Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes
Simple Summary
Abstract
1. Introduction
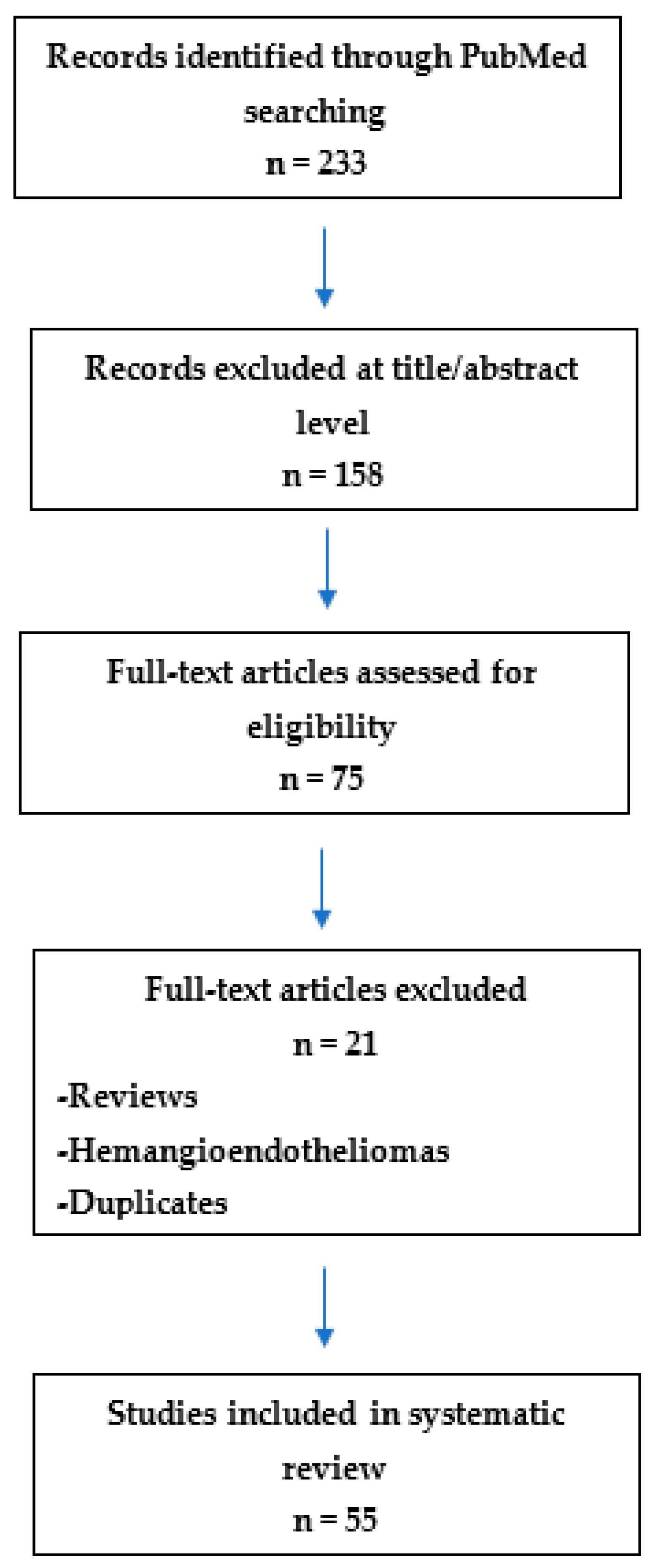
2. Materials and Methods
3. Results
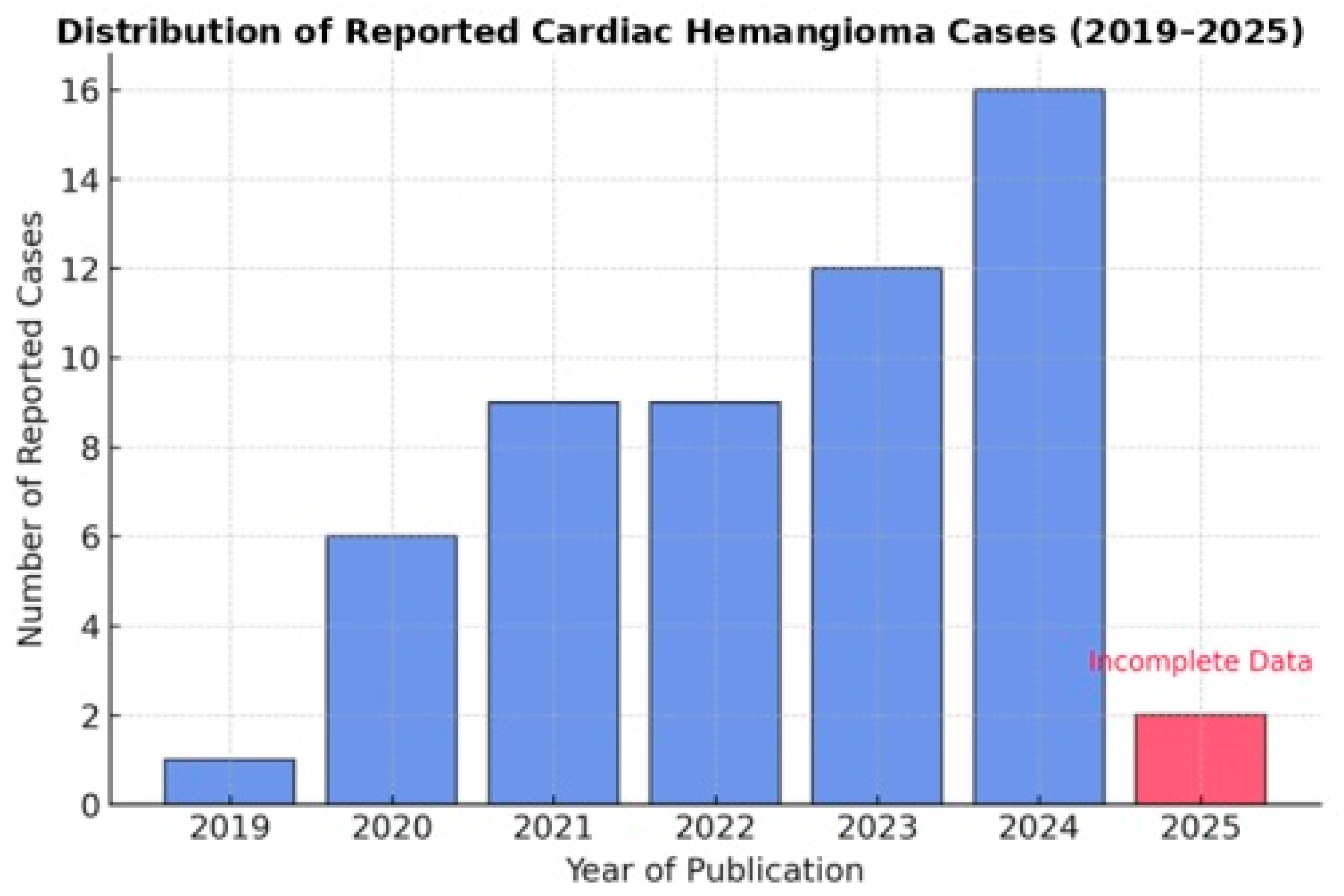
3.1. Trends in the Reporting of Cardiac Hemangiomas: A Growing Incidence or Improved Detection?
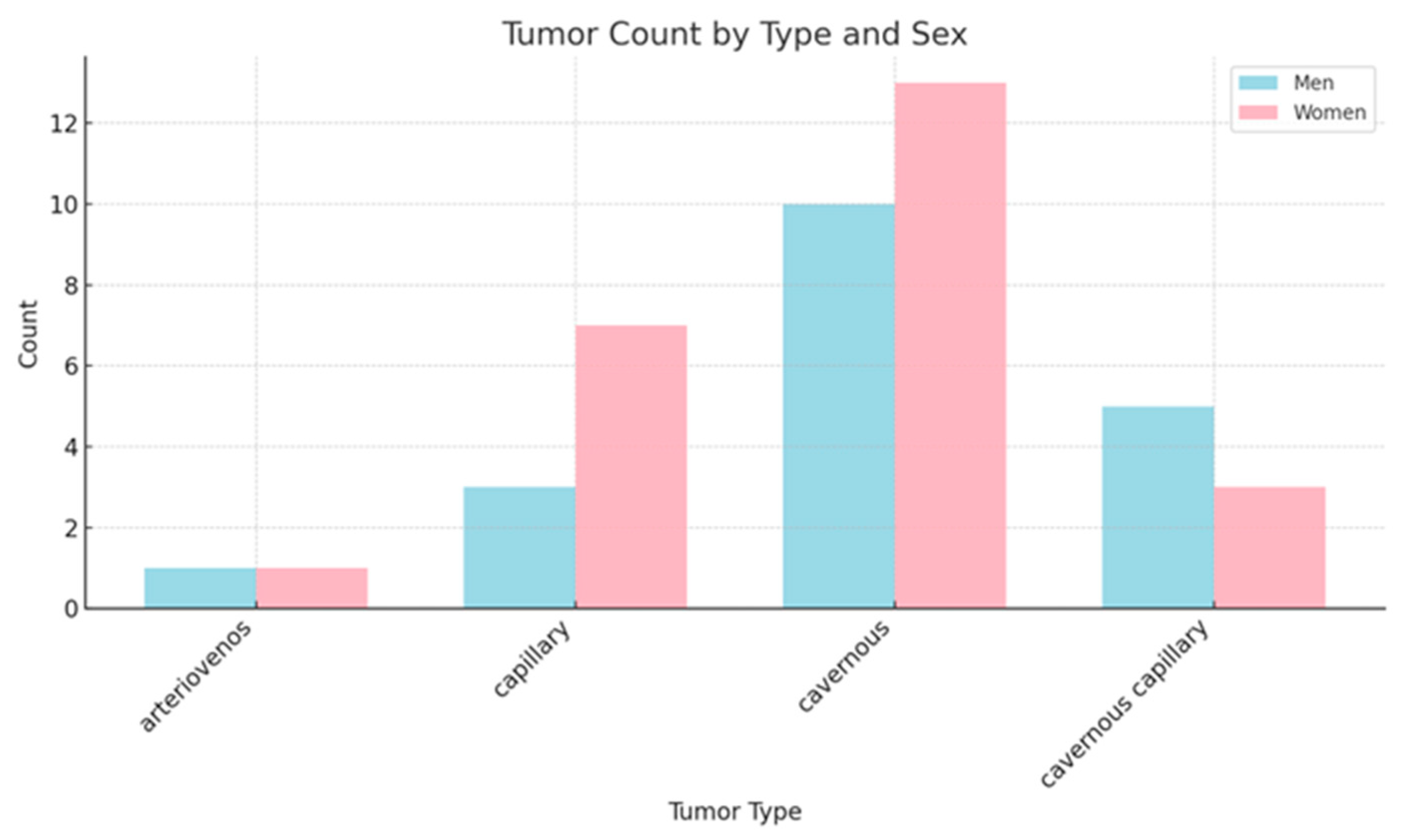
3.2. Distribution and Classification of Hemangioma Subtypes: A Data-Driven Analysis
- The most prevalent subtype is cavernous hemangioma, comprising 23 cases, characterized by large, dilated vascular spaces;
- Capillary hemangiomas are represented by 10 cases;
- Cavernous-capillary hemangiomas account for another 8 cases, demonstrating mixed histological features;
- Arteriovenous hemangiomas are the least common, with only 2 reported cases;
- The designation “ns” (12 cases) denotes “not specified,” which may reflect either incomplete reporting or diagnostic uncertainty.
