Human Cancers Derived from Either Genetic or Lifestyle Factors Are Initiated by Impaired Estrogen Signaling
Simple Summary
Abstract
1. Introduction
2. Theories Suggesting Various Initiators of Insulin Resistance
3. The Origin of Insulin Resistance Is a Defect in Estrogen Signaling, While Hyperinsulinemia Is a Compensatory Effort to Improve Estrogen Regulation
4. Impaired Estrogen Signaling Is the Origin of Genomic Instability and Insulin Resistance in BRCA Gene Mutation Carriers
5. Polycystic Ovary Syndrome Originates from the Disruption of Estrogen Signaling via CYP19A Gene Mutation
6. In Type-1 Diabetes, the Characteristic Triad of Insulin Resistance, Fertility Disorder, and Increased Risk for Malignancies Reveals the Impact of Impaired Estrogen Signaling
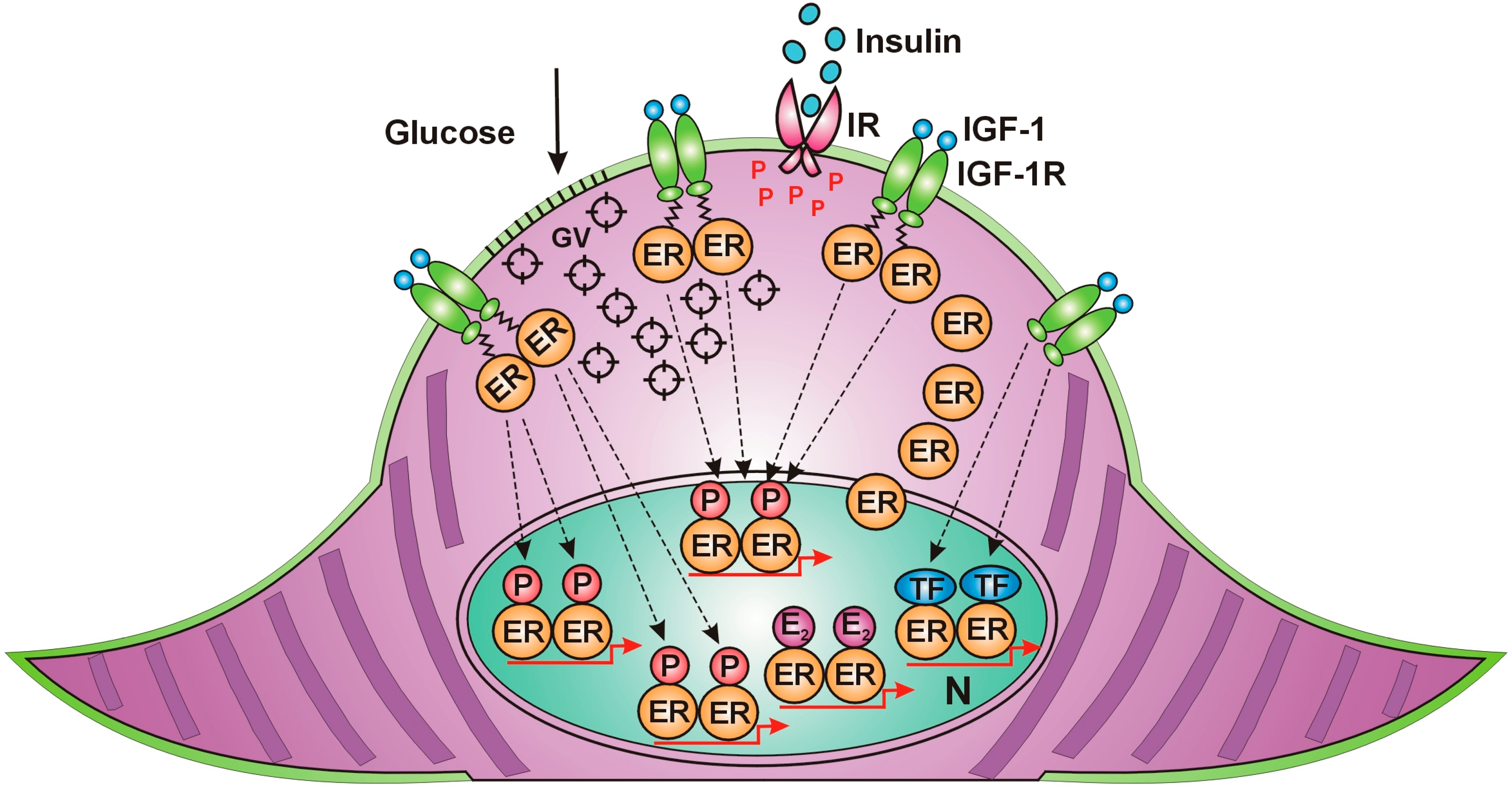
7. Estrogen Is the Principal Regulator of All Cellular Functions in Mammals
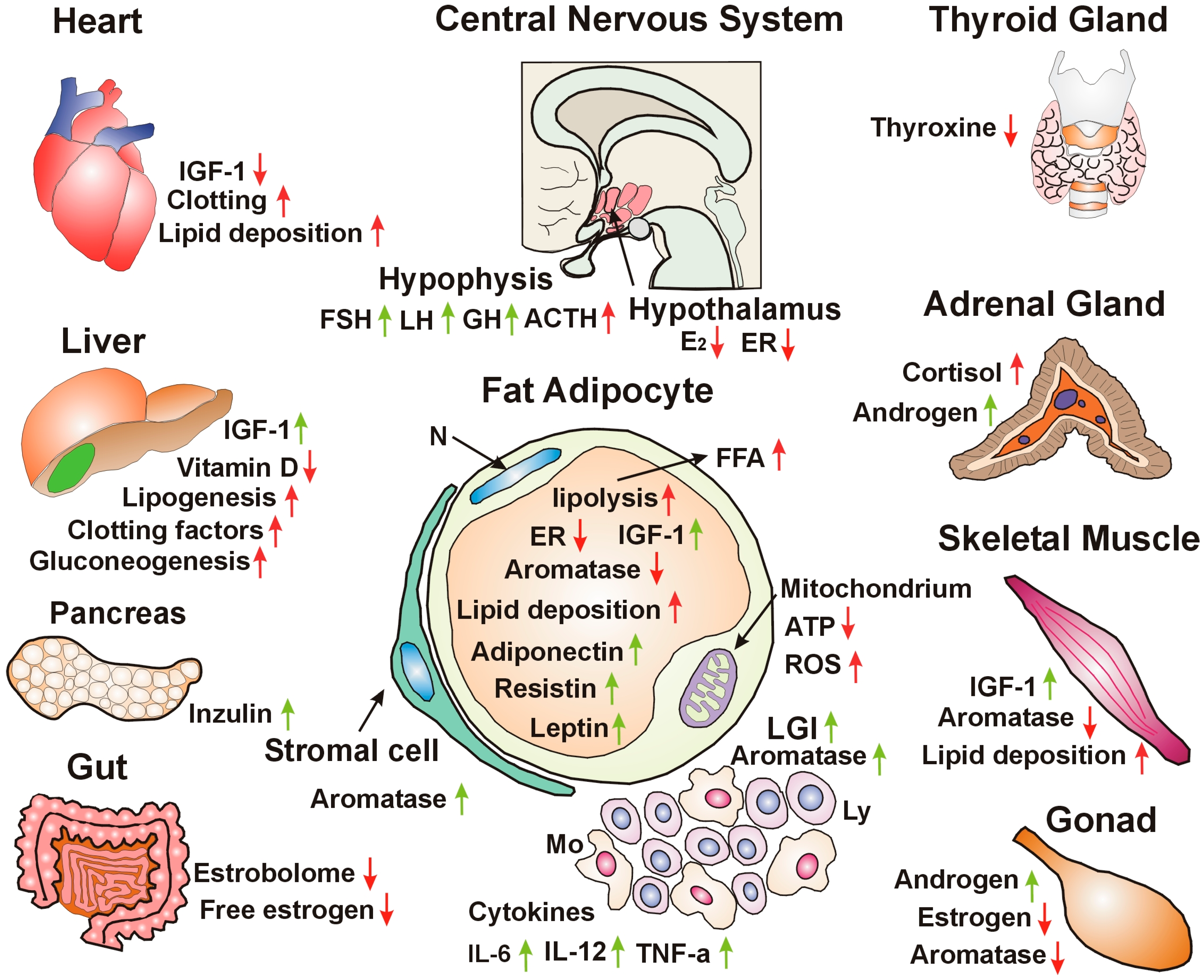
8. Adipose Tissue Ensures Metabolic Balance and Energy Homeostasis via Estrogen Regulation
9. Skeletal Muscle Contraction Improves Insulin Sensitivity Through Rapid Unliganded Activation of ERs via the IGF-1 Receptor
10. Estrogen Regulation of Multiple Functions of the Liver
11. Hypothalamic Estrogen Signaling Is the Central Regulator of Somatic, Reproductive, and Mental Health
12. The Origin of Cancer Development from Unhealthy Lifestyle Factors and Bad Habits: Impaired Estrogen Signaling and Associated Insulin Resistance
13. Discussion
14. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene–Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef]
- Sinkala, M. Mutational landscape of cancer-driver genes across human cancers. Sci. Rep. 2023, 13, 12742. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. DNA Damage Responses in Tumors Are Not Proliferative Stimuli, but Rather They Are DNA Repair Actions Requiring Supportive Medical Care. Cancers 2024, 16, 1573. [Google Scholar] [CrossRef] [PubMed]
- Beatson, G. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Lancet 1896, 148, 104–107. [Google Scholar] [CrossRef]
- Boyd, S. Oophorectomy in Cancer of the Breast. BMJ 1902, 1, 110–111. [Google Scholar] [CrossRef]
- Suba, Z. Estrogen Regulated Genes Compel Apoptosis in Breast Cancer Cells, Whilst Stimulate Antitumor Activity in Peritumoral Immune Cells in a Janus-Faced Manner. Curr. Oncol. 2024, 31, 4885–4907. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Role of insulin resistance in human disease. Nutrition 1997, 13, 64. [Google Scholar] [CrossRef]
- Prasad, H.; Ryan, D.A.; Celzo, M.F.; Stapleton, D. Metabolic Syndrome: Definition and Therapeutic Implications. Postgrad. Med. 2012, 124, 21–30. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef]
- Nolan, C.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Szablewski, L. Insulin Resistance: The Increased Risk of Cancers. Curr. Oncol. 2024, 31, 998–1027. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender Differences in Insulin Resistance: New Knowledge and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Low Estrogen Exposure and/or Defective Estrogen Signaling Induces Disturbances in Glucose Uptake and Energy Expenditure. J. Diabetes Metab. 2013, 4, 5. [Google Scholar] [CrossRef]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef]
- Betai, D.; Ahmed, A.S.; Saxena, P.; Rashid, H.; Patel, H.; Shahzadi, A.; Mowo-Wale, A.G.; Nazir, Z. Gender Disparities in Cardiovascular Disease and Their Management: A Review. Cureus 2024, 16, e59663. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Sex Steroids, Cardiovascular Disease, and Hypertension. Hypertension 2005, 45, 170–174. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Selvaraj, R.C.; Cioffi, G.; Waite, K.A.; Jackson, S.S.; Barnholtz-Sloan, J.S. A Pan-Cancer Analysis of Age and Sex Differences in Cancer Incidence and Survival in the United States, 2001–2020. Cancers 2025, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Gender-related hormonal risk factors for oral cancer. Pathol. Oncol. Res. 2007, 13, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers 2019, 11, 1402. [Google Scholar] [CrossRef]
- Lee, H.-M.; Lee, H.-J.; Chang, J.-E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines 2022, 10, 2116. [Google Scholar] [CrossRef] [PubMed]
- Rho, O.; Kim, D.J.; Kiguchi, K.; DiGiovanni, J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol. Carcinog. 2011, 50, 264–279. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A. Insulin Resistance and Hyperinsulinemia: The Egg and the Chicken. J. Clin. Endocrinol. Metab. 2021, 106, 1897–1899. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem Rev. 2005, 26, 19–39. [Google Scholar] [PubMed] [PubMed Central]
- Johnson, A.M.; Olefsky, J.M. The Origins and Drivers of Insulin Resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl. Res. 2016, 167, 228–256. [Google Scholar] [CrossRef]
- Elkanawati, R.Y.; Sumiwi, S.A.; Levita, J. Impact of Lipids on Insulin Resistance: Insights from Human and Animal Studies. Drug Des. Dev. Ther. 2024, 18, 3337–3360. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Lee, H.-Y. Mechanisms linking gut microbial metabolites to insulin resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Freeman, A.M.; Acevedo, L.A. Pennings N. Insulin Resistance; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Zhang, A.M.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311, Erratum in Diabetes Metab. J. 2021, 45, 622. https://doi.org/10.4093/dmj.2021.0131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.J.C.M.M.; van der Lely, A.J. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83–89. [Google Scholar] [CrossRef]
- Giustina, A.; Berardelli, R.; Gazzaruso, C.; Mazziotti, G. Insulin and GH–IGF-I axis: Endocrine pacer or endocrine disruptor? Acta Diabetol. 2014, 52, 433–443. [Google Scholar] [CrossRef]
