Profiling Invasiveness in Head and Neck Cancer: Recent Contributions of Genomic and Transcriptomic Approaches
Abstract
:1. Introduction
2. The Profile of the Invasive HNSCC
2.1. Recent Genomic and Transcriptomic Findings—Impact on Invasiveness of HPV-driven HNSCCs
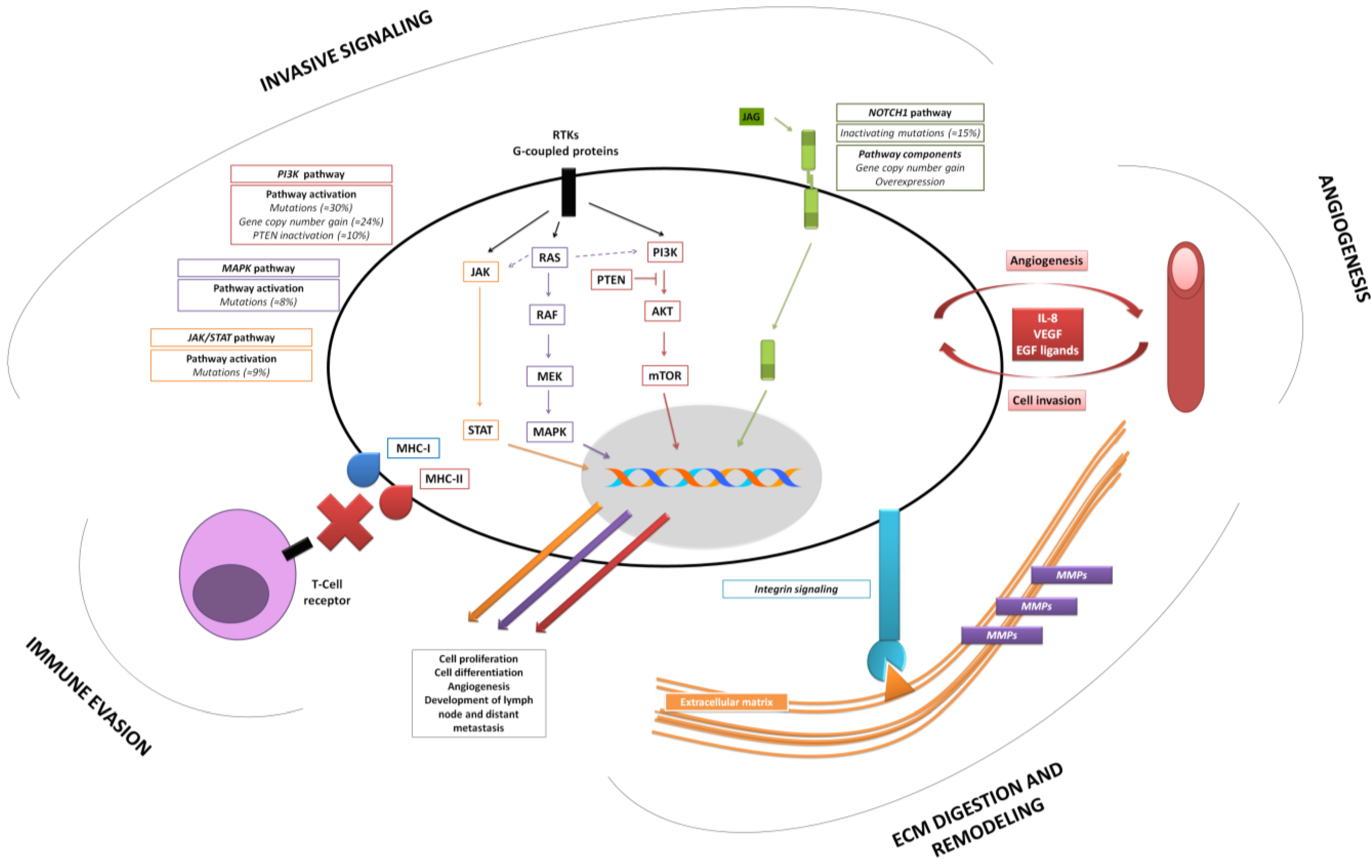
2.2. Acquiring an Invasive Phenotype—Relevance of Cell Differentiation in HNSCCs
2.3. NOTCH1 Functional Duality as an Emergent Link between Initiation and Invasion of HNSCC
2.4. New Insights into Old Players: Implications of the Mutational Landscape of Mitogenic Pathways
2.5. Execution of Tumor Cell Invasion—Basic Features Underlined by Gene-Expression Profiling Studies
| Reference(s) | Function | Sense of Regulation in HNSCC | Gene(s) |
|---|---|---|---|
| Ye
et al. [65]; Nagata et al. [66]; Kainuma et al. [67]; Kondoh et al. [68]; Choi and Chen [69] | Digestion and remodeling of ECM |  | MMP-1 |
| MMP-3 | |||
| MMP-9 | |||
| MMP-10 | |||
| MMP-13 | |||
| MMP-2 | |||
| uPA | |||
| ITGA3 | |||
| ITGA5 | |||
| Ye
et al. [65]; Gottschlich et al. [70] | Chemotaxis Lymphocyte activation |  | IL-8 |
| CXCL1 | |||
| CD28 | |||
| CD3D | |||
| CD4 | |||
| IL-18 | |||
| IL-2 | |||
| Kondoh
et al. [68]; Yu et al. [53] | Antigen presentation |  | MHC-I |
| MHC-II | |||
| Gottschlich
et al. [70]; Yu et al. [71] | Angiogenesis |  | VEGF signaling |
| IL-8 | |||
| Gottschlich
et al. [70]; Sun et al. [39] | Signal transduction |  | EGFR |
| STAT-3 | |||
| PI3K | |||
| NOTCH |
 —Up-regulation;
—Up-regulation;  —Down-regulation; MMP—Matrix metalloproteinase; ITGA—Integrin alpha; MHC—Major histocompatibility complex; VEGF—Vascular endothelial growth factor; IL-8—Interleukin-8; CXCL1—Chemokine (C-X-C motif) ligand 1.
—Down-regulation; MMP—Matrix metalloproteinase; ITGA—Integrin alpha; MHC—Major histocompatibility complex; VEGF—Vascular endothelial growth factor; IL-8—Interleukin-8; CXCL1—Chemokine (C-X-C motif) ligand 1.2.6. Paving the Tracks of Invasion—Relevance of Integrin Signaling
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar]
- Goodwin, W.J., Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: When do the ends justify the means? Laryngoscope 2000, 110, 1–18. [Google Scholar]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the united states. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar]
- Ferlito, A.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Mondin, V. Incidence and sites of distant metastases from head and neck cancer. ORL J. Otorhinolaryngol. Relat. Spec. 2001, 63, 202–207. [Google Scholar]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar]
- Hornberg, J.J.; Bruggeman, F.J.; Westerhoff, H.V.; Lankelma, J. Cancer: A systems biology disease. Bio. Syst. 2006, 83, 81–90. [Google Scholar]
- Bruggeman, F.J.; Westerhoff, H.V. The nature of systems biology. Trends Microbiol. 2007, 15, 45–50. [Google Scholar]