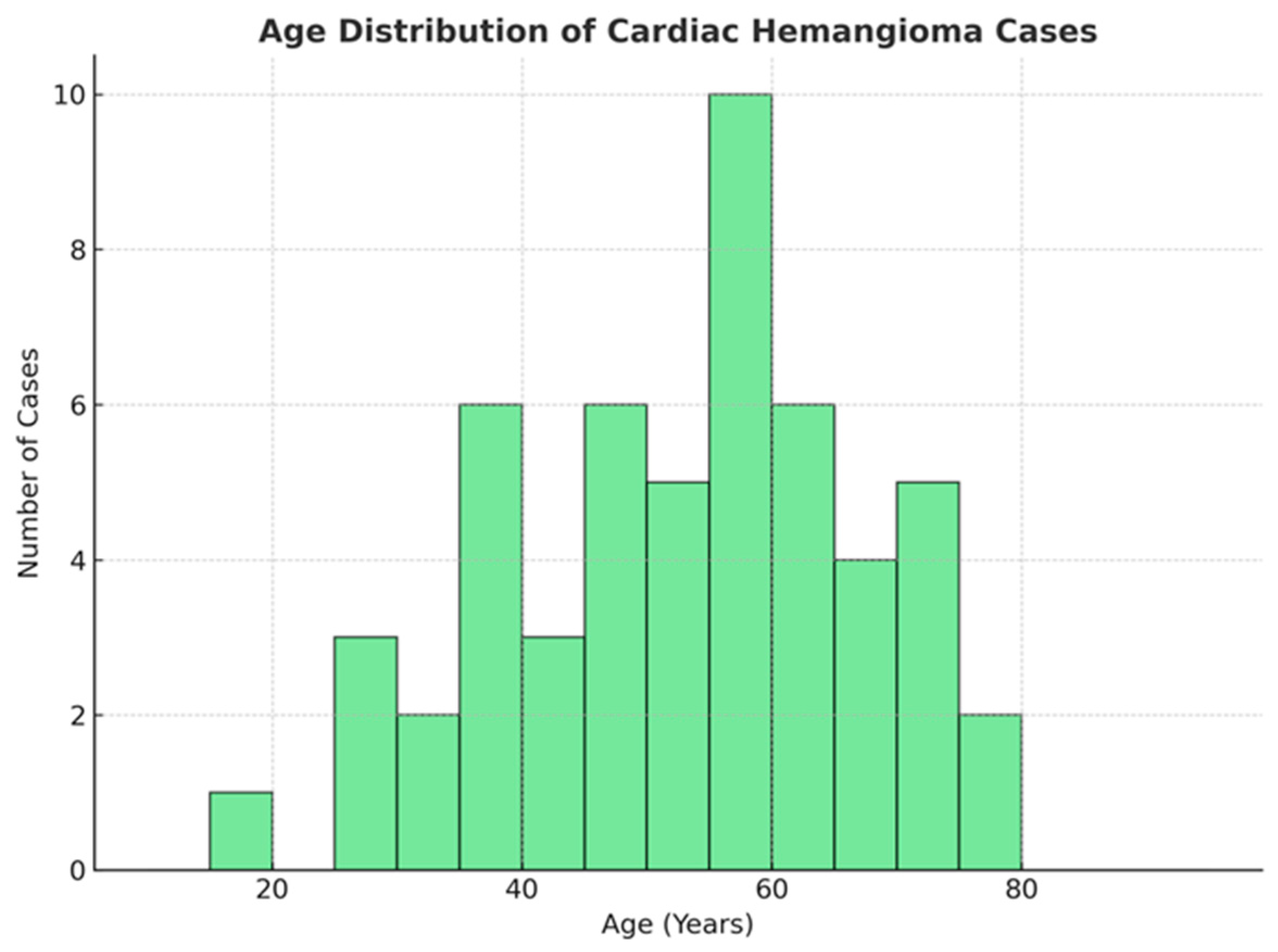
3.3. Statistical Analysis of the Distribution of Cardiac Hemangiomas by Age and Gender
- Mean age: 52.5 years, indicating that middle-aged and older adults are the most frequently affected;
- Median age: 52 years, demonstrating a central tendency closely aligned with the mean;
- Interquartile range: 44 to 64 years, suggesting that 50% of patients fall within this age range;
- Standard deviation: 16.97 years, reflecting a moderate degree of variability around the mean.
- Women: 32 cases (58.2%);
- Men: 23 cases (41.8%).
3.4. Clinical Presentation and Symptomatology of Cardiac Hemangiomas
- Asymptomatic cases: 12 (21.8%), suggesting that these cases were likely discovered incidentally, often during imaging conducted for unrelated conditions;
- Dyspnea: present in 8 cases (14.5%), making it the most prevalent symptom among symptomatic patients;
- Chest pain: reported in 6 cases (10.9%), either as an isolated symptom or in combination with other manifestations;
- Other symptoms: palpitations, dizziness, syncope, stroke, etc., less frequently observed.