- Macvanin, M.; Gluvic, Z.; Radovanovic, J.; Essack, M.; Gao, X.; Isenovic, E.R. New insights on the cardiovascular effects of IGF-1. Front. Endocrinol. 2023, 14, 1142644. [Google Scholar] [CrossRef]
- Houston, E.J.; Templeman, N.M. Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS. J. Endocrinol. 2025, 265, e240269. [Google Scholar] [CrossRef]
- Rangraze, I.R.; El-Tanani, M.; Rabbani, S.A.; Babiker, R.; Matalka, I.I.; Rizzo, M. Diabetes and its Silent Partner: A Critical Review of Hyperinsulinemia and its Complications. Curr. Diabetes Rev. 2025, 21, e15733998311738. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Overnutrition, Hyperinsulinemia and Ectopic Fat: It Is Time for A Paradigm Shift in the Management of Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 5488. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. DNA stabilization by the upregulation of estrogen signaling in BRCA gene mutation carriers. Drug Des. Dev. Ther. 2015, 9, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2018, 68, 291–304. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Gregorio, K.C.R.; Laurindo, C.P.; Machado, U.F. Estrogen and Glycemic Homeostasis: The Fundamental Role of Nuclear Estrogen Receptors ESR1/ESR2 in Glucose Transporter GLUT4 Regulation. Cells 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Gunter, M.J.; Wylie-Rosett, J.; Ho, G.Y.F.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes/Metab. Res. Rev. 2009, 25, 3–12. [Google Scholar] [CrossRef]
- Suba, Z. Compensatory Estrogen Signal Is Capable of DNA Repair in Antiestrogen-Responsive Cancer Cells via Activating Mutations. J. Oncol. 2020, 2020, 5418365. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Insulin/IGF-1 signaling promotes immunosuppression via the STAT3 pathway: Impact on the aging process and age-related diseases. Inflamm. Res. 2021, 70, 1043–1061. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hammarstedt, A.; Vandenput, L.; Saarinen, N.; Ryberg, H.; Windahl, S.H.; Farman, H.H.; Jansson, J.-O.; Movérare-Skrtic, S.; Smith, U.; et al. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am. J. Physiol. Metab. 2017, 313, E450–E462. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Kim, J.Y.; Singh, M.; Shin, Y.-K.; Kim, J.; Kumbrink, J.; Wu, Y.; Lee, M.-J.; Kirsch, K.H.; Fried, S.K.; et al. Insulin Inhibits Lipolysis in Adipocytes via the Evolutionarily Conserved mTORC1-Egr1-ATGL-Mediated Pathway. Mol. Cell. Biol. 2013, 33, 3659–3666. [Google Scholar] [CrossRef]
- Ko, S.-H.; Kim, H.-S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Wang, H.; Shi, F.; Zheng, L.; Zhou, W.; Mi, B.; Wu, S.; Feng, X. Gut microbiota has the potential to improve health of menopausal women by regulating estrogen. Front. Endocrinol. 2025, 16, 1562332. [Google Scholar] [CrossRef]
- Tao, Z.; Cheng, Z. Hormonal regulation of metabolism—recent lessons learned from insulin and estrogen. Clin. Sci. 2023, 137, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Liu, W.H.; Liu, Y.; Wang, L.; Xiao, Y. PID1 alters the antilipolytic action of insulin and increases lipolysis via inhibition of AKT/PKA pathway activation. PLoS ONE 2019, 14, e0214606, Erratum in PLoS ONE 2019, 17, e0218721. https://doi.org/10.1371/journal.pone.0218721. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Semo, D.; Reinecke, H.; Godfrey, R. Gut microbiome regulates inflammation and insulin resistance: A novel therapeutic target to improve insulin sensitivity. Signal Transduct. Target. Ther. 2024, 9, 35. [Google Scholar] [CrossRef]
- Rishabh; Bansal, S.; Goel, A.; Gupta, S.; Malik, D.; Bansal, N.; Chaudhary, R. Unravelling the Crosstalk between Estrogen Deficiency and Gut-biota Dysbiosis in the Development of Diabetes Mellitus. Curr. Diabetes Rev. 2024, 20, 69–79. [Google Scholar] [CrossRef]
- Link, C.D. Is There a Brain Microbiome? Neurosci. Insights 2021, 16, 26331055211018709. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Huang, X.; Pan, C.-H.; Yin, F.; Peng, J.; Yang, L. The Role of Estrogen in Mitochondrial Disease. Cell. Mol. Neurobiol. 2025, 45, 68. [Google Scholar] [CrossRef]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G.; et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792, Erratum in Nature 1996, 22, 379–749. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.R. Cancer Susceptibility and the Functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.J.; Kennedy, R.D.; Hosey, A.M.; Harkin, D.P. The Complex Relationship between BRCA1 and ERα in Hereditary Breast Cancer. Clin. Cancer Res. 2009, 15, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Di, L.-J. BRCA1 And Estrogen/Estrogen Receptor In Breast Cancer: Where They Interact? Int. J. Biol. Sci. 2014, 10, 566–575. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Van De, V.M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The pathology of familial breast cancer: Predictive value of immunohisto-chemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- dos Santos, E.S.; Lallemand, F.; Petitalot, A.; Caputo, S.M.; Rouleau, E. HRness in Breast and Ovarian Cancers. Int. J. Mol. Sci. 2020, 21, 3850. [Google Scholar] [CrossRef]
- Spillman, M.A.; Bowcock, A.M. BRCA1 and BRCA2 mRNA levels are coordinately elevated in human breast cancer cells in response to estrogen. Oncogene 1996, 13, 1639–1645. [Google Scholar] [PubMed]
- Suba, Z. Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: Preventive and therapeutic implications. OncoTargets Ther. 2014, 7, 147–164. [Google Scholar] [CrossRef]
- Fan, S.; Wang, J.-A.; Yuan, R.; Ma, Y.; Meng, Q.; Erdos, M.R.; Pestell, R.G.; Yuan, F.; Auborn, K.J.; Goldberg, I.D.; et al. BRCA1 Inhibition of Estrogen Receptor Signaling in Transfected Cells. Science 1999, 284, 1354–1356. [Google Scholar] [CrossRef]