- Stricker, T.; Catenacci, D.V.; Seiwert, T.Y. Molecular profiling of cancer—The future of personalized cancer medicine: A primer on cancer biology and the tools necessary to bring molecular testing to the clinic. Semin. Oncol. 2011, 38, 173–185. [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar]
- Nichols, A.C.; Palma, D.A.; Chow, W.; Tan, S.; Rajakumar, C.; Rizzo, G.; Fung, K.; Kwan, K.; Wehrli, B.; Winquist, E.; et al. High frequency of activating pik3ca mutations in human papillomavirus-positive oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 617–622. [Google Scholar]
- Oganesyan, G.; Saha, S.K.; Guo, B.; He, J.Q.; Shahangian, A.; Zarnegar, B.; Perry, A.; Cheng, G. Critical role of TRAF3 in the toll-like receptor-dependent and -independent antiviral response. Nature 2006, 439, 208–211. [Google Scholar]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappab signaling. Cell 2008, 132, 344–362. [Google Scholar]
- Sepiashvili, L.; Bruce, J.P.; Huang, S.H.; O’Sullivan, B.; Liu, F.F.; Kislinger, T. Novel insights into head and neck cancer using next-generation “omic” technologies. Cancer Res. 2015, 75, 480–486. [Google Scholar]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-kappab activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets 2010, 14, 45–55. [Google Scholar]
- Smith, A.; Teknos, T.N.; Pan, Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 287–292. [Google Scholar]
- Chen, C.; Zimmermann, M.; Tinhofer, I.; Kaufmann, A.M.; Albers, A.E. Epithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinoma. Cancer Lett. 2013, 338, 47–56. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar]
- Scheel, C.; Weinberg, R.A. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin. Cancer Biol. 2012, 22, 396–403. [Google Scholar]
- Chung, C.H.; Parker, J.S.; Karaca, G.; Wu, J.; Funkhouser, W.K.; Moore, D.; Butterfoss, D.; Xiang, D.; Zanation, A.; Yin, X.; et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 2004, 5, 489–500. [Google Scholar]
- Chung, C.H.; Parker, J.S.; Ely, K.; Carter, J.; Yi, Y.; Murphy, B.A.; Ang, K.K.; El-Naggar, A.K.; Zanation, A.M.; Cmelak, A.J.; et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappab signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006, 66, 8210–8218. [Google Scholar]
- Ginos, M.A.; Page, G.P.; Michalowicz, B.S.; Patel, K.J.; Volker, S.E.; Pambuccian, S.E.; Ondrey, F.G.; Adams, G.L.; Gaffney, P.M. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004, 64, 55–63. [Google Scholar]
- Yan, B.; Yang, X.; Lee, T.L.; Friedman, J.; Tang, J.; van Waes, C.; Chen, Z. Genome-wide identification of novel expression signatures reveal distinct patterns and prevalence of binding motifs for p53, nuclear factor-kappab and other signal transcription factors in head and neck squamous cell carcinoma. Genome Biol. 2007, 8, R78. [Google Scholar]