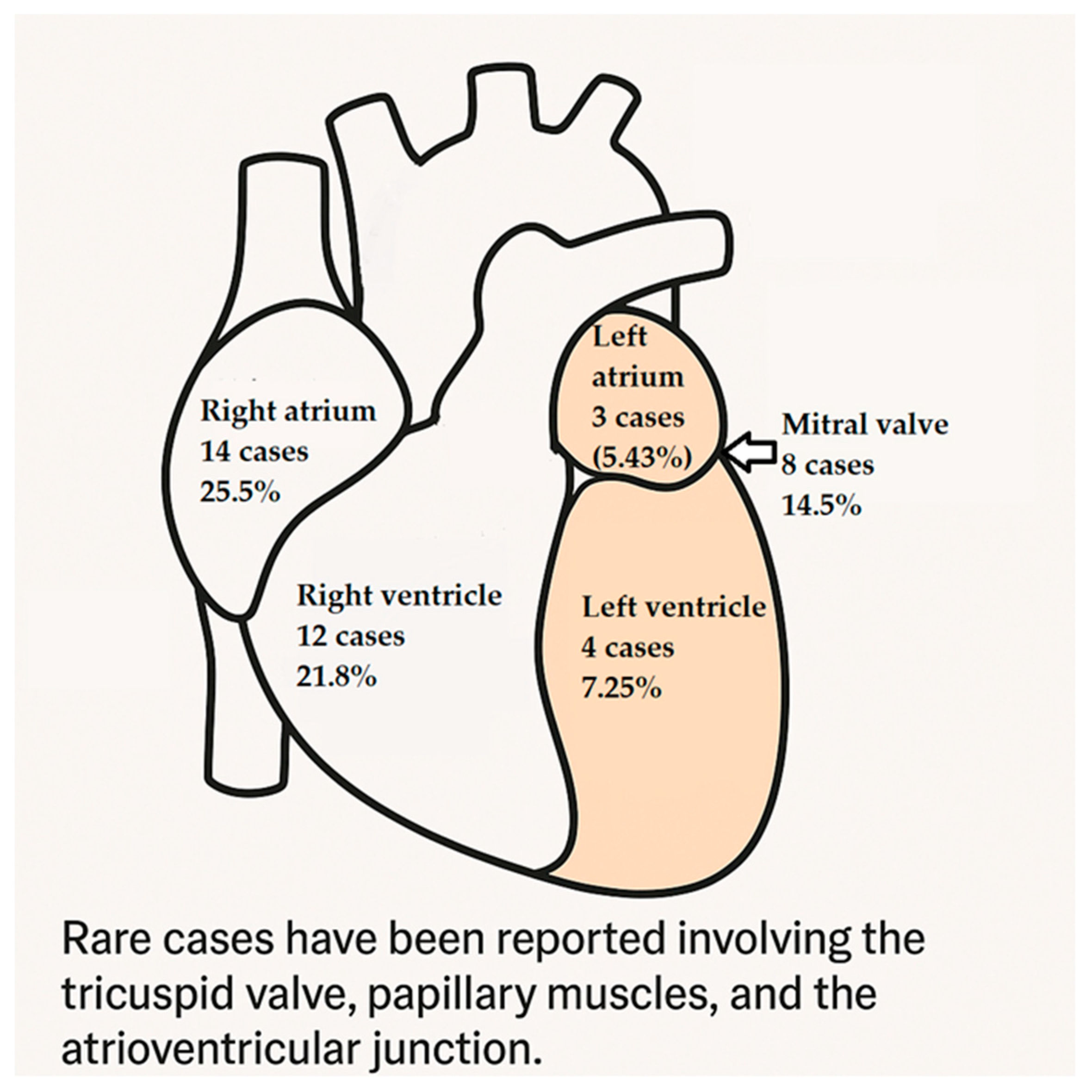
3.5. Anatomical Distribution of Cardiac Hemangiomas: Predilection for Specific Heart Chambers and Structures?
- Right atrium: the most commonly affected site, with 14 cases (25.5%);
- Right ventricle: the second most common site, with 12 cases (21.8%);
- Mitral valve: involved in 8 cases (14.5%), indicating the tumor’s potential to affect valvular structures;
- Left ventricle and left atrium: less frequently affected, with 4 and 3 cases, respectively.
3.6. Size Variability of Cardiac Hemangiomas
- Mean size: 3.76 cm, suggesting that the majority of tumors are relatively small;
- Median size: 3.15 cm, indicating that half of the tumors are below this dimension;
- Range: 0.68 cm to 11.05 cm, reflecting a broad spectrum in tumor dimensions;
- Interquartile Range: 1.7 cm to 4.95 cm, signifying that 50% of the tumors fall within this middle range.
3.7. Diagnostic Modalities in Cardiac Hemangiomas: Echocardiography as the Primary Tool?
- Echocardiography: 45 cases (81.8%)—underscoring its role as the primary diagnostic modality, likely attributable to its widespread availability, non-invasive nature, and high efficacy in detecting cardiac masses;
- Computed Tomography: 7 cases (12.7%)—typically employed as a complementary imaging technique, providing detailed anatomical information;
- Chest X-ray: 3 cases (5.5%)—infrequently utilized, potentially identifying indirect signs rather than directly visualizing the tumor.
3.8. Co-Occurrence of Cardiac Hemangiomas with Other Tumors: Incidence and Potential Associations
- Liver hemangioma: 2 cases;
- Malignancies: rare occurrences, including colon cancer and endometrial cancer (1 case each);
- Hematological disorders: one patient had polycythemia and another had myelodysplastic syndrome, hinting at possible hematological links;
- Multiple cardiac hemangiomas at the same site: reported in 3 cases.
3.9. Surgical Management of Cardiac Hemangiomas: Predominance, Rationale, and Exceptions
- Surgery performed: 48 cases (87.3%)—reflecting a clear preference for surgical excision, likely due to the potential for complications such as obstruction, embolism, or arrhythmias;
- No surgery: 5 cases (9.1%)—possibly due to factors like patient comorbidities, asymptomatic presentation, conservative management decisions, or necropsy report;
- Biopsy only: 2 cases (3.6%)—indicating situations where tissue diagnosis was obtained without full excision, possibly due to diagnostic uncertainty or inoperability.
3.10. Follow-Up Duration and Recurrence Patterns in Cardiac Hemangioma Management: Insights and Correlations