- Xu, J.; Fan, S.; Rosen, E.M. Regulation of the Estrogen-Inducible Gene Expression Profile by the Breast Cancer Susceptibility Gene BRCA1. Endocrinology 2005, 146, 2031–2047. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fan, S.; Hu, C.; Meng, Q.; Fuqua, S.A.; Pestell, R.G.; Tomita, Y.A.; Rosen, E.M. BRCA1 regulates acetylation and ubiquitination of estrogen recep-tor-alpha. Mol. Endocrinol. 2010, 24, 76–90. [Google Scholar] [CrossRef]
- Fan, S.; Ma, Y.X.; Wang, C.; Yuan, R.-Q.; Meng, Q.; Wang, J.-A.; Erdos, M.; Goldberg, I.D.; Webb, P.; Kushner, P.J.; et al. p300 Modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res. 2002, 62, 141–151. [Google Scholar] [PubMed]
- Wang, C.; Fan, S.; Li, Z.; Fu, M.; Rao, M.; Ma, Y.; Lisanti, M.P.; Albanese, C.; Katzenellenbogen, B.S.; Kushner, P.J.; et al. Cyclin D1 Antagonizes BRCA1 Repression of Estrogen Receptor α Activity. Cancer Res. 2005, 65, 6557–6567. [Google Scholar] [CrossRef]
- Fan, S.; Ma, Y.X.; Wang, C.; Yuan, R.-Q.; Meng, Q.; Wang, J.-A.; Erdos, M.; Goldberg, I.D.; Webb, P.; Kushner, P.J.; et al. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 2001, 20, 77–87. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. Toward a unified concept of mammary carcinogenesis. In Progress in Clinical and Biological Research; Aldaz, M.C., Gould, M.N., McLachlan, J., Slaga, T.J., Eds.; Wiley-Liss: New York, NY, USA, 1997. [Google Scholar]
- Hosey, A.M.; Gorski, J.J.; Murray, M.M.; Quinn, J.E.; Chung, W.Y.; Stewart, G.E.; James, C.R.; Farragher, S.M.; Mulligan, J.M.; Scott, A.N.; et al. Molecular Basis for Estrogen Receptor Deficiency in BRCA1-Linked Breast Cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Burga, L.N.; Hu, H.; Juvekar, A.; Tung, N.M.; Troyan, S.L.; Hofstatter, E.W.; Wulf, G.M. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 2011, 13, R30. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, C.; Riegel, A.T.; Fan, S.; Rosen, E.M. Growth Factor Signaling Pathways Modulate BRCA1 Repression of Estrogen Receptor-α Activity. Mol. Endocrinol. 2007, 21, 1905–1923. [Google Scholar] [CrossRef]
- Ghosh, S.; Lu, Y.; Katz, A.; Hu, Y.; Li, R. Tumor suppressor BRCA1 inhibits a breast cancer-associated promoter of the aromatase gene (CYP19) in human adipose stromal cells. Am. J. Physiol. Metab. 2007, 292, E246–E252. [Google Scholar] [CrossRef]
- Sau, A.; Lau, R.; Cabrita, M.A.; Nolan, E.; Crooks, P.A. Persistent Activation of NF-κB in BRCA1-Deficient Mammary Progenitors Drives Aberrant Proliferation and Accumulation of DNA Damage. Cell Stem Cell 2016, 19, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Annab, L.A.; Afshari, C.A.; Lee, W.-H.; Boyer, T.G. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 9587–9592. [Google Scholar] [CrossRef]
- Arnold, A.; Papanikolaou, A. Cyclin D1 in Breast Cancer Pathogenesis. J. Clin. Oncol. 2005, 23, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; El-Halaby, A.A.; Zhang, H.; Yang, Q.; Laughlin, T.S.; Rothberg, P.G.; Skinner, K.; Hicks, D.G. p53 alteration in morphologically normal/benign breast luminal cells in BRCA carriers with or without history of breast cancer. Hum. Pathol. 2017, 68, 22–25. [Google Scholar] [CrossRef]
- Oktay, K.; Kim, J.Y.; Barad, D.; Babayev, S.N. Association of BRCA1 Mutations With Occult Primary Ovarian Insufficiency: A Possible Explanation for the Link Between Infertility and Breast/Ovarian Cancer Risks. J. Clin. Oncol. 2010, 28, 240–244. [Google Scholar] [CrossRef]
- Lin, W.T.; Beattie, M.; Chen, L.; Oktay, K.; Crawford, S.L.; Gold, E.B.; Cedars, M.; Rosen, M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non–clinic-based sample of women in northern California. Cancer 2013, 119, 1652–1659. [Google Scholar] [CrossRef]
- kConFab; Chand, A.L.; Simpson, E.R.; Clyne, C.D. Aromatase expression is increased in BRCA1mutation carriers. BMC Cancer 2009, 9, 148. [Google Scholar] [CrossRef]
- Bruno, E.; Manoukian, S.; Venturelli, E.; Oliverio, A.; Rovera, F.; Iula, G.; Morelli, D.; Peissel, B.; Azzolini, J.; Roveda, E.; et al. Adherence to Mediterranean Diet and Metabolic Syndrome in BRCA Mutation Carriers. Integr. Cancer Ther. 2017, 17, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lentscher, J.A.; Decherney, A.H. Clinical Presentation and Diagnosis of Polycystic Ovarian Syndrome. Clin. Obstet. Gynecol. 2020, 64, 3–11. [Google Scholar] [CrossRef]
- Zańko, A.; Siewko, K.; Krętowski, A.J.; Milewski, R. Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. Int. J. Environ. Res. Public Health 2022, 20, 732. [Google Scholar] [CrossRef]
- Dong, J.; Rees, D.A. Polycystic ovary syndrome: Pathophysiology and therapeutic opportunities. BMJ Med. 2023, 2, e000548. [Google Scholar] [CrossRef]
- Blackwood, S.J.; Tischer, D.; Pontén, M.; Moberg, M.; Katz, A. Relationship Between Insulin Sensitivity and Hyperinsulinemia in Early Insulin Resistance is Sex-dependent. J. Clin. Endocrinol. Metab. 2025. [Google Scholar] [CrossRef]
- Hernández-Jiménez, J.L.; Barrera, D.; Espinoza-Simón, E.; González, J.; Ortíz-Hernández, R.; Escobar, L.; Echeverría, O.; Torres-Ramírez, N. Polycystic ovarian syndrome: Signs and feedback effects of hyperandrogenism and insulin resistance. Gynecol. Endocrinol. 2021, 38, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Aromatase and estrogen receptor α deficiency. Fertil. Steril. 2014, 101, 323–329. [Google Scholar] [CrossRef]
- Suba, Z. Diverse pathomechanisms leading to the breakdown of cellular estrogen surveillance and breast cancer development: New therapeutic strategies. Drug Des. Dev. Ther. 2014, 8, 1381–1390. [Google Scholar] [CrossRef]