- De Cecco, L.; Bossi, P.; Locati, L.; Canevari, S.; Licitra, L. Comprehensive gene expression meta-analysis of head and neck squamous cell carcinoma microarray data defines a robust survival predictor. Ann. Oncol. 2014, 25, 1628–1635. [Google Scholar]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in Notch1. Science 2011, 333, 1154–1157. [Google Scholar]
- Miele, L.; Golde, T.; Osborne, B. Notch signaling in cancer. Curr. Mol. Med. 2006, 6, 905–918. [Google Scholar]
- Koch, U.; Radtke, F. Notch signaling in solid tumors. Curr. Top. Dev. Biol. 2010, 92, 411–455. [Google Scholar]
- Hu, Y.Y.; Zheng, M.H.; Zhang, R.; Liang, Y.M.; Han, H. Notch signaling pathway and cancer metastasis. Adv. Exp. Med. Biol. 2012, 727, 186–198. [Google Scholar]
- Garcia, A.; Kandel, J.J. Notch: A key regulator of tumor angiogenesis and metastasis. Histol. Histopathol. 2012, 27, 151–156. [Google Scholar]
- Bailey, J.M.; Singh, P.K.; Hollingsworth, M.A. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell. Biochem. 2007, 102, 829–839. [Google Scholar]
- South, A.P.; Cho, R.J.; Aster, J.C. The double-edged sword of Notch signaling in cancer. Semin. Cell Dev. Biol. 2012, 23, 458–464. [Google Scholar]
- Extance, A. Alzheimer’s failure raises questions about disease-modifying strategies. Nat. Rev. Drug Discov. 2010, 9, 749–751. [Google Scholar]
- Sun, W.; Gaykalova, D.A.; Ochs, M.F.; Mambo, E.; Arnaoutakis, D.; Liu, Y.; Loyo, M.; Agrawal, N.; Howard, J.; Li, R.; et al. Activation of the Notch pathway in head and neck cancer. Cancer Res. 2014, 74, 1091–1104. [Google Scholar]
- Hayward, P.; Kalmar, T.; Arias, A.M. Wnt/Notch signalling and information processing during development. Development 2008, 135, 411–424. [Google Scholar]
- Li, Y.; Ma, J.; Qian, X.; Wu, Q.; Xia, J.; Miele, L.; Sarkar, F.H.; Wang, Z. Regulation of emt by Notch signaling pathway in tumor progression. Curr. Cancer Drug Targets 2013, 13, 957–962. [Google Scholar]
- Wang, N.J.; Sanborn, Z.; Arnett, K.L.; Bayston, L.J.; Liao, W.; Proby, C.M.; Leigh, I.M.; Collisson, E.A.; Gordon, P.B.; Jakkula, L.; et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17761–17766. [Google Scholar]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar]
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’Assoro, A.B.; et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzyme Regul. 2006, 46, 249–279. [Google Scholar]
- Meyer, S.C.; Levine, R.L. Molecular pathways: Molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin. Cancer Res. 2014, 20, 2051–2059. [Google Scholar]
- Lui, V.W.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013, 3, 761–769. [Google Scholar]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar]
- Gonzalez-Angulo, A.M.; Ferrer-Lozano, J.; Stemke-Hale, K.; Sahin, A.; Liu, S.; Barrera, J.A.; Burgues, O.; Lluch, A.M.; Chen, H.; Hortobagyi, G.N.; et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol. Cancer Ther. 2011, 10, 1093–1101. [Google Scholar]
