Survival Benefits of GLP-1 Receptor Agonists in Patients with Neuroendocrine Neoplasms: A Large-Scale Propensity-Matched Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Characteristics
2.2. Study Population
2.3. Propensity Score Matching Analysis
2.4. Stratification
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Follow-Up Time
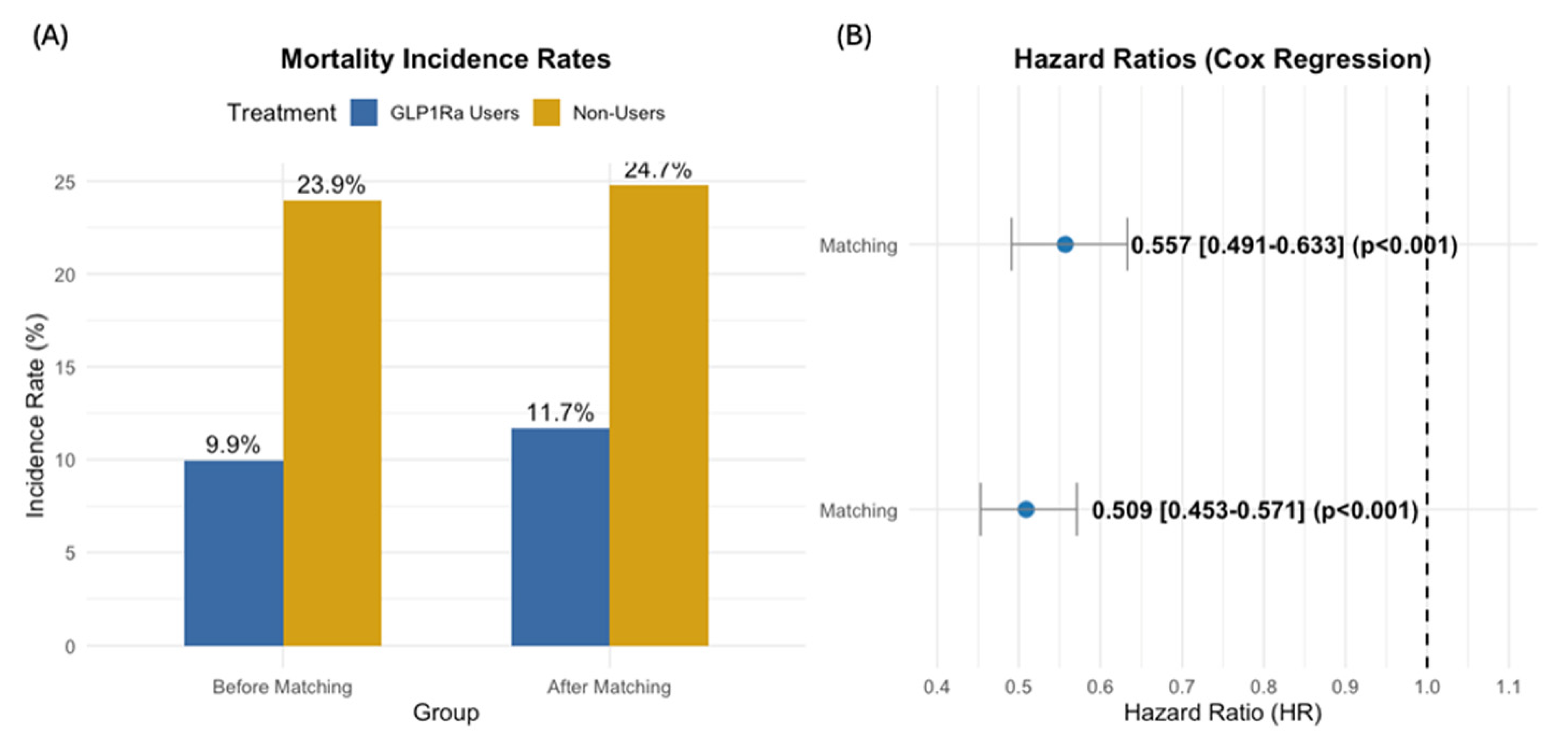
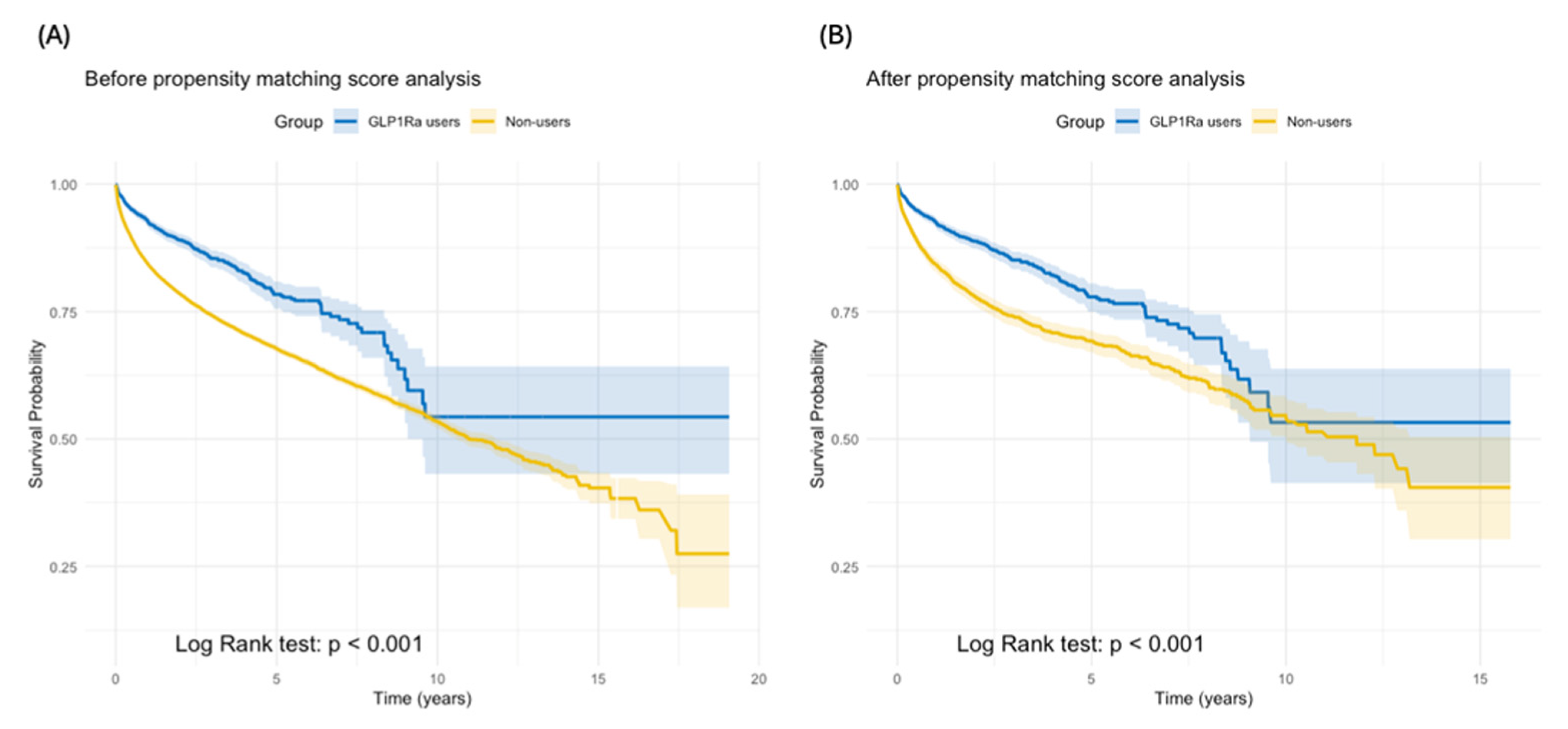
3.3. Overall Survival
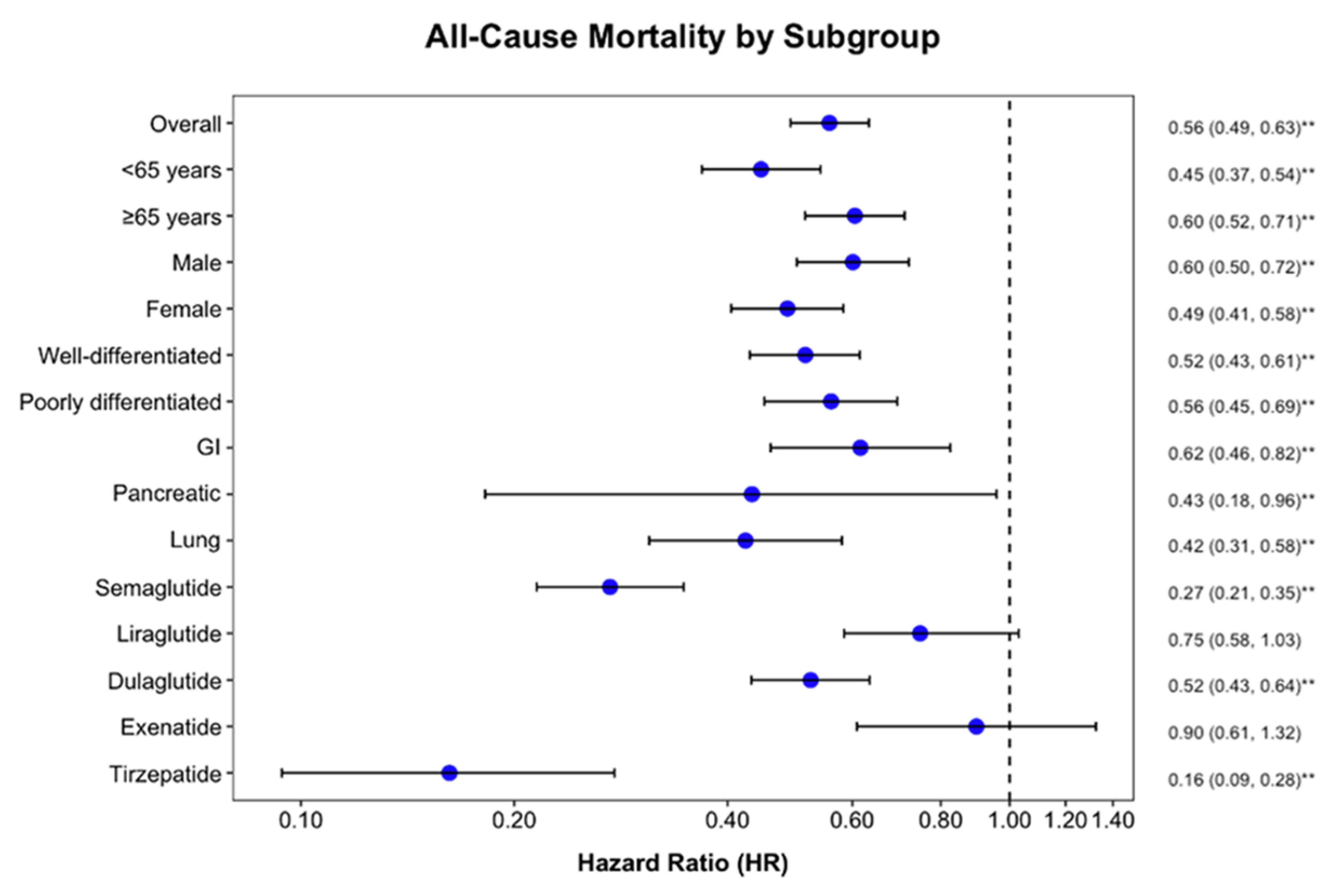
3.4. Subgroup Analysis
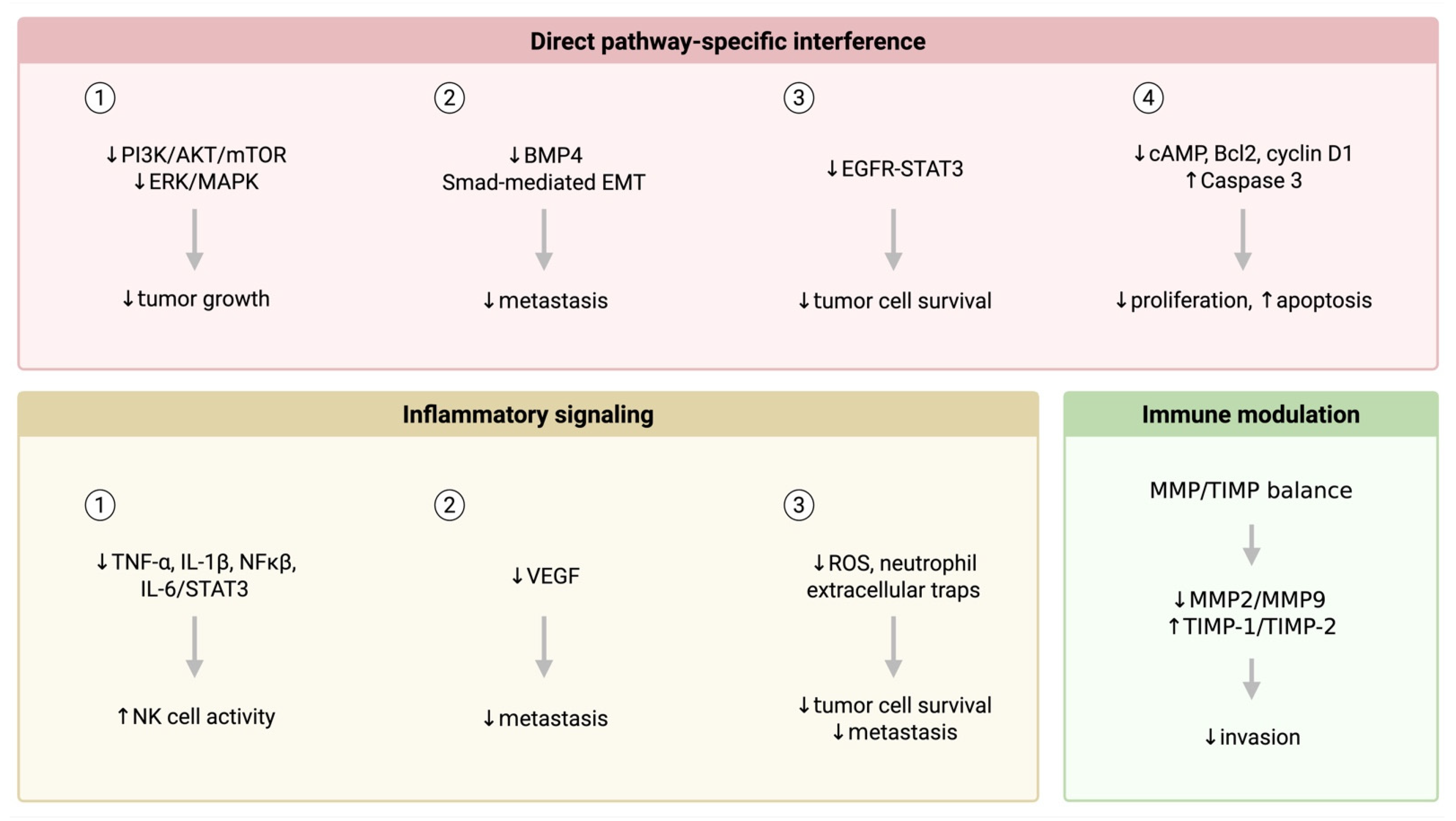
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Taal, B.G.; Visser, O. Epidemiology of Neuroendocrine Tumours. Neuroendocrinology 2004, 80 (Suppl. 1), 3–7. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Mariam, Z.; Niazi, K.S. Glucagon—like peptide agonists: A prospective review. Endocrinol. Diabetes Metab. 2024, 7, e462. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Bezin, J.; Gouverneur, A.; Pénichon, M.; Mathieu, C.; Garrel, R.; Hillaire-Buys, D.; Pariente, A.; Faillie, J.-L. GLP-1 Receptor Agonists and the Risk of Thyroid Cancer. Diabetes Care 2023, 46, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, R.; Wang, L.; Tian, Q.; Tao, M.; Ke, J.; Liu, Y.; Hou, W.; Zhang, L.; Yang, J.; et al. Activation of glucagon-like peptide-1 receptor inhibits growth and promotes apoptosis of human pancreatic cancer cells in a cAMP-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1431–E1441. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, H.; Wolf, I.; Israeli, S.; Haimsohn, M.; Ferber, S.; Karasik, A.; Kaufman, B.; Rubinek, T. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res. Treat. 2012, 132, 449–461. [Google Scholar] [CrossRef]