- Quaynor, S.D.; Stradtman, E.W.J.; Kim, H.-G.; Shen, Y.; Chorich, L.P.; Schreihofer, D.A.; Layman, L.C. Delayed Puberty and Estrogen Resistance in a Woman with Estrogen Receptor α Variant. N. Engl. J. Med. 2013, 369, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Dentamaro, I.; Carbonara, R.; Bulzis, G.; Dachille, A.; Caputo, P.; Riccardi, R.; Locorotondo, M.; Mandurino, C.; Ciccone, M.M. Cardiovascular Risk in Women With PCOS. Int. J. Endocrinol. Metab. 2012, 10, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.T.; Hartzema, A.G.; Brophy, J.M.; Etminan, M.; Delaney, J.A. Risk of venous thromboembolism in women with polycystic ovary syndrome: A population-based matched cohort analysis. Can. Med. Assoc. J. 2012, 185, E115–E120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef]
- Yuk, J.; Noh, J.H.; Han, G.H.; Yoon, S.H.; Kim, M. Risk of cancers in women with polycystic ovary syndrome: Cohort study based on health insurance database in South Korea. Int. J. Gynecol. Obstet. 2025. [Google Scholar] [CrossRef]
- Ignatov, A.; Ortmann, O. Endocrine Risk Factors of Endometrial Cancer: Polycystic Ovary Syndrome, Oral Contraceptives, Infertility, Tamoxifen. Cancers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Estrogen Receptors in Polycystic Ovary Syndrome. Cells 2021, 10, 459. [Google Scholar] [CrossRef]
- Casper, R.F.M.; Mitwally, M.F.M. Use of the Aromatase Inhibitor Letrozole for Ovulation Induction in Women With Polycystic Ovarian Syndrome. Clin. Obstet. Gynecol. 2011, 54, 685–695. [Google Scholar] [CrossRef]
- Epstein, F.H.; Atkinson, M.A.; Maclaren, N.K. The Pathogenesis of Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar] [CrossRef]
- Lemos, J.R.N.; Hirani, K.; von Herrath, M. Immunological and virological triggers of type 1 diabetes: Insights and implications. Front. Immunol. 2024, 14, 1326711. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, K.-J.; Peng, Y.-S.; Chen, P.-C.; Yang, Y.-H. Type 1 diabetes impairs female fertility even before it is diagnosed. Diabetes Res. Clin. Pract. 2018, 143, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cichocka, E.; Maj-Podsiadło, A.; Gumprecht, J. Polycystic ovary syndrome and type 1 diabetes — The current state of knowledge. Endokrynol. Polska 2024, 75, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Facondo, P.; Di Lodovico, E.; Delbarba, A.; Anelli, V.; Pezzaioli, L.C.; Filippini, E.; Cappelli, C.; Corona, G.; Ferlin, A. The impact of diabetes mellitus type 1 on male fertility: Systematic review and meta-analysis. Andrology 2021, 10, 426–440. [Google Scholar] [CrossRef]
- Khadilkar, A.; Oza, C.; Mondkar, S.A. Insulin Resistance in Adolescents and Youth With Type 1 Diabetes: A Review of Problems and Solutions. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231206730. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Lukaszuk, B.; Chabowski, A. The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? Int. J. Environ. Res. Public Health 2020, 17, 8651. [Google Scholar] [CrossRef]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.-J.; Mauvais-Jarvis, F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef]
- Contreras, J.L.; Smyth, C.A.; Bilbao, G.; Young, C.J.; Thompson, J.A.; Eckhoff, D.E. 17β-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: Molecular mechanisms and islet functionality1. Transplantation 2002, 74, 1252–1259. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef]
- Scheithauer, T.P.; Dallinga-Thie, G.M.; de Vos, W.M.; Nieuwdorp, M.; van Raalte, D.H. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol. Metab. 2016, 5, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children With Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 14650. [Google Scholar] [CrossRef]
- Gong, I.Y.; Cheung, M.C.; Read, S.; Na, Y.; Lega, I.C.; Lipscombe, L.L. Association between diabetes and haematological malignancies: A population-based study. Diabetologia 2021, 64, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Ventelä, J.; Korja, M.; Auvinen, A.; Lohi, O.; Nikkilä, A. Clustering of childhood acute leukemia in Finland: A nationwide register-based study. Cancer Causes Control. 2025, 36, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Todor, S.B.; Ichim, C. Microbiome Modulation in Pediatric Leukemia: Impact on Graft-Versus-Host Disease and Treatment Outcomes: A Narrative Review. Children 2025, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Swafford, A.D.-E.; Howson, J.M.; Davison, L.J.; Wallace, C.; Smyth, D.J.; Schuilenburg, H.; Maisuria-Armer, M.; Mistry, T.; Lenardo, M.J.; Todd, J.A. An Allele of IKZF1 (Ikaros) Conferring Susceptibility to Childhood Acute Lymphoblastic Leukemia Protects Against Type 1 Diabetes. Diabetes 2011, 60, 1041–1044. [Google Scholar] [CrossRef]
- Churchman, M.L.; Qian, M.; Kronnie, G.T.; Zhang, R.; Yang, W.; Zhang, H.; Lana, T.; Tedrick, P.; Baskin, R.; Verbist, K.; et al. Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer Cell 2018, 33, 937–948.e8. [Google Scholar] [CrossRef]
- Read, K.A.; Jones, D.M.; Freud, A.G.; Oestreich, K.J. Established and emergent roles for Ikaros transcription factors in lymphoid cell development and function. Immunol. Rev. 2020, 300, 82–99. [Google Scholar] [CrossRef]
- Noble, J.A. Fifty years of HLA-associated type 1 diabetes risk: History, current knowledge, and future directions. Front. Immunol. 2024, 15, 1457213. [Google Scholar] [CrossRef]
- Sharp, S.A.; Rich, S.S.; Wood, A.R.; Jones, S.E.; Beaumont, R.N.; Harrison, J.W.; Schneider, D.A.; Locke, J.M.; Tyrrell, J.; Weedon, M.N.; et al. Development and Standardization of an Improved Type 1 Diabetes Genetic Risk Score for Use in Newborn Screening and Incident Diagnosis. Diabetes Care 2019, 42, 200–207. [Google Scholar] [CrossRef]
- Qu, H.; Hakonarson, H. Sex as a modifier of genetic risk for type 1 diabetes. Diabetes, Obes. Metab. 2025, 27, 6857–6868. [Google Scholar] [CrossRef]