- Daneshmand, M.; Hanson, J.E.; Nabavi, M.; Hilton, J.F.; Vandermeer, L.; Kanji, F.; Dent, S.F.; Clemons, M.; Lorimer, I.A. Detection of pik3ca mutations in breast cancer bone metastases. ISRN Oncol. 2012, 2012, 492578. [Google Scholar]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar]
- Psyrri, A.; Seiwert, T.Y.; Jimeno, A. Molecular pathways in head and neck cancer: EGFR, PI3K, and more. Am. Soc. Clin. Oncol. Educ. Book 2013. [Google Scholar] [CrossRef]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar]
- Yu, Y.H.; Kuo, H.K.; Chang, K.W. The evolving transcriptome of head and neck squamous cell carcinoma: A systematic review. PLoS ONE 2008, 3, e3215. [Google Scholar]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar]
- Takeuchi, K.; Ito, F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol. Pharm. Bull. 2011, 34, 1774–1780. [Google Scholar]
- Nisa, L.; Aebersold, D.M.; Giger, R.; Zimmer, Y.; Medova, M. Biological, diagnostic and therapeutic relevance of the met receptor signaling in head and neck cancer. Pharmacol. Ther. 2014, 143, 337–349. [Google Scholar]
- Maiti, G.P.; Mondal, P.; Mukherjee, N.; Ghosh, A.; Ghosh, S.; Dey, S.; Chakrabarty, J.; Roy, A.; Biswas, J.; Roychoudhury, S.; et al. Overexpression of egfr in head and neck squamous cell carcinoma is associated with inactivation of SH3Gl2 and CDC25A genes. PLoS ONE 2013, 8, e63440. [Google Scholar]
- Radhakrishnan, R.; Solomon, M.; Satyamoorthy, K.; Martin, L.E.; Lingen, M.W. Tissue microarray—A high-throughput molecular analysis in head and neck cancer. J. Oral Pathol. Med. 2008, 37, 166–176. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar]
- Hua, H.; Li, M.; Luo, T.; Yin, Y.; Jiang, Y. Matrix metalloproteinases in tumorigenesis: An evolving paradigm. Cell. Mol. Life Sci. 2011, 68, 3853–3868. [Google Scholar]
- Cai, K.Q.; Yang, W.L.; Capo-Chichi, C.D.; Vanderveer, L.; Wu, H.; Godwin, A.K.; Xu, X.X. Prominent expression of metalloproteinases in early stages of ovarian tumorigenesis. Mol. Carcinog. 2007, 46, 130–143. [Google Scholar]
- Di Carlo, A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol. Lett. 2013, 5, 1677–1681. [Google Scholar]
- Bouchet, S.; Bauvois, B. Neutrophil gelatinase-associated lipocalin (NGAL), pro-matrix metalloproteinase-9 (pro-MMP-9) and their complex pro-MMP-9/NGAL in leukaemias. Cancers 2014, 6, 796–812. [Google Scholar]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar]
- Ye, H.; Yu, T.; Temam, S.; Ziober, B.L.; Wang, J.; Schwartz, J.L.; Mao, L.; Wong, D.T.; Zhou, X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics 2008, 9, 69. [Google Scholar]
- Nagata, M.; Fujita, H.; Ida, H.; Hoshina, H.; Inoue, T.; Seki, Y.; Ohnishi, M.; Ohyama, T.; Shingaki, S.; Kaji, M.; et al. Identification of potential biomarkers of lymph node metastasis in oral squamous cell carcinoma by cDNA microarray analysis. Int. J. Cancer 2003, 106, 683–689. [Google Scholar]