- Most common follow-up duration: 12 months (9 cases), suggesting a general tendency toward a standard one-year surveillance period;
- Short-term follow-up: in 16 cases, follow-up was limited to 6 months or less, potentially due to early discharge following successful surgical outcomes or low-risk clinical profiles;
- Long-term follow-up: a minority of cases documented extended follow-up durations—70, 120, and even 168 months (14 years)—demonstrating a commitment to long-term outcome assessment in select instances;
- Unspecified follow-up (“ns”): in 19 cases, follow-up duration was not reported, impairing comprehensive evaluation of long-term prognosis.
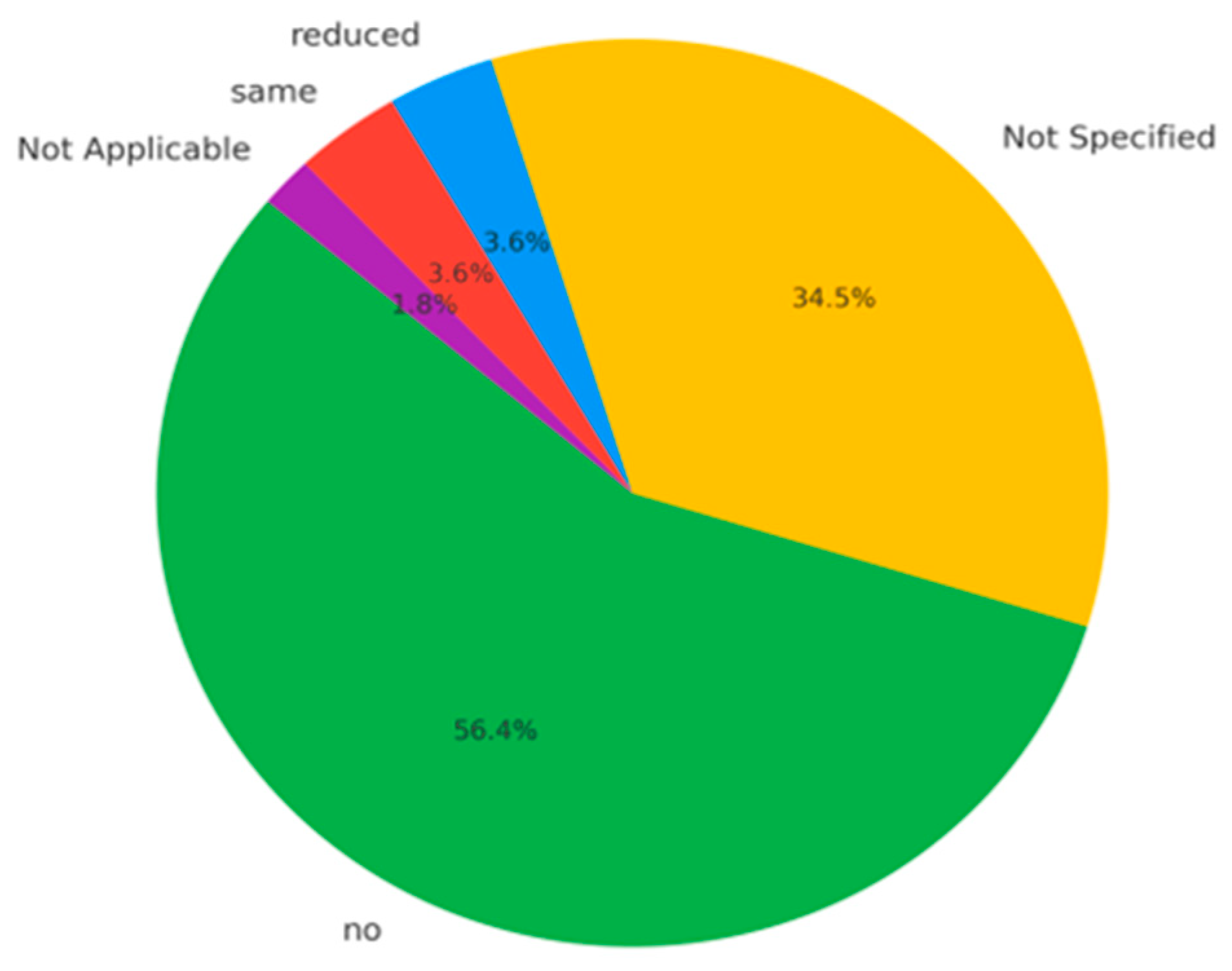
- No recurrence: reported in 34 cases (61.8%), supporting the notion that complete surgical excision is typically curative;
- Unreported recurrence status: 18 cases (32.7%) lacked sufficient data, limiting full assessment of recurrence trends;
- Reduced tumor size: noted in 2 cases (3.6%), possibly reflecting partial regression or a benign post-treatment course;
- Stable disease: also reported in 2 cases (3.6%), indicating no progression over time.
- Positive correlation+
- Between follow-up duration and stable disease: there is a moderate positive correlation (r = +0.51) between follow-up duration and the classification of disease as stable, suggesting that longer periods of clinical follow-up are associated with a higher likelihood of observing sustained disease stability over time. This relationship implies that patients monitored over extended intervals tend to exhibit consistent disease status without significant progression or regression, highlighting the potential role of follow-up duration as an indicator of clinical stability in certain conditions.
- Negative correlations−
- Between follow-up duration and surgical treatment (r = −0.53): patients who underwent surgical intervention tended to have shorter follow-up;
- Between surgical treatment and tumor size reduction (r = −0.51): surgical excision is linked to a lower chance of observing tumor shrinkage after treatment, likely because the tumor is completely removed during surgery. Since there is no remaining mass, further reduction cannot be measured. This highlights that surgical treatment aims to fully eliminate the tumor, unlike non-surgical methods, which often reduce tumor size gradually [77].
3.11. Comparative Subgroup Analysis
4. Discussion
5. Conclusions
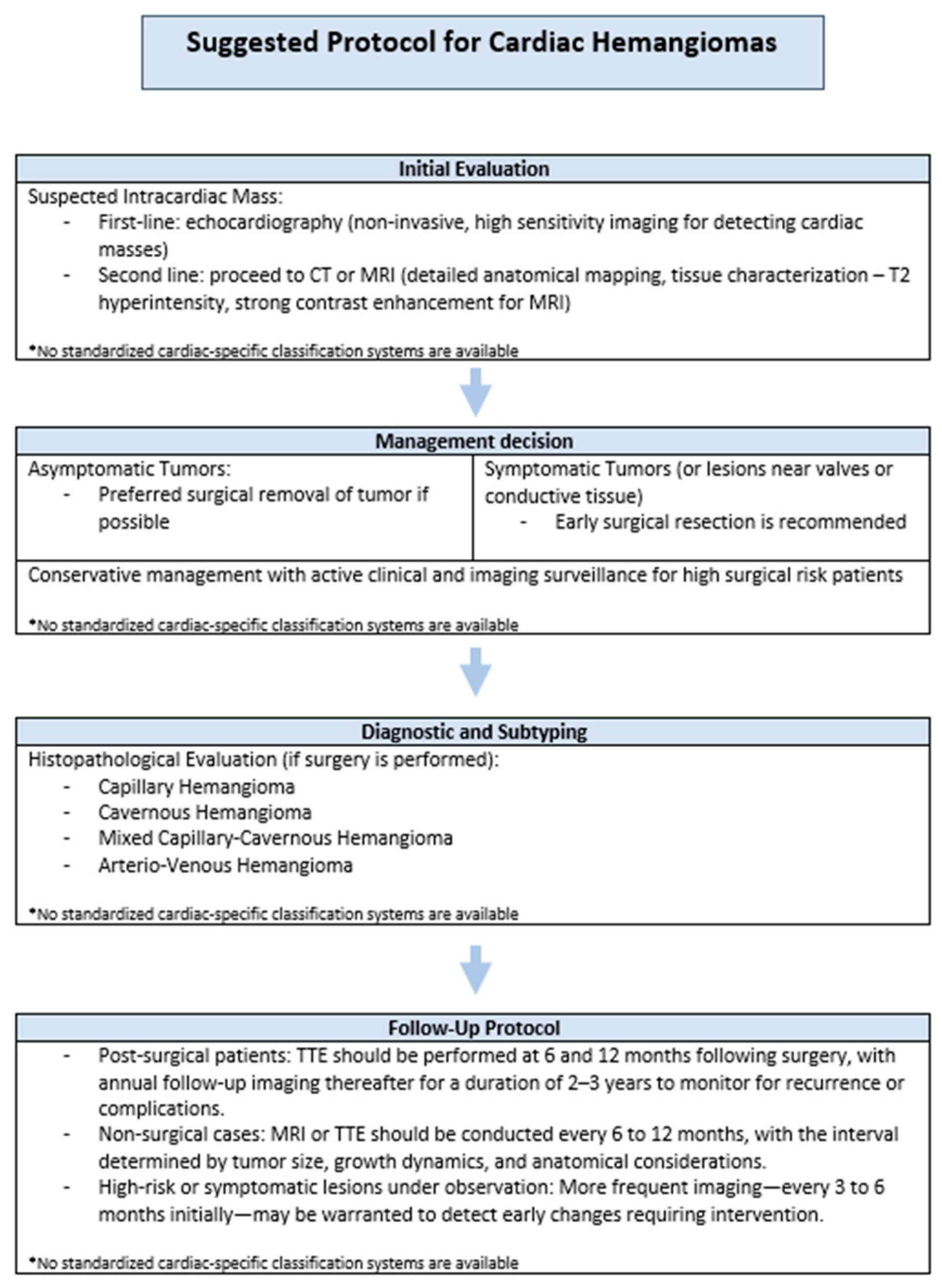
5.1. Clinical Recommendations
- —
- Post-surgical patients: TTE should be performed at 6 and 12 months following surgery, with annual follow-up imaging thereafter for a duration of 2–3 years to monitor for recurrence or complications.