- Cases, A.I.; Ohtsuka, T.; Fujino, M.; Ideno, N.; Kozono, S.; Zhao, M.; Ohuchida, K.; Aishima, S.; Nomura, M.; Oda, Y. Expression of Glucagon-Like Peptide 1 Receptor and its Effects on Biologic Behavior in Pancreatic Neuroendocrine Tumors. Pancreas 2014, 43, 1–6. [Google Scholar] [CrossRef]
- Pollak, M.N.; Zeng, J.; Huang, L.; Wang, Y.; Rahbani, J.; Kazak, L. Tirzepatide inhibits tumor growth in mice with diet-induced obesity. bioRxiv 2024, 84 (Suppl. 7), LB376. [Google Scholar]
- Wang, L.; Xu, R.; Kaelber, D.C.; Berger, N.A. Glucagon-Like Peptide 1 Receptor Agonists and 13 Obesity-Associated Cancers in Patients with Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2421305. [Google Scholar] [CrossRef]
- Levy, S.; Attia, A.; Elshazli, R.M.; Abdelmaksoud, A.; Tatum, D.; Aiash, H.; Toraih, E.A. Differential Effects of GLP-1 Receptor Agonists on Cancer Risk in Obesity: A Nationwide Analysis of 1.1 Million Patients. Cancers 2024, 17, 78. [Google Scholar] [CrossRef]
- Lin, A.; Ding, Y.; Li, Z.; Jiang, A.; Liu, Z.; Wong, H.Z.H.; Cheng, Q.; Zhang, J.; Luo, P. Glucagon-like peptide 1 receptor agonists and cancer risk: Advancing precision medicine through mechanistic understanding and clinical evidence. Biomark. Res. 2025, 13, 50. [Google Scholar] [CrossRef]
- Wu, P.; He, D.; Chang, H.; Zhang, X. Epidemiologic trends of and factors associated with overall survival in patients with neuroendocrine tumors over the last two decades in the USA. Endocr. Connect. 2023, 12, e230331. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Ibrahim, R.S.; Arabi, B.; Brockmueller, A.; Shakibaei, M.; Büsselberg, D. The effect of GLP-1R agonists on the medical triad of obesity, diabetes, and cancer. Cancer Metastasis Rev. 2024, 43, 1297–1314. [Google Scholar] [CrossRef]