- Qian, L.; Shi, H.; Ding, M. Comparative analysis of gene expression profiles in children with type 1 diabetes mellitus. Mol. Med. Rep. 2019, 19, 3989–4000. [Google Scholar] [CrossRef]
- Słomiński, B.; Myśliwska, J.; Ryba-Stanisławowska, M.; Skrzypkowska, M.; Myśliwiec, M. Estrogen receptor α gene polymorphism and vascular complications in girls with type 1 diabetes mellitus. Mol. Cell. Biochem. 2017, 437, 153–161. [Google Scholar] [CrossRef]
- Wang, B.; Fu, Z.-Y.; Ma, Y.-T.; Huang, D.; Liu, F.; Dong, C.-L.; Wang, T.; Meng, Y.-J. Identification of a CYP19 Gene Single-Nucleotide Polymorphism Associated with a Reduced Risk of Coronary Heart Disease. Genet. Test. Mol. Biomark. 2016, 20, 2–10. [Google Scholar] [CrossRef]
- Ryba, M.; Malinowska, E.; Rybarczyk-Kapturska, K.; Brandt, A.; Myśliwiec, M.; Myśliwska, J. The association of the IVS1-397T>C estrogen receptor α polymorphism with the regulatory conditions in longstanding type 1 diabetic girls. Mol. Immunol. 2011, 49, 324–328. [Google Scholar] [CrossRef]
- Maggi, A. Liganded and unliganded activation of estrogen receptor and hormone replacement therapies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.W.; Washburn, T.; Sewall, C.; DiAugustine, R.; Lindzey, J.; Couse, J.F.; Korach, K.S. Physiological coupling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc. Natl. Acad. Sci. USA 1996, 93, 12626–12630. [Google Scholar] [CrossRef]
- Levin, E.R. Bidirectional Signaling between the Estrogen Receptor and the Epidermal Growth Factor Receptor. Mol. Endocrinol. 2003, 17, 309–317. [Google Scholar] [CrossRef]
- Suba, Z. Amplified Crosstalk Between Estrogen Binding and GFR Signaling Mediated Pathways of ER Activation Drives Responses in Tumors Treated with Endocrine Disruptors. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 428–444. [Google Scholar] [CrossRef]
- Suba, Z. Rosetta Stone for Cancer Cure: Comparison of the Anticancer Capacity of Endogenous Estrogens, Synthetic Estrogens and Antiestrogens. Oncol. Rev. 2023, 17, 10708. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Activating Mutations of ESR1, BRCA1 and CYP19 Aromatase Genes Confer Tumor Response in Breast Cancers Treated with Antiestrogens. Recent Pat. Anti-Cancer Drug Discov. 2017, 12, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.A.; Gustafsson, J.Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tiano, J.P.; Mauvais-Jarvis, F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat. Rev. Endocrinol. 2012, 8, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and Exercise May Enhance β-Cell Function and Mass via Insulin Receptor Substrate 2 Induction in Ovariectomized Diabetic Rats. Endocrinology 2005, 146, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Campello, R.S.; Fátima, L.A.; Barreto-Andrade, J.N.; Lucas, T.F.; Mori, R.C.; Porto, C.S.; Machado, U.F. Estradiol-induced regulation of GLUT4 in 3T3-L1 cells: Involvement of ESR1 and AKT activation. J. Mol. Endocrinol. 2017, 59, 257–268. [Google Scholar] [CrossRef]
- Steiner, B.M.; Berry, D.C. The Regulation of Adipose Tissue Health by Estrogens. Front. Endocrinol. 2022, 13, 889923. [Google Scholar] [CrossRef]
- Lizcano, F.; Guzmán, G. Estrogen Deficiency and the Origin of Obesity during Menopause. BioMed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Luu-The, V.; Labrie, C.; Simard, J.; Cusan, L.; Gomez, J.-L.; Candas, B. DHEA and the Intracrine Formation of Androgens and Estrogens in Peripheral Target Tissues: Its Role during Aging. Steroids 1998, 63, 322–328. [Google Scholar] [CrossRef]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.Å.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front. Endocrinol. 2022, 13, 828780. [Google Scholar] [CrossRef]
- Dieudonné, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for functional estrogen receptors α and β in human adipose cells: Regional specificities and regulation by estrogens. Am. J. Physiol. Physiol. 2004, 286, C655–C661. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The role of estrogen in adipose tissue metabolism: Insights into glucose homeostasis regulation [Review]. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef]
- Ahmed, F.; Kamble, P.G.; Hetty, S.; Fanni, G.; Vranic, M.; Sarsenbayeva, A.; Kristófi, R.; Almby, K.; Svensson, M.K.; Pereira, M.J.; et al. Role of Estrogen and Its Receptors in Adipose Tissue Glucose Metabolism in Pre- and Postmenopausal Women. J. Clin. Endocrinol. Metab. 2022, 107, e1879–e1889. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Leclercq, G. Recent insights into the effect of natural and environmental estrogens on mammary development and carcinogenesis. Int. J. Dev. Biol. 2011, 55, 869–878. [Google Scholar] [CrossRef]
- Wang, H.; Leng, Y.; Gong, Y. Bone Marrow Fat and Hematopoiesis. Front. Endocrinol. 2018, 9, 694. [Google Scholar] [CrossRef]
- Wang, P.; Mariman, E.; Renes, J.; Keijer, J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell. Physiol. 2008, 216, 3–13. [Google Scholar] [CrossRef]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen Signaling in Metabolic Inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef]
- Boon, W.C.; Jenny, D.Y.; Chow, J.D.Y.; Simpson, E.R. The Multiple Roles of Estrogens and the Enzyme Aromatase. Prog. Brain Res. 2010, 181, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal Hormones Determine Sensitivity to Central Leptin and Insulin. Diabetes 2006, 55, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Pajvani, U.B.; Berg, A.H.; Lin, Y.; Jelicks, L.A.; Laplante, M.; Nawrocki, A.R.; Rajala, M.W.; Parlow, A.F.; Cheeseboro, L.; et al. A Transgenic Mouse with a Deletion in the Collagenous Domain of Adiponectin Displays Elevated Circulating Adiponectin and Improved Insulin Sensitivity. Endocrinology 2004, 145, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Smith, A.; Woodside, B.; Abizaid, A. Ghrelin and the Control of Energy Balance in Females. Front. Endocrinol. 2022, 13, 904754. [Google Scholar] [CrossRef]