- —
- Non-surgical cases: MRI or TTE should be conducted every 6 to 12 months, with the interval determined by tumor size, growth dynamics, and anatomical considerations.
- —
- High-risk or symptomatic lesions under observation: More frequent imaging—every 3 to 6 months initially—may be warranted to detect early changes requiring intervention.
5.2. Study Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| avg_age_m | Average male age |
| avg_age_w | Average women age |
| count_m | Number of males |
| count_w | Number of women |
| CT | Computer Tomography |
| max_age_m | Highest age in male group |
| max_age_w | Highest age in women group |
| min_age_m | Lowest age in male group |
| min_age_w | Lowest age in women group |
| MRI | Magnetic Resonance Imaging |
| na | not applicable |
| ns | not specified |
| WHO | World Health Organization |
Appendix A
| No. | Article | Year | Type | Age | Sex | Clinical Presentation | Site | Size (cm) | Way of Diags | Other Tumors | Surgical Treatment | Follow Up Time (Months) | Follow Up (Recurrence) | Observation/ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [19] | 2025 | cavernous | 38 | w | palpitations | left ventricular apex | 8/5/1.7 | CT | no | yes | 36 | no | engulfed the LAD |
| 2 | [20] | 2023 | cavernous | 44 | w | asymptomatic | right atrium | 11.5/9.2 | Rx | no | yes | 12 | no | gigantic |
| 3 | [21] | 2023 | ns | 23 | w | chest pain | right atrium | 7/6.4 | CT | no | yes | ns | ns | postpartum, thrombosis, biopsy-angiosarcoma |
| 4 | [22] | 2023 | cavernous | 53 | m | dyspnea | right atrium | 2/1.5 and 1.5/1.3 | Eco | 2 same site | yes | 6 | no | mitral stenosis |
| 5 | [23] | 2023 | ns | 50 | w | dizziness palpitations | right atrium | 2/1.8 | Eco | no | yes | 1 | no | postop AV block |
| 6 | [24] | 2020 | cavernous capillary | 34 | w | asymptomatic | papillary muscle | 0.9/0.7/0.6 | Eco | no | yes | ns | ns | na |
| 7 | [25] | 2024 | cavernous capillary | 70 | m | chills cold sweats | mitral valve | 1.5/1.5/1.5 | Eco | no | yes | ns | ns | na |
| 8 | [26] | 2023 | cavernous | 87 | w | dyspnea dizziness weakness | left atrium | 2.1/1.5 | Eco | no | yes | ns | ns | na |
| 9 | [27] | 2023 | capillary | 56 | w | dyspnea palpitations | mitral valve | 1.5/1.3 | Eco | no | yes | 2 | no | mitral stenosis hemodialysis tracheostomy |
| 10 | [28] | 2024 | cavernous capillary | 79 | m | dyspnea palpitations | tricuspid valve | 4.1/5.8 | Eco | polycythemia | yes | 12 | no | anasarca |
| 11 | [77] | 2022 | cavernous | 45 | w | dyspnea fatigue cough | right atrium and right ventricle | 8.6/13.5 | Rx | no | no | 120 | reduced | tried surgery, radiotherapy, 10 years follow up |
| 12 | [29] | 2023 | capillary | 52 | m | fatigue | right atrium | 3.5/3.1 | Eco | no | yes | ns | ns | epicardium tumor |
| 13 | [30] | 2024 | ns | 64 | w | palpitations dyspnea | left ventricular septum | 3.0/2.0 | Eco | no | yes | 12 | no | elective knee surgery |
| 14 | [31] | 2023 | cavernous | 61 | m | shoulder pain | left atrium | 6 | CT | no | yes | 4 | no | epicardium tumor pericarditis |
| 15 | [83] | 2023 | capillary | 71 | w | dyspnea | left atrium and left ventricle | ns | Rx | no | biopsy | ns | ns | supportive care |
| 16 | [10] | 2020 | cavernous capillary | 14 | m | asymptomatic | right ventricle | 2.2/1.2 | Eco | no | yes | 2 | no | complete atrioventricular canal defect repaired, not shown 1 year previous to diagnosis |
| 17 | [32] | 2021 | ns | 56 | w | dyspnea chest pain | right ventricle | 4.0/3.0/3.0 | Eco | no | yes | 4 | no | na |
| 18 | [33] | 2020 | cavernous | 56 | w | fatigue chest pain palpitations syncope | right atrium | 3.0/2.0 | Eco | no | yes | ns | ns | na |
| 19 | [34] | 2025 | cavernous | 60 | w | syncope | right ventricle | 4.5/3.5 | Eco | no | yes | 24 | no | na |
| 20 | [35] | 2022 | cavernous | 65 | m | palpitation | right ventricle | 2.0/2.0/1 | Eco | no | yes | 12 | no | na |
| 21 | [35] | 2022 | capillary | 58 | w | chest pain | right atrium | 2.0/1/1 | Eco | no | yes | 12 | no | coronary disease |
| 22 | [11] | 2024 | capillary | 76 | m | dyspnea | right atrium | 4.7/3.7/3.7 | Eco | no | yes | ns | ns | postop atrial fibrillation and pneumonia |
| 23 | [36] | 2022 | cavernous | 55 | w | chest pain dyspnea | right atrium | 11.3/7.7/7.1 | Eco | no | yes | 1 | no | na |
| 24 | [37] | 2023 | cavernous | 35 | m | fever sweats | right atrium | 6/6.7 | Eco | no | yes | 10 | no | na |
| 25 | [84] | 2024 | ns | 21 | m | chest pain dyspnea | left ventricle | 2.5/3.8 | Eco | colon cancer | no | 5 | na | na |
| 26 | [38] | 2019 | cavernous | 32 | m | asymptomatic | atrio-ventricular groove | 7.7/6.7 | Eco | no | yes | ns | ns | enclosing right coronary artery |