- Wulbrand, U.; Remmert, G.; Zöfel, P.; Wied, M.; Arnold, R.; Fehmann, H.C. mRNA expression patterns of insulin—like growth factor system components in human neuroendocrine tumours. Eur. J. Clin. Investig. 2000, 30, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Höpfner, M.; Baradari, V.; Huether, A.; Schöfl, C.; Scherübl, H. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr. -Relat. Cancer 2006, 13, 135–149. [Google Scholar] [CrossRef]
- Ranallo, N.; Iamurri, A.P.; Foca, F.; Liverani, C.; De Vita, A.; Mercatali, L.; Calabrese, C.; Spadazzi, C.; Fabbri, C.; Cavaliere, D.; et al. Prognostic and Predictive Role of Body Composition in Metastatic Neuroendocrine Tumor Patients Treated with Everolimus: A Real-World Data Analysis. Cancers 2022, 14, 3231. [Google Scholar] [CrossRef]
- Shilyansky, J.S.; Chan, C.J.; Xiao, S.; Gribovskaja-Rupp, I.; Quelle, D.E.; Howe, J.R.; Dillon, J.S.; Ear, P.H. GLP-1R agonist promotes proliferation of neuroendocrine neoplasm cells expressing GLP-1 receptors. Surgery 2025, 179, 108943. [Google Scholar] [CrossRef]
- Yang, Z.; Lv, Y.; Yu, M.; Mei, M.; Xiang, L.; Zhao, S.; Li, R. GLP-1 receptor agonist-associated tumor adverse events: A real-world study from 2004 to 2021 based on FAERS. Front. Pharmacol. 2022, 13, 925377. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Campbell-Thompson, M.; Gurlo, T.; Dawson, D.W.; Atkinson, M.; Butler, P.C. Marked Expansion of Exocrine and Endocrine Pancreas with Incretin Therapy in Humans with Increased Exocrine Pancreas Dysplasia and the Potential for Glucagon-Producing Neuroendocrine Tumors. Diabetes 2013, 62, 2595–2604. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Wei, R.; Xiu, D.; Tao, M.; Ke, J.; Liu, Y.; Yang, J.; Hong, T. Activation of glucagon—like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes. Metab. 2014, 16, 850–860. [Google Scholar] [CrossRef]
- Eads, R.J. Poorly Differentiated Neuroendocrine Tumors. Hematol. Oncol. Clin. North Am. 2016, 30, 151–162. [Google Scholar] [CrossRef]
- Thomas, K.E.H.; Voros, B.A.; Boudreaux, J.P.; Thiagarajan, R.; Woltering, E.A.; Ramirez, R.A. Current Treatment Options in Gastroenteropancreatic Neuroendocrine Carcinoma. Oncologist 2019, 24, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, H.J.; Borniger, C.J. Neuroendocrine and Behavioral Consequences of Hyperglycemia in Cancer. Endocrinology 2020, 161, bqaa047. [Google Scholar] [CrossRef]
- Tsoli, M.; Chatzellis, E.; Koumarianou, A.; Kolomodi, D.; Kaltsas, G. Current best practice in the management of neuroendocrine tumors. Ther. Adv. Endocrinol. Metab. 2019, 10, 204201881880469. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, A. Neuroendocrine Tumors: Treatment and Management. Cancers 2022, 14, 4048. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Soni, B.; Khetan, A.; Mishra, S.; Sharma, B.; Bhojwani, R. Duodenal neuroendocrine tumours in morbidly obese. J. Minimal Access Surg. 2021, 17, 249–252. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Cantone, M.C.; Guarnotta, V.; Mazzilli, R.; Verde, L.; Vetrani, C.; Colao, A.; Faggiano, A. Neuroendocrine Tumors: A Comprehensive Review on Nutritional Approaches. Cancers 2022, 14, 4402. [Google Scholar] [CrossRef]