- Suba, Z. Crossroad between obesity and cancer: A defective signaling function of heavily lipid-laden adipocytes. In Crosstalk in Biological Processes; El-Esawi, M.A., Ed.; InTechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Lueprasitsakul, P.; Latour, D.; Longcope, C. Aromatase activity in human adipose tissue stromal cells: Effect of growth factors. Steroids 1990, 55, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular mechanisms underlying metabolic syndrome: The expanding role of the adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol. Cells 2014, 37, 365–371. [Google Scholar] [CrossRef]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res. 2002, 4, 65–69. [Google Scholar] [CrossRef]
- Hope, M.C.; Unger, C.A.; Kettering, M.C.; Socia, C.E.; Aladhami, A.K.; Rice, B.C.; Niamira, D.S.; Wiznitzer, B.P.; Altomare, D.; Cotham, W.E.; et al. Adipose Tissue Estrogen Receptor-Alpha Overexpression Ameliorates High-Fat Diet–Induced Adipose Tissue Inflammation. J. Endocr. Soc. 2025, 9, bvaf134. [Google Scholar] [CrossRef]
- Blum, W.F.; Alherbish, A.; Alsagheir, A.; El Awwa, A.; Kaplan, W.; Koledova, E.; Savage, M.O. The growth hormone–insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr. Connect. 2018, 7, R212–R222. [Google Scholar] [CrossRef]
- Holly, J.M.; Perks, C.M. Insulin-like growth factor physiology: What we have learned from human studies. Endocrinol. Metab. Clin. N. Am. 2012, 41, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Tseng, Y.-H.; Kahn, C.R. Insulin and Insulin-like Growth Factor-1 Receptors Act as Ligand-specific Amplitude Modulators of a Common Pathway Regulating Gene Transcription. J. Biol. Chem. 2010, 285, 17235–17245. [Google Scholar] [CrossRef] [PubMed]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V.; Reineri, S.; Ricci, L.; Friard, O.; Testori, A.; et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4892–4897. [Google Scholar] [CrossRef]
- Barthelemy, J.; Bogard, G.; Wolowczuk, I. Beyond energy balance regulation: The underestimated role of adipose tissues in host defense against pathogens. Front. Immunol. 2023, 14, 1083191. [Google Scholar] [CrossRef]
- Millas, I.; Barros, M.D. Estrogen receptors and their roles in the immune and respiratory systems. Anat. Rec. 2021, 304, 1185–1193. [Google Scholar] [CrossRef]
- Bradley, D.; Deng, T.; Shantaram, D.; Hsueh, W.A. Orchestration of the Adipose Tissue Immune Landscape by Adipocytes. Annu. Rev. Physiol. 2024, 86, 199–223. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 2011, 14, 58–66. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.-J.; Chen, G.-X. Current understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 2020, 11, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Pereira, R.M.; de Moura, L.P.; Muñoz, V.R.; da Silva, A.S.R.; Gaspar, R.S.; Ropelle, E.R.; Pauli, J.R. Molecular mechanisms of glucose uptake in skeletal muscle at rest and in response to exercise. Mot. Rev. Educ. Física 2017, 23, e101609. [Google Scholar] [CrossRef]
- Merino, B.; García-Arévalo, M. Sexual hormones and diabetes: The impact of estradiol in pancreatic β cell. Int. Rev. Cell Mol. Biol. 2021, 359, 81–138. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zugaza, J.L.; Aleman, I.T. The signaling landscape of insulin-like growth factor 1. J. Biol. Chem. 2024, 301, 108047. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid steroid hormone actions via membrane receptors. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Karas, R.H. Rapid progress for non-nuclear estrogen receptor signaling. J. Clin. Investig. 2010, 120, 2277–2279. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Cordoba-Chacon, J.; Kineman, R.D. Growth Hormone and Insulin-Like Growth Factor 1 Regulation of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, 1812–1824. [Google Scholar] [CrossRef]
- Fernández-Pérez, L.; Guerra, B.; Díaz-Chico, J.C.; Flores-Morales, A. Estrogens Regulate the Hepatic Effects of Growth Hormone, a Hormonal Interplay with Multiple Fates. Front. Endocrinol. 2013, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Chaturantabut, S.; Shwartz, A.; Evason, K.J.; Cox, A.G.; Labella, K.; Schepers, A.G.; Yang, S.; Acuña, M.; Houvras, Y.; Mancio-Silva, L.; et al. Estrogen Activation of G-Protein–Coupled Estrogen Receptor 1 Regulates Phosphoinositide 3-Kinase and mTOR Signaling to Promote Liver Growth in Zebrafish and Proliferation of Human Hepatocytes. Gastroenterology 2019, 156, 1788–1804.e13. [Google Scholar] [CrossRef]
- Faulds, M.H.; Zhao, C.; Dahlman-Wright, K.; Gustafsson, J. The diversity of sex steroid action: Regulation of metabolism by estrogen signaling. J. Endocrinol. 2011, 212, 3–12. [Google Scholar] [CrossRef]
- Yakar, S.; Adamo, M.L. Insulin-Like Growth Factor 1 Physiology. Endocrinol. Metab. Clin. N. Am. 2012, 41, 231–247. [Google Scholar] [CrossRef]
- Kahlert, S.; Nuedling, S.; van Eickels, M.; Vetter, H.; Meyer, R.; Grohé, C. Estrogen Receptor α Rapidly Activates the IGF-1 Receptor Pathway. J. Biol. Chem. 2000, 275, 18447–18453. [Google Scholar] [CrossRef]
- Flores, B.; Trivedi, H.D.; Robson, S.C.; Bonder, A. Hemostasis, bleeding and thrombosis in liver disease. J. Transl. Sci. 2017, 3. [Google Scholar] [CrossRef]
- Muciño-Bermejo, J.; Carrillo-Esper, R.; Méndez-Sánchez, N.; Uribe, M. Thrombosis and hemorrhage in the critically ill cirrhotic patients: Five years retrospective prevalence study. Ann. Hepatol. 2015, 14, 93–98. [Google Scholar] [CrossRef]
- Kostallari, E.; Schwabe, R.F.; Guillot, A. Inflammation and immunity in liver homeostasis and disease: A nexus of hepatocytes, nonparenchymal cells and immune cells. Cell. Mol. Immunol. 2025, 22, 1205–1225. [Google Scholar] [CrossRef]