| 27 | [39] | 2022 | cavernous | 66 | w | fever infection Enterococcus faecalis | mitral valve | 1.92/2.83 | Eco | no | yes | 6 | no | endocarditis on mitral prosthesis |
| 28 | [40] | 2024 | cavernous capillary | 52 | w | asymptomatic | right ventricle | 6/3.9/4.6 | Eco | no | yes | 4 | no | required tricuspid prosthesis |
| 29 | [41] | 2023 | cavernous | 49 | w | palpitation dizziness syncope | right ventricle | 1.1/1.36 0.77/0.58 | Eco | 2 (same site) | yes | 12 | no | na |
| 30 | [42] | 2024 | ns | 41 | w | chest pain | tricuspid valve | 2.5/2.5/2 | Eco | no | yes | 24 | no | na |
| 31 | [43] | 2022 | cavernous | 71 | m | abdominal pain | right ventricle | 1.3/1.7/1.4 | Eco | no | yes | ns | ns | na |
| 32 | [44] | 2020 | cavernous | 52 | w | asymptomatic | right atrium | 10.5/7.5 | Eco | no | yes | ns | ns | na |
| 33 | [45] | 2024 | capillary | 78 | m | asymptomatic | mitral valve | 0.5/0.5 | Eco | no | yes | 9 | no | aortic aneurism |
| 34 | [46] | 2024 | ns | 50 | m | dyspnea | right atrium | 7.5/7/7 | Eco | no | yes | 36 | no | assist device cardiomyopathy |
| 35 | [47] | 2020 | cavernous capillary | 50 | w | asymptomatic | right ventricle | 3.6/1.6 | Eco | no | yes | 5 | no | na |
| 36 | [48] | 2024 | ns | 41 | m | stroke | right atrium | 4.77/2.51 | Eco | no | yes | 6 | no | na |
| 37 | [86] | 2020 | cavernous capillary | 76 | m | asymptomatic | left ventricle | 4.7/5 | Eco | no | no | 12 | same | enclosing diagonal artery |
| 38 | [49] | 2021 | cavernous | 69 | w | dyspnea | right atrium | 8.0/7/4 | CT | endometrial cancer | yes | 70 | no | na |
| 39 | [50] | 2021 | capillary | 64 | w | dyspnea chest pain cough | right atrium and right ventricle | 4.1/5.3 | Eco | no | yes | ns | ns | na |
| 40 | [85] | 2021 | ns | 36 | m | dyspnea | left ventricle | 4/2.5 | Eco | no | no | 168 | same | enclosing LAD |
| 41 | [16] | 2023 | cavernous capillary | 64 | m | pericardial effusions | right ventricle | 1.9 | Eco | mielodysplastic syndrome | yes | 3 | no | extended to pulmonary artery |
| 42 | [12] | 2021 | cavernous | 79 | w | dysartria perioral numbness | mitral valve | 1/0.7/0.5/0.5 | Eco | 2 (same site) | yes | ns | ns | multiple infarcts zone in frontal left lobe |
| 43 | [51] | 2024 | cavernous | 68 | m | palpitation dyspnea | right ventricle | ns | Eco | no | yes | ns | ns | na |
| 44 | [52] | 2024 | capillary | 52 | w | chest pain | right ventricle | 3/2.5/1.5 | Eco | no | yes | ns | ns | na |
| 45 | [81] | 2022 | ns | <1 | w | asymptomatic | atrio-ventricular junction | 3.5/1.2/0.9 | Eco | no | biopsy | ns | ns | DSA |
| 46 | [13] | 2021 | capillary | 49 | w | dyspnea chest pain | left atrium | 9/6.5 | Eco | no | yes | ns | ns | extended to LAD |
| 47 | [53] | 2022 | cavernous | 44 | w | dyspnea | mitral valve | 2/3.0 | Eco | no | yes | ns | ns | na |
| 48 | [54] | 2024 | ns | 19 | w | palpitation | mitral valve | 1/1.5 | Eco | no | yes | ns | ns | na |
| 49 | [55] | 2024 | arteriovenous | 52 | m | chest pain | right ventricle | 6.0/4 | Eco | no | yes | 6 | no | mitral valve annuloplasty |
| 50 | [56] | 2021 | arteriovenous | 55 | w | asymptomatic | right ventricle | 1.5/1.4 | Eco | no | yes | 13 | no | na |
| 51 | [57] | 2021 | cavernous | 14 | m | chest pain | right ventricle | 1/1.2 | Eco | no | yes | 8 | no | na |
| 52 | [14] | 2024 | cavernous | 44 | m | palpitation dyspnea | right atrium | 7.5/6/5 | CT | no | yes | 12 | no | extended to IVC |
| 53 | [82] | 2024 | ns | <1 | w | tachycardia | mitral valve | 0.7/0.8 | Eco | liver hemangioma | no | 12 | reduced | medical treatment |
| 54 | [58] | 2021 | capillary | 48 | w | dyspnea | right ventricle | 4.3/3 | CT | no | yes | 6 | no | pericarditis |
| 55 | [15] | 2022 | cavernous | 44 | m | asymptomatic | right atrium | 4.5/3 | CT | liver hemangioma | yes | 4 | no | extended to IVC |
Appendix B
References
- Richter, G.T.; Friedman, A.B. Hemangiomas and Vascular Malformations: Current Theory and Management. Int. J. Pediatr. 2012, 2012, 645678. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Teng, P.; Xu, H.; Ma, L.; Ni, Y. Cardiac Hemangioma: A Comprehensive Analysis of 200 Cases. Ann. Thorac. Surg. 2015, 99, 2246–2252. [Google Scholar] [CrossRef]
- Brizard, C.; Latremouille, C.; Jebara, V.A.; Acar, C.; Fabiani, J.N.; Deloche, A.; Carpentier, A.F. Cardiac Hemangiomas. Ann. Thorac. Surg. 1993, 56, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, T.; Jaggi, N.; Kalra, A.; Bansal, K.; Sharma, S.P. Hemangioma: Review of Literature. J. Contemp. Dent. Pract. 2013, 14, 1000–1007. [Google Scholar]
- Abramso, D.I. Blood Vessels and Lymphatics; Academic Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Fletcher, C.D.M. Diagnostic Histopathology of Tumors; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Patrut, M.E.; Manole, G.; Dinca, G.V. Tumorile Cardiace; Coresi: Bucharest, Romania, 2007. [Google Scholar]