- Capurso, G.; Falconi, M.; Panzuto, F.; Rinzivillo, M.; Boninsegna, L.; Bettini, R.; Corleto, V.; Borgia, P.; Pederzoli, P.; Scarpa, A.; et al. Risk Factors for Sporadic Pancreatic Endocrine Tumors. Am. J. Gastroenterol. 2009, 104, 3034–3041. [Google Scholar] [CrossRef]
- Haugvik, S.P.; Hedenström, P.; Korsæth, E.; Valente, R.; Hayes, A.; Siuka, D.; Maisonneuve, P.; Gladhaug, I.P.; Lindkvist, B.; Capurso, G. Diabetes, Smoking, Alcohol Use, and Family History of Cancer as Risk Factors for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Neuroendocrinology 2015, 101, 133–142. [Google Scholar] [CrossRef]
| Characteristic | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| GLP-1Ra Users (n = 3046) | Non-Users (n = 28,878) | p-Value | GLP-1Ra Users (n = 3043) | Non-Users (n = 3043) | p-Value | |
| Demographics | ||||||
| Age, mean (SD) | ||||||
| Age at index, years | 62.4 (11.1) | 64.2 (12.8) | <0.001 | 62.4 (11.0) | 62.3 (12.1) | 0.62 |
| Sex, n (%) | <0.001 | 0.48 | ||||
| Female | 1737 (57.0) | 14,438 (50.0) | 1735 (57.0) | 1765 (58.0) | ||
| Male | 1192 (39.1) | 13,109 (45.4) | 1191 (39.1) | 1174 (38.6) | ||
| Race, n (%) | 0.22 | 0.06 | ||||
| White | 2132 (70.0) | 19,815 (68.7) | 2129 (70.0) | 2200 (72.3) | ||
| Black/African American | 484 (15.9) | 4604 (16.0) | 484 (15.9) | 470 (15.4) | ||
| Asian | 74 (2.4) | 734 (2.5) | 74 (2.4) | 57 (1.9) | ||
| Diagnosis, n (%) | ||||||
| Type 2 diabetes mellitus | 2419 (79.4) | 11,136 (38.6) | <0.001 | 2416 (79.4) | 2425 (79.7) | 0.77 |