- Bryzgalova, G.; Lundholm, L.; Portwood, N.; Gustafsson, J.Å.; Khan, A.; Efendic, S.; Dahlman-Wright, K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am. J. Physiol. Metab. 2008, 295, E904–E912. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Carreau, S.; Ellouz, F.; Masmoudi, H.; El Feki, A. Protective effect of 17β-estradiol on oxidative stress and liver dysfunction in aged male rats. J. Physiol. Biochem. 2007, 63, 195–201. [Google Scholar] [CrossRef]
- Anagnostis, P.; Stevenson, J.C.; Crook, D.; Johnston, D.G.; Godsland, I.F. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015, 81, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Lambrinoudaki, I.; Stevenson, J.C.; Goulis, D.G. Menopause-associated risk of cardiovascular disease. Endocr. Connect. 2022, 11, e210537. [Google Scholar] [CrossRef]
- Cetin, E.G.; Demir, N.; Sen, I. The Relationship between Insulin Resistance and Liver Damage in non-alcoholic Fatty Liver Patients. SiSli Etfal Hast. Tip Bulteni/Med. Bull. Sisli Hosp. 2020, 54, 411–415. [Google Scholar] [CrossRef]
- Talamantes, S.; Lisjak, M.; Gilglioni, E.H.; Llamoza-Torres, C.J.; Ramos-Molina, B.; Gurzov, E.N. Non-alcoholic fatty liver disease and diabetes mellitus as growing aetiologies of hepatocellular carcinoma. JHEP Rep. 2023, 5, 100811. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mittal, A.; Jain, V.R.; Bharadwaj, A.; Modi, S.; Ahuja, G.; Jain, A.; Kumar, K. Integrative Functions of the Hypothalamus: Linking Cognition, Emotion and Physiology for Well-being and Adaptability. Ann. Neurosci. 2024, 32, 128–142. [Google Scholar] [CrossRef]
- Zeng, Y.; Rong, R.; You, M.; Zhu, P.; Zhang, J.; Xia, X. Light-eye-body axis: Exploring the network from retinal illumination to systemic regulation. Theranostics 2025, 15, 1496–1523. [Google Scholar] [CrossRef]
- Alvord, V.M.; Kantra, E.J.; Pendergast, J.S. Estrogens and the circadian system. Semin. Cell Dev. Biol. 2022, 126, 56–65. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocr. 2014, 35, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocr. 2018, 30, e12590. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Irizarry, V.C.T.; Jiang, Y.; He, Y.; Xu, P. Hypothalamic Estrogen Signaling and Adipose Tissue Metabolism in Energy Homeostasis. Front. Endocrinol. 2022, 13, 898139. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A. Control of energy homeostasis by the lateral hypothalamic area. Trends Neurosci. 2023, 46, 738–749. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.; Yuan, Y.; Rong, Y.; Pang, R.; Li, Q. Neural and hormonal mechanisms of appetite regulation during eating. Front. Nutr. 2025, 12, 1484827. [Google Scholar] [CrossRef]
- Butera, P.C. Estradiol and the control of food intake. Physiol. Behav. 2010, 99, 175–180. [Google Scholar] [CrossRef]
- Islami, F.; Marlow, E.C.; Thomson, B.; McCullough, M.L.; Rumgay, H.; Gapstur, S.M.; Patel, A.V.; Soerjomataram, I.; Jemal, A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, 2019. CA A Cancer J. Clin. 2024, 74, 405–432. [Google Scholar] [CrossRef] [PubMed]
- Personal Habits and Indoor Combustions. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Working Group; IARC Press: Lyon, France, 2012; Volume 100E. [Google Scholar]
- Cena, H.; Fonte, M.L.; Turconi, G. Relationship between smoking and metabolic syndrome. Nutr. Rev. 2011, 69, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Artese, A.; Stamford, B.A.; Moffatt, R.J. Cigarette Smoking: An Accessory to the Development of Insulin Resistance. Am. J. Lifestyle Med. 2017, 13, 602–605. [Google Scholar] [CrossRef]
- Barbieri, R.L.; Gochberg, J.; Ryan, K.J. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J. Clin. Investig. 1986, 77, 1727–1733. [Google Scholar] [CrossRef]
- Biegon, A.; Alia-Klein, N.; Fowler, J.S. Potential Contribution of Aromatase Inhibition to the Effects of Nicotine and Related Compounds on the Brain. Front. Pharmacol. 2012, 3, 185. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, Y.; Guan, Q.; Gao, L.; Lee, K.O.; Zhao, J. Ethanol Feeding Impairs Insulin-Stimulated Glucose Uptake in Isolated Rat Skeletal Muscle: Role of Gs α and cAMP. Alcohol. Clin. Exp. Res. 2005, 29, 1450–1456. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Perry, I.J.; Alberti, K.G.M.M. Alcohol consumption and the incidence of type II diabetes. J. Epidemiol. Community Health 2002, 56, 542–548. [Google Scholar] [CrossRef]
- Johnson, C.H.; Golla, J.P.; Dioletis, E.; Singh, S.; Ishii, M.; Charkoftaki, G.; Thompson, D.C.; Vasiliou, V. Molecular Mechanisms of Alcohol-Induced Colorectal Carcinogenesis. Cancers 2021, 13, 4404. [Google Scholar] [CrossRef]
- Orywal, K.; Szmitkowski, M. Alcohol dehydrogenase and aldehyde dehydrogenase in malignant neoplasms. Clin. Exp. Med. 2016, 17, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N.; Sarkar, D.K. Effects of Alcohol on the Endocrine System. Endocrinol. Metab. Clin. N. Am. 2013, 42, 593–615. [Google Scholar] [CrossRef]
- Fanfarillo, F.; Caronti, B.; Lucarelli, M.; Francati, S.; Tarani, L.; Ceccanti, M.; Piccioni, M.G.; Verdone, L.; Caserta, M.; Venditti, S.; et al. Alcohol Consumption and Breast and Ovarian Cancer Development: Molecular Pathways and Mechanisms. Curr. Issues Mol. Biol. 2024, 46, 14438–14452. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Haley, R.L.; Blackburn, M.L.; Badger, T.M.; Ronis, M.J. Ethanol Impairs Estrogen Receptor Signaling Resulting in Accelerated Activation of Senescence Pathways, Whereas Estradiol Attenuates the Effects of Ethanol in Osteoblasts. J. Bone Miner. Res. 2009, 24, 221–230. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; Neuhouser, M.L. Obesity and the Risk for Premenopausal and Postmenopausal Breast Cancer. Cancer Prev. Res. 2012, 5, 515–521. [Google Scholar] [CrossRef]
