Prediction of Bone Metastasis in Prostate Cancer Using Blood Glucose-6-Phosphate Dehydrogenase Activity: A Retrospective Medical Record Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. G6PD Activity Test
2.3. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Logistic Regression Analysis for Predictors of Bone Metastasis
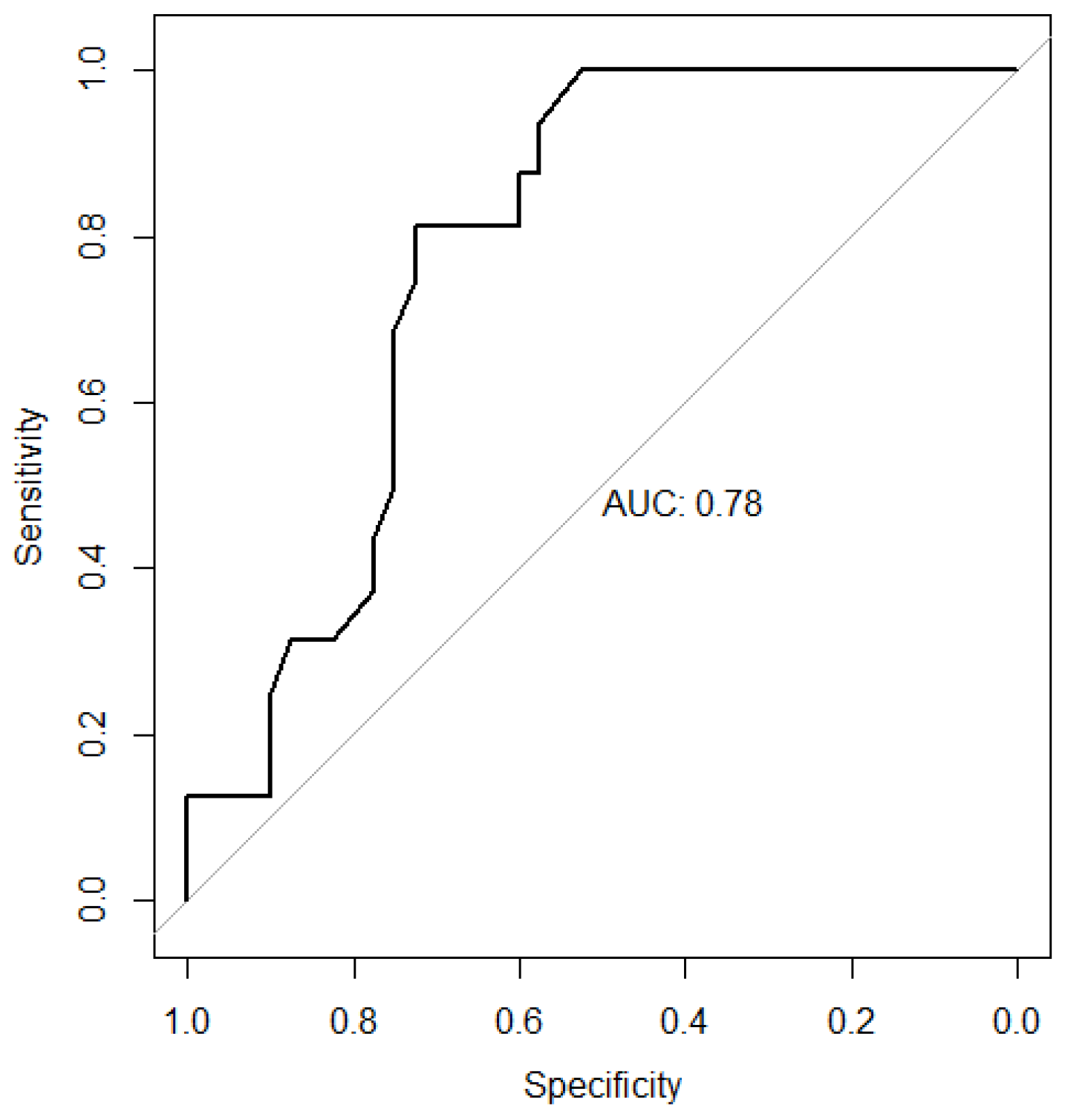
3.3. Receiver Operating Characteristic Curve for G6PD Activity
3.4. Diagnostic Performance of G6PD Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| G6PD | Glucose-6-phosphate dehydrogenase |
| SREs | Skeletal-related events |
| ROS | reactive oxygen species |
| ROC | Receiver operating characteristic |
| CI | Confidence interval |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| eNOS | endothelial nitric oxide synthase |
| VEGF | vascular endothelial growth factor |
| AR | androgen receptor |
| BALP | Bone-specific alkaline phosphatase |
| PINP | pro-collagen type I N-terminal propeptide |
| PSA | prostate-specific antigen |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS ONE 2020, 15, e0234927. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.M.; Smith, M.; Coleman, R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef]
- Oefelein, M.G.; Ricchiuti, V.; Conrad, W.; Resnick, M.I. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 2002, 168, 1005–1007. [Google Scholar] [CrossRef]
- Saad, F.; Eastham, J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 2010, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Dong, Y.; Chen, F.; Niu, M.; Liu, Z.; Wang, C. Cinobufotalin Capsule Combined with Zoledronic Acid in the Treatment of Pain Symptoms and Clinical Efficacy in Prostate Cancer Patients with Bone Metastases: A Retrospective Study. Arch. Esp. Urol. 2024, 77, 242–248. [Google Scholar] [CrossRef]
- Baldessari, C.; Pipitone, S.; Molinaro, E.; Cerma, K.; Fanelli, M.; Nasso, C.; Oltrecolli, M.; Pirola, M.; D’Agostino, E.; Pugliese, G. Bone metastases and health in prostate cancer: From pathophysiology to clinical implications. Cancers 2023, 15, 1518. [Google Scholar] [CrossRef]