| Type 1 diabetes mellitus | 350 (11.5) | 1190 (4.1) | <0.001 | 348 (11.4) | 325 (10.7) | 0.34 |
| Obesity | 1949 (64.0) | 9587 (33.2) | <0.001 | 1946 (64.0) | 1946 (64.0) | 1.00 |
| Primary hypertension | 2496 (81.9) | 18,031 (62.5) | <0.001 | 2493 (81.9) | 2480 (81.5) | 0.66 |
| Cerebrovascular diseases | 450 (14.8) | 3340 (11.6) | <0.001 | 450 (14.8) | 402 (13.2) | 0.07 |
| Kidney disease * | 993 (32.6) | 6071 (21.0) | <0.001 | 993 (32.6) | 924 (30.4) | 0.06 |
| Procedures, n (%) | ||||||
| Surgery | 2669 (87.6) | 20,395 (70.7) | <0.001 | 2666 (87.6) | 2646 (87.0) | 0.44 |
| Radiation therapy | 167 (5.5) | 1640 (5.7) | 0.64 | 167 (5.5) | 138 (4.5) | 0.09 |
| Chemotherapy | 265 (8.7) | 2143 (7.4) | 0.011 | 265 (8.7) | 236 (7.8) | 0.053 |
| Medication, n (%) | ||||||
| Antineoplastics | 701 (23.0) | 5099 (17.7) | <0.001 | 698 (22.9) | 639 (21.0) | 0.07 |
| Subgroup | Categories | Count per Group | GLP-1Ra Users | Non-Users (Controls) | p-Value |
|---|---|---|---|---|---|
| Overall | Overall | 3043 | 356 (11.7%) | 753 (24.7%) | <0.001 |
| Age | <65 years | 1838 | 144 (7.8%) | 403 (21.9%) | <0.001 |
| ≥65 years | 1508 | 228 (15.1%) | 442 (29.3%) | <0.001 | |
| Sex | Male | 1228 | 180 (14.7%) | 345 (28.1%) | <0.001 |
| Female | 1814 | 166 (9.2%) | 424 (23.4%) | <0.001 | |
| Type of NEN | NET | 1885 | 173 (9.2%) | 429 (22.8%) | <0.001 |
| NEC | 524 | 129 (24.6%) | 234 (44.7%) | <0.001 | |
| Primary tumor site | GI | 865 | 66 (7.6%) | 159 (18.4%) | <0.001 |
| Pancreatic | 65 | 10 (15.4%) | 23 (35.4%) | 0.009 | |
| Lung | 563 | 54 (9.6%) | 154 (27.4%) | <0.001 | |