- Suba, Z. Circulatory Estrogen Level Protects Against Breast Cancer in Obese Women. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 154–167. [Google Scholar] [CrossRef]
- Gong, D.; Lai, W.-F. Dietary patterns and type 2 diabetes: A narrative review. Nutrition 2025, 140, 112905. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Markozannes, G.; Kanellopoulou, A.; Critselis, E.; Alhardan, S.; Karafousia, V.; Kasimis, J.C.; Katsaraki, C.; Papadopoulou, A.; Zografou, M.; et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 2021, 12, 4579. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Sjøberg, K.A.; Frøsig, C.; Kjøbsted, R.; Sylow, L.; Kleinert, M.; Betik, A.C.; Shaw, C.S.; Kiens, B.; Wojtaszewski, J.F.; Rattigan, S.; et al. Exercise Increases Human Skeletal Muscle Insulin Sensitivity via Coordinated Increases in Microvascular Perfusion and Molecular Signaling. Diabetes 2017, 66, 1501–1510. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Sandhu, A.P.S.; Tanvir; Singh, K.; Singh, S.; Antaal, H.; Luthra, S.; Singla, A.; Nijjar, G.S.; Kaur, Y.; Aulakh, S.K. Decoding Cancer Risk: Understanding Gene-Environment Interactions in Cancer Development. Cureus 2024, 16, e64936. [Google Scholar] [CrossRef]
- Wadgaonkar, P. Targeting the Right Player via Nanotechnology. In Cancer Epigenetics and Nanomedicine; Academic Press: Cambridge, MA, USA, 2024; pp. 69–92. [Google Scholar]
- Kotsopoulos, J. BRCA Mutations and Breast Cancer Prevention. Cancers 2018, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Espinola, O.P.; Tobias, D.K.; Manson, J.E. The Role of Lifestyle Interventions for the Prevention and Treatment of Type 2 Diabetes: A Narrative Review. Am. J. Lifestyle Med. 2025. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Hansda, A.K.; Biswas, B.; Goswami, R. 17-β Estradiol (E2) distinctly regulates the expression of IL-4 and IL-13 in Th2 cells via modulating the interplay between GATA3 and PU.1. Cytokine 2023, 173, 156440. [Google Scholar] [CrossRef]
| Theories | References |
|---|---|
| Insulin resistance is the primary alteration | Wilcox 2005 [30] |
| Insulin resistance has various causal factors | Johnson et al. 2013 [31] |
| Low-grade inflammation is the origin of insulin resistance | Khodabandehloo et al. 2016 [32] |
| Lipotoxicity may impair glucose uptake | Elkanawati et al. 2024 [33] |
| Altered gut microbiome causes insulin resistance | Jang et al. 2021 [34] |
| Mitochondrial oxidative stress causes insulin resistance | Fazakerley et al. 2018 [35] |
| Impaired GLUT4 trafficking causes insulin resistance | van Gerven et al. 2023 [36] |
| Hyperinsulinemia is the primary alteration attributed to energy rich diet | Freeman et al. 2025 [37] Janssen 2025 [38] Zhang et al. 2021 [39] |
| Interplay among insulin, GH and IGF-I leads to hyperinsulinemia and insulin resistance | Nijenhuis-Noort et al. 2024 [40] |
| Hyperinsulinemia improves insulin resistance via activation of IGF-1 signaling | Giustina et al. 2015 [41] Macvanin et al. 2023 [42] |
| Findings | References |
|---|---|
| Estrogen controls genome stabilization, cell proliferation, and glucose supply | Suba 2015 [46] |
| Restoration of estrogen signaling improves glucose uptake and metabolic homeostasis | Yan et al. 2019 [47] Kurylowitz 2023 [48] |
| Insulin and estrogen signaling work together to regulate GLUT4 transport | Gregorio et al. 2021 [49] |
| Insulin-like growth factor 1(IGF-1) has a direct effect on glucose uptake both with and without insulin | Rajpathak et al. 2009 [50] |
| IGF-1 signaling is capable of the unliganded activation of estrogen receptors even in the absence of estrogen | Suba 2020 [51] |
| In insulin resistance, simultaneously increased insulin and IGF-1 signaling stimulate inflammation | Salminen et al. 2021 [52] |
| In insulin resistance, increasing expression of pro-inflammatory cytokines stimulates aromatase expression and estrogen concentration | Ohlsson et al. 2017 [53] |
| In insulin resistance, increased estrogen levels restore glucose uptake and a decreasing estrogen concentration reduces inflammation | Suba 2024 [8] |
| Estrogen and insulin simultaneously regulate the balance between lipolysis and lipogenesis. In insulin resistance, impaired estrogen function liberates free fatty acids. | Saponaro et al. 2015 [54] |
| In obesity, abundant free fatty acids in the circulation are pathologically deposited in non-adipose organs, a process attributed to weak estrogen signaling. | Chakrabarti et al. 2013 [55] Ro et al. 2020 [56] |
| The estrobolome, gut bacteria with β-glucuronidase activity, reactivates bound estrogens, improves insulin sensitivity, and supports the survival of pancreatic β-cells. | Wang et al. 2025 [57] |
| Insulin and estrogen signaling regulate autophagy and metabolism in mitochondria in close partnership. In insulin resistance, weak estrogen signaling leads to impaired mitochondrial function. | Tao et al. 2023 [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Suba, Z. Human Cancers Derived from Either Genetic or Lifestyle Factors Are Initiated by Impaired Estrogen Signaling. Cancers 2026, 18, 78. https://doi.org/10.3390/cancers18010078
Suba Z. Human Cancers Derived from Either Genetic or Lifestyle Factors Are Initiated by Impaired Estrogen Signaling. Cancers. 2026; 18(1):78. https://doi.org/10.3390/cancers18010078
Chicago/Turabian StyleSuba, Zsuzsanna. 2026. "Human Cancers Derived from Either Genetic or Lifestyle Factors Are Initiated by Impaired Estrogen Signaling" Cancers 18, no. 1: 78. https://doi.org/10.3390/cancers18010078
APA StyleSuba, Z. (2026). Human Cancers Derived from Either Genetic or Lifestyle Factors Are Initiated by Impaired Estrogen Signaling. Cancers, 18(1), 78. https://doi.org/10.3390/cancers18010078






