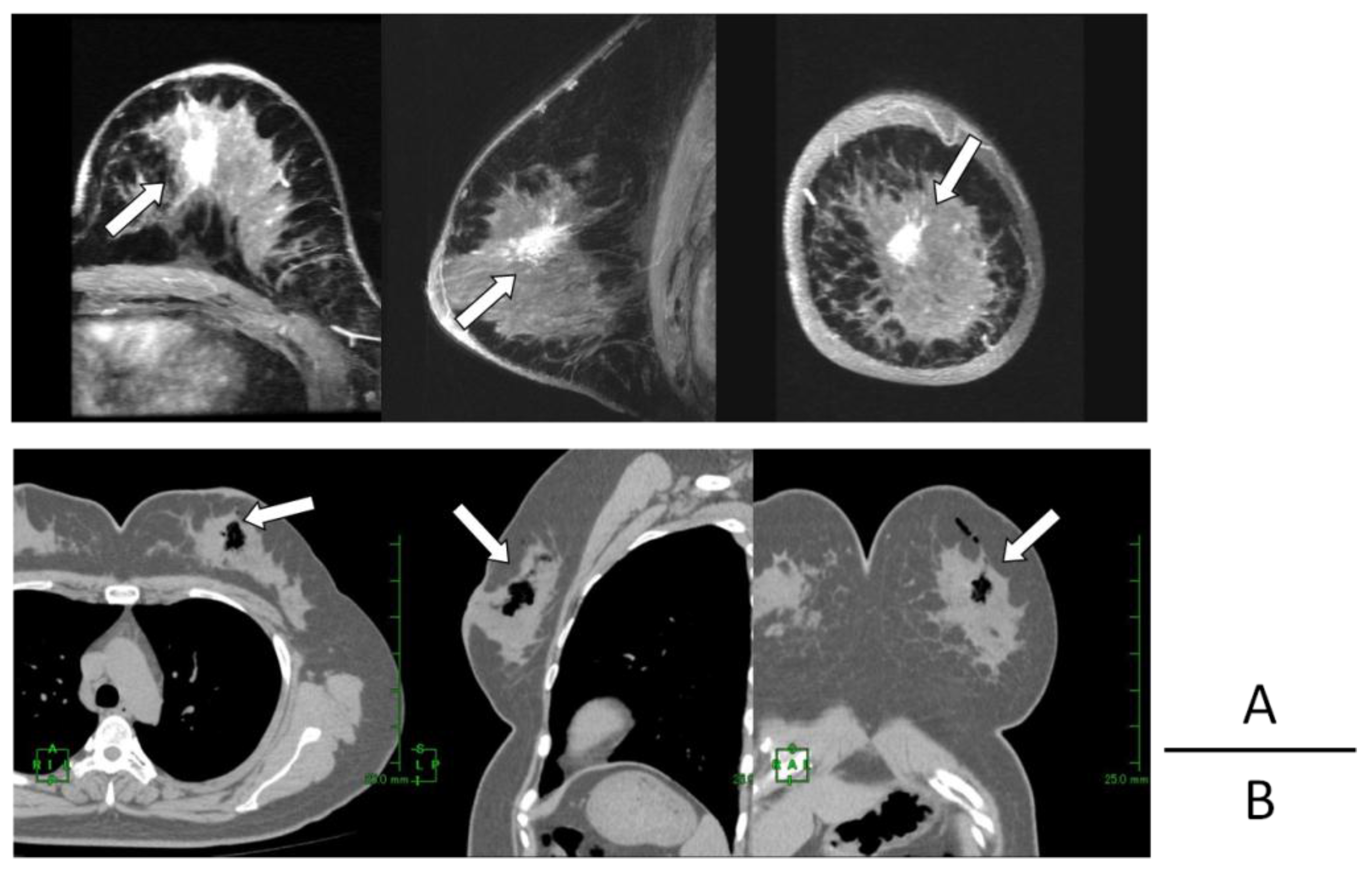

| Case | Age/Gender | TNM | Diseased Site | Oxygen Distribution |
|---|---|---|---|---|
| 1 | 61/F | T2N1M0 | Rt.B | good |
| 2 | 48/F | T4cN1M0 | Lt.C | good |
| 3 | 70/F | T2N0M0 | Lt.CD | good |
| 4 | 41/F | T2N3M0 | Lt.BD | good |
| 5 | 39/F | T1cN0M0 | Lt.C | good |
| 6 | 38/F | T2N1M0 | Lt.AC | good |
| 7 | 50/F | T2N1M0 | Rt.AC | good |
| 8 | 51/F | T2N0M0 | Rt.D | good |
| 9 | 54/F | T1cN0M0 | Lt.A | good |
| 10 | 56/F | T3N1M0 | Lt.C | good |
| Tissue | Hounsfield Unit |
|---|---|
| bone | 400~1000 |
| soft tissue | 40~80 |
| blood | 30~50 |
| water | 0 |
| fat | −60~−100 |
| lung | −800~−900 |
| air | −1000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, N.; Ogawa, Y.; Kubota, K.; Okino, K.; Akima, R.; Morita-Tokuhiro, S.; Tsuzuki, A.; Yaogawa, S.; Nishioka, A.; Miyamura, M. Computed Tomography Demonstration of the Production and Distribution of Oxygen Gas Following Intratumoral Injection of a New Radiosensitizer (KORTUC) for Patients with Breast Cancer—Is Intratumoral Injection Not an Ideal Approach to Solve the Major Problem of Tumor Hypoxia in Radiotherapy? Cancers 2016, 8, 43. https://doi.org/10.3390/cancers8040043
Hayashi N, Ogawa Y, Kubota K, Okino K, Akima R, Morita-Tokuhiro S, Tsuzuki A, Yaogawa S, Nishioka A, Miyamura M. Computed Tomography Demonstration of the Production and Distribution of Oxygen Gas Following Intratumoral Injection of a New Radiosensitizer (KORTUC) for Patients with Breast Cancer—Is Intratumoral Injection Not an Ideal Approach to Solve the Major Problem of Tumor Hypoxia in Radiotherapy? Cancers. 2016; 8(4):43. https://doi.org/10.3390/cancers8040043
Chicago/Turabian StyleHayashi, Naoya, Yasuhiro Ogawa, Kei Kubota, Kazuhiro Okino, Ryo Akima, Shiho Morita-Tokuhiro, Akira Tsuzuki, Shin Yaogawa, Akihito Nishioka, and Mitsuhiko Miyamura. 2016. "Computed Tomography Demonstration of the Production and Distribution of Oxygen Gas Following Intratumoral Injection of a New Radiosensitizer (KORTUC) for Patients with Breast Cancer—Is Intratumoral Injection Not an Ideal Approach to Solve the Major Problem of Tumor Hypoxia in Radiotherapy?" Cancers 8, no. 4: 43. https://doi.org/10.3390/cancers8040043
APA StyleHayashi, N., Ogawa, Y., Kubota, K., Okino, K., Akima, R., Morita-Tokuhiro, S., Tsuzuki, A., Yaogawa, S., Nishioka, A., & Miyamura, M. (2016). Computed Tomography Demonstration of the Production and Distribution of Oxygen Gas Following Intratumoral Injection of a New Radiosensitizer (KORTUC) for Patients with Breast Cancer—Is Intratumoral Injection Not an Ideal Approach to Solve the Major Problem of Tumor Hypoxia in Radiotherapy? Cancers, 8(4), 43. https://doi.org/10.3390/cancers8040043