| GLP-1Ra analogues | Semaglutide | 1636 | 83 (5.1%) | 425 (26%) | <0.001 |
| Liraglutide | 644 | 115 (17.9%) | 137 (21.3%) | 0.12 | |
| Dulaglutide | 1199 | 156 (13%) | 330 (27.5%) | <0.001 | |
| Exenatide | 211 | 52 (24.6%) | 51 (24.2%) | 0.91 | |
| Tirzepatide | 592 | 15 (2.5%) | 156 (26.4%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawzy, M.S.; Alenezy, A.; Jishu, J.A.; Khan, I.; Dessouky, A.; Abdelmaksoud, A.; Limbach, K.E.; Toraih, E.A. Survival Benefits of GLP-1 Receptor Agonists in Patients with Neuroendocrine Neoplasms: A Large-Scale Propensity-Matched Cohort Study. Cancers 2025, 17, 1593. https://doi.org/10.3390/cancers17091593
Fawzy MS, Alenezy A, Jishu JA, Khan I, Dessouky A, Abdelmaksoud A, Limbach KE, Toraih EA. Survival Benefits of GLP-1 Receptor Agonists in Patients with Neuroendocrine Neoplasms: A Large-Scale Propensity-Matched Cohort Study. Cancers. 2025; 17(9):1593. https://doi.org/10.3390/cancers17091593
Chicago/Turabian StyleFawzy, Manal S., Awwad Alenezy, Jessan A. Jishu, Issa Khan, Ahmad Dessouky, Ahmed Abdelmaksoud, Kristen E. Limbach, and Eman A. Toraih. 2025. "Survival Benefits of GLP-1 Receptor Agonists in Patients with Neuroendocrine Neoplasms: A Large-Scale Propensity-Matched Cohort Study" Cancers 17, no. 9: 1593. https://doi.org/10.3390/cancers17091593
APA StyleFawzy, M. S., Alenezy, A., Jishu, J. A., Khan, I., Dessouky, A., Abdelmaksoud, A., Limbach, K. E., & Toraih, E. A. (2025). Survival Benefits of GLP-1 Receptor Agonists in Patients with Neuroendocrine Neoplasms: A Large-Scale Propensity-Matched Cohort Study. Cancers, 17(9), 1593. https://doi.org/10.3390/cancers17091593