- Kainuma, K.; Katsuno, S.; Hashimoto, S.; Oguchi, T.; Suzuki, N.; Asamura, K.; Usami, S. Differences in the expression of genes between normal tissue and squamous cell carcinomas of head and neck using cancer-related gene cDNA microarray. Acta Otolaryngol. 2006, 126, 967–974. [Google Scholar]
- Kondoh, N.; Ishikawa, T.; Ohkura, S.; Arai, M.; Hada, A.; Yamazaki, Y.; Kitagawa, Y.; Shindoh, M.; Takahashi, M.; Ando, T.; et al. Gene expression signatures that classify the mode of invasion of primary oral squamous cell carcinomas. Mol. Carcinog. 2008, 47, 744–756. [Google Scholar]
- Choi, P.; Chen, C. Genetic expression profiles and biologic pathway alterations in head and neck squamous cell carcinoma. Cancer 2005, 104, 1113–1128. [Google Scholar]
- Gottschlich, S.; Ambrosch, P.; Cordes, C.; Gorogh, T.; Schreiber, S.; Hasler, R. Gene expression profiling of head and neck squamous cell carcinoma using cDNA microarrays. Int. J. Oncol. 2006, 29, 605–613. [Google Scholar]
- Zaidel-Bar, R.; Itzkovitz, S.; Ma’ayan, A.; Iyengar, R.; Geiger, B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007, 9, 858–867. [Google Scholar]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for rhogtpases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar]
- Li, H.; Peyrollier, K.; Kilic, G.; Brakebusch, C. Rho GTPases and cancer. BioFactors 2014, 40, 226–235. [Google Scholar]
- Lathia, J.D.; Chigurupati, S.; Thundyil, J.; Selvaraj, P.K.; Mughal, M.R.; Woodruff, T.M.; Chan, S.L.; Karamyan, V.T.; Mattson, M.P.; Arumugam, T.V. Pivotal role for beta-1 integrin in neurovascular remodelling after ischemic stroke. Exp. Neurol. 2010, 221, 107–114. [Google Scholar]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, L.; Aebersold, D.M.; Giger, R.; Caversaccio, M.D.; Borner, U.; Medová, M.; Zimmer, Y. Profiling Invasiveness in Head and Neck Cancer: Recent Contributions of Genomic and Transcriptomic Approaches. Cancers 2015, 7, 585-597. https://doi.org/10.3390/cancers7020585
Nisa L, Aebersold DM, Giger R, Caversaccio MD, Borner U, Medová M, Zimmer Y. Profiling Invasiveness in Head and Neck Cancer: Recent Contributions of Genomic and Transcriptomic Approaches. Cancers. 2015; 7(2):585-597. https://doi.org/10.3390/cancers7020585
Chicago/Turabian StyleNisa, Lluís, Daniel Matthias Aebersold, Roland Giger, Marco Domenico Caversaccio, Urs Borner, Michaela Medová, and Yitzhak Zimmer. 2015. "Profiling Invasiveness in Head and Neck Cancer: Recent Contributions of Genomic and Transcriptomic Approaches" Cancers 7, no. 2: 585-597. https://doi.org/10.3390/cancers7020585
APA StyleNisa, L., Aebersold, D. M., Giger, R., Caversaccio, M. D., Borner, U., Medová, M., & Zimmer, Y. (2015). Profiling Invasiveness in Head and Neck Cancer: Recent Contributions of Genomic and Transcriptomic Approaches. Cancers, 7(2), 585-597. https://doi.org/10.3390/cancers7020585