- Ying, M.; Mao, J.; Sheng, L.; Wu, H.; Bai, G.; Zhong, Z.; Pan, Z. Biomarkers for prostate cancer bone metastasis detection and prediction. J. Pers. Med. 2023, 13, 705. [Google Scholar] [CrossRef]
- Ban, J.; Fock, V.; Aryee, D.N.; Kovar, H. Mechanisms, diagnosis and treatment of bone metastases. Cells 2021, 10, 2944. [Google Scholar] [CrossRef]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef]
- Harrison, I.P.; Selemidis, S. Understanding the biology of reactive oxygen species and their link to cancer: NADPH oxidases as novel pharmacological targets. Clin. Exp. Pharmacol. Physiol. 2014, 41, 533–542. [Google Scholar] [CrossRef]
- Whitburn, J.; Rao, S.R.; Morris, E.V.; Tabata, S.; Hirayama, A.; Soga, T.; Edwards, J.R.; Kaya, Z.; Palmer, C.; Hamdy, F.C. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci. Adv. 2022, 8, eabf9096. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Salminen, E.; Kallioinen, M.; Ala-Houhala, M.; Vihinen, P.; Tiitinen, S.; Varpula, M.; Vahlberg, T. Survival markers related to bone metastases in prostate cancer. Anticancer Res. 2006, 26, 4879–4884. [Google Scholar]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagase, K.; Yoshimitsu, M.; Magara, T.; Nojiri, Y.; Kato, H.; Kobayashi, T.; Teramoto, Y.; Yasuda, M.; Wada, H. Glucose-6-phosphate dehydrogenase correlates with tumor immune activity and programmed death ligand-1 expression in Merkel cell carcinoma. J. Immunother. Cancer 2020, 8, e001679. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, C.; Lin, J.; Liu, B. Analysis of Factors Influencing Bone Metastasis in Prostate Cancer and Diagnostic Value of Serum PSA, CysC and DD. Arch. Esp. Urol. 2024, 77, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Lein, M.; Stephan, C.; Von Hösslin, K.; Semjonow, A.; Sinha, P.; Loening, S.A.; Schnorr, D. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: Diagnostic and prognostic implications. Int. J. Cancer 2004, 111, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, N.; De Jong, I.; Breeuwsma, A.; Van der Veer, E. Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: A longitudinal approach. J. Urol. 2007, 178, 849–853. [Google Scholar] [CrossRef]
- Aihara, M.; Lebovitz, R.M.; Wheeler, T.M.; Kinner, B.M.; Ohori, M.; Scardino, P.T. Prostate specific antigen and Gleason grade: An immunohistochemical study of prostate cancer. J. Urol. 1994, 151, 1558–1564. [Google Scholar] [CrossRef]
- Rattazzi, M.C. Glucose 6-phosphate dehydrogenase from human erythrocytes: Molecular weight determination by gel filtration. Biochem. Biophys. Res. Commun. 1968, 31, 16–24. [Google Scholar] [CrossRef]
- Peters, A.L.; Noorden, C.J.V. Glucose-6-phosphate dehydrogenase deficiency and malaria: Cytochemical detection of heterozygous G6PD deficiency in women. J. Histochem. Cytochem. 2009, 57, 1003–1011. [Google Scholar] [CrossRef]
- Ju, H.-Q.; Lin, J.-F.; Tian, T.; Xie, D.; Xu, R.-H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The multiple roles of glucose-6-phosphate dehydrogenase in tumorigenesis and cancer chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef]
- Tsouko, E.; Khan, A.; White, M.; Han, J.; Shi, Y.; Merchant, F.; Sharpe, M.; Xin, L.; Frigo, D. Regulation of the pentose phosphate pathway by an androgen receptor–mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis 2014, 3, e103. [Google Scholar] [CrossRef]
- Leopold, J.A.; Walker, J.; Scribner, A.W.; Voetsch, B.; Zhang, Y.-Y.; Loscalzo, A.J.; Stanton, R.C.; Loscalzo, J. Glucose-6-phosphate dehydrogenase modulates vascular endothelial growth factor-mediated angiogenesis. J. Biol. Chem. 2003, 278, 32100–32106. [Google Scholar] [CrossRef] [PubMed]
- Pretlow, T.G.; Harris, B.E.; Bradley, E.L., Jr.; Bueschen, A.J.; Lloyd, K.L.; Pretlow, T.P. Enzyme activities in prostatic carcinoma related to Gleason grades. Cancer Res. 1985, 45, 442–446. [Google Scholar]
- Fujita, K.; Nonomura, N. Role of androgen receptor in prostate cancer: A review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Pujana-Vaquerizo, M.; Bozal-Basterra, L.; Carracedo, A. Metabolic adaptations in prostate cancer. Br. J. Cancer 2024, 131, 1250–1262. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Soto, M.; Guarner-Lans, V.; Manzano-Pech, L.; Soria-Castro, E. The Possible Role of Glucose-6-Phosphate Dehydrogenase in the SARS-CoV-2 Infection. Cells 2022, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liew, C.W.; Handy, D.E.; Zhang, Y.; Leopold, J.A.; Hu, J.; Guo, L.; Kulkarni, R.N.; Loscalzo, J.; Stanton, R.C. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. FASEB J. 2010, 24, 1497. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Temel, Y.; Çiftci, M. In Vitro Effects of Some Chemotherapeutics on Human Erythrocyte Glucose-6-Phosphate Dehydrogenase Enzyme. Acs Omega 2024, 9, 48292–48298. [Google Scholar] [CrossRef] [PubMed]
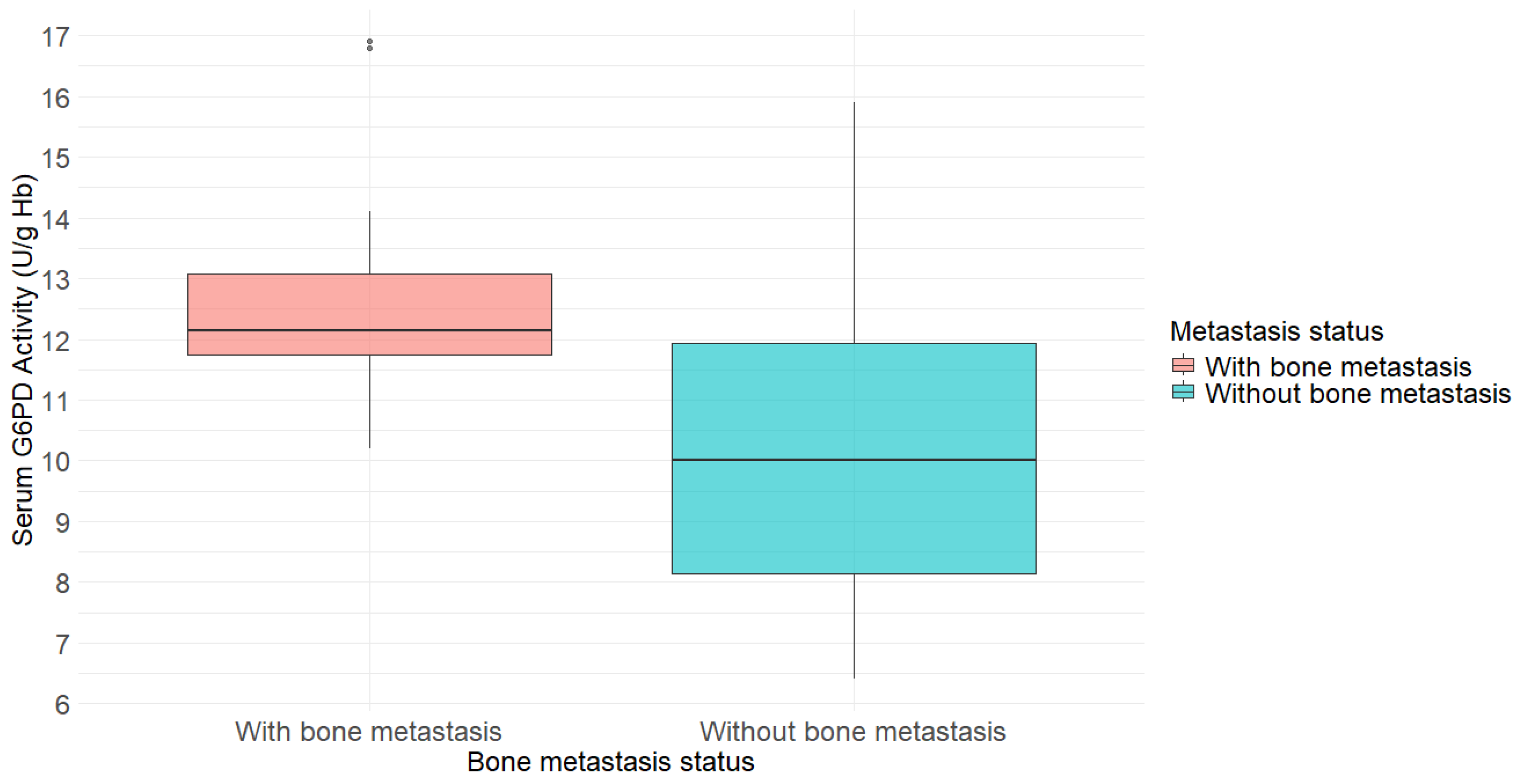
| Without Bone Metastasis (N = 40) | With Bone Metastasis (N = 16) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) 1 | 66.6 ± 8.4 | 68.4 ± 6.3 | 0.383 |
| Clinical outcomes | |||
| G6PD activity (U/g Hb) 2 | 10.0 (8.2–11.9) | 12.2 (11.8–13.1) | 0.001 |
| Cutoffs for G6PD Activity (U/g Hb) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 9.5 | 1.00 | 0.45 | 0.42 | 1.00 |
| 10.5 | 0.94 | 0.58 | 0.47 | 0.96 |
| 11.5 | 0.81 | 0.73 | 0.54 | 0.91 |
| 12.5 | 0.38 | 0.78 | 0.40 | 0.76 |
| 13.5 | 0.19 | 0.90 | 0.43 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yeom, C.-H.; Lee, J.; Bae, K.-J.; Kim, K.; Choi, J.; Lim, M.-H. Prediction of Bone Metastasis in Prostate Cancer Using Blood Glucose-6-Phosphate Dehydrogenase Activity: A Retrospective Medical Record Review. Cancers 2026, 18, 41. https://doi.org/10.3390/cancers18010041
Yeom C-H, Lee J, Bae K-J, Kim K, Choi J, Lim M-H. Prediction of Bone Metastasis in Prostate Cancer Using Blood Glucose-6-Phosphate Dehydrogenase Activity: A Retrospective Medical Record Review. Cancers. 2026; 18(1):41. https://doi.org/10.3390/cancers18010041
Chicago/Turabian StyleYeom, Chang-Hwan, Jiewon Lee, Keun-Joo Bae, Kangseok Kim, Jongsoon Choi, and Myeong-Hun Lim. 2026. "Prediction of Bone Metastasis in Prostate Cancer Using Blood Glucose-6-Phosphate Dehydrogenase Activity: A Retrospective Medical Record Review" Cancers 18, no. 1: 41. https://doi.org/10.3390/cancers18010041
APA StyleYeom, C.-H., Lee, J., Bae, K.-J., Kim, K., Choi, J., & Lim, M.-H. (2026). Prediction of Bone Metastasis in Prostate Cancer Using Blood Glucose-6-Phosphate Dehydrogenase Activity: A Retrospective Medical Record Review. Cancers, 18(1), 41. https://doi.org/10.3390/cancers18010041