- Dema, A.; Taban, S.; Cornianu, M. Morfopatologie Generala; Victor Babes: Timisoara, Romania, 2019. [Google Scholar]
- Bangal, K.; Keshav, M.; Joshi, S.S.; Murthy, K. Hemangioma (Arteriovenous Type) Forming an Interventricular Septal Mass. Ann. Card. Anaesth. 2023, 26, 237–238. [Google Scholar] [CrossRef]
- Caicedo, D.; Oshiro, K.; Glickstein, J.S.; Krishnan, U.; DiLorenzo, M.P. Cardiac Hemangioma in an Asymptomatic Teenager with a History of Congenital Heart Disease. CASE 2020, 4, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, J.; Loobuyck, V.; Silvestri, V.; Chaput, A.; Altes, A. Right Atrial Cardiac Hemangioma: A Multidisciplinary Pathway From Symptoms to Surgery. JACC Case Rep. 2024, 29, 102920. [Google Scholar] [CrossRef]
- Parkash, O.; Ying, G.W.; Ram, A.; Vemireddy, L.P.; Zahra, F. A Rare Case of Cavernous Hemangioma of the Mitral Valve Presenting as Multifocal Embolic Brain Infarcts. Cureus 2021, 13, e17721. [Google Scholar] [CrossRef]
- Yildirim, S.; Işik, M.; Tanyeli, Ö.; Görmüş, N. Giant Left Atrial Capillary Haemangioma Invading Left-Main Coronary Artery. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 631–633. [Google Scholar] [CrossRef]
- Cheaban, R.; Piran, M.; Opacic, D.; Gummert, J.F.; Rojas, S.V. Epicardial Cavernous Haemangioma; A Case Report of a Unique Incidental Finding. Eur. Heart J. Case Rep. 2024, 8, ytae146. [Google Scholar] [CrossRef]
- Harrison, S. Rare Case of Cavernous Haemangioma of the Right Atrium with Probable Hepatic Haemangioma. Case Rep. Cardiol. 2022, 2022, 9214196. [Google Scholar] [CrossRef]
- Abdul-Rahman, D.G.; Hajisaeed, S.R.; Othman, Y.N.; Anwar, E.O.; Majeed, Z.S.; Ali, R.K.; Muhamad, H.N.; Qadir, O.O. Patient with Myelodysplastic Syndrome Presented with Recurrent Pericardial Effusion Diagnosed as Epicardial Hemangioma; Case Report of a Rare Diagnosis with Rare Presentation. Radiol. Case Rep. 2023, 18, 2253–2258. [Google Scholar] [CrossRef]
- Reynen, K. Frequency of Primary Tumors of the Heart. Am. J. Cardiol. 1996, 77, 107. [Google Scholar] [CrossRef] [PubMed]
- Parato, V.M.; Nocco, S.; Alunni, G. Imaging of Cardiac Masses: An Updated Overview. J. Cardiovasc. Echogr. 2022, 32, 65–75. [Google Scholar] [CrossRef]
- Qamar, F.; Kharsa, C.; Letham, P.; Aoun, J.; Goel, S.S.; Kleiman, N.S.; Reardon, M.J.; Atkins, M.D. Cardiac Cavernous Hemangioma. JACC Case Rep. 2025, 30, 102956. [Google Scholar] [CrossRef]
- Kondo, Y.; Yasutsune, T.; Kado, Y.; Jinzai, Y.; Takigawa, T.; Kishigami, T.; Inaba, Y.; Nishimura, Y. Giant Cardiac Hemangioma in the Right Atrium: An Asymptomatic Surgical Case. Gen. Thorac. Cardiovasc. Surg. Cases 2023, 2, 63. [Google Scholar] [CrossRef]
- Shah, M.; Russo, L.P.; Haddad, D.; Chang, J.; Okere, A. Cardiac Hemangioma: A Rare Tumor Presenting as Postpartum Chest Pain. Cureus 2023, 15, e44407. [Google Scholar] [CrossRef]
- Xie, T.; Masroor, M.; Chen, X.; Liu, F.; Zhang, J.; Yang, D.; Liu, C.; Xiang, M. Rheumatism as a Cause of Cardiac Hemangioma: A Rare Case Report and Review of Literature with Special Focus on Etiology. BMC Cardiovasc. Disord. 2023, 23, 203. [Google Scholar] [CrossRef]
- Batko, J.; Rams, D.J.; Bartuś, K.; Bartoszcze, A.; Litwinowicz, R.A. Cardiac Hemangioma in the Atrioventricular Node Localization. Kardiochirurgia Torakochirurgia Pol. 2023, 20, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.Y.; Silva, D.R.d.; Cardoso, A.P.T.; Stolf, N.A.G.; Pozzan, G. Cardiac Papillary Muscle Hemangioma. Autops. Case Rep. 2020, 10, e2020169. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Hu, Y.N. Cardiac Hemangioma Mimicking Infective Endocarditis. Diagnostics 2024, 14, 2109. [Google Scholar] [CrossRef] [PubMed]
- Berdica, L.; Kola, E.; Nakuci, D.; Horjeti, E.; Alimehmeti, M. Cardiac Hemangioma Presenting as a Primary Cardiac Tumor. Cardio-Oncology 2023, 9, 3. [Google Scholar] [CrossRef]
- Osada, H.; Yamazaki, K.; Suzuki, T.; Tomotsuka, S.; Sugimoto, A.; Fujimoto, M.; Minatoya, K. Cardiac Capillary Hemangioma Originating from the Mitral Valve. JTCVS Tech. 2023, 20, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Rattenni, F.; Arlati, F.G.; Galanti, A.; Sansone, F.; Clerici, A.; Triggiani, M.; Muneretto, C. Advanced Presentation of Cardiac Hemangioma. J. Cardiothorac. Surg. 2024, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Kalinic, N.; Tomanic, T.C.; Redzek, A.; Sobot, N.; Skrbic, R.; Radomir, B.; Bjeljac, I.; Jonjev, Z.; Maric, S.; Miljevic, I.B.; et al. Pericardial Hemangioma: An Extremely Rare Cardiac Tumor. Kardiol. Pol. 2024, 82, 105–106. [Google Scholar] [CrossRef]
- Ilcheva, L.; Cholubek, M.; Loiero, D.; Dzemali, O. Cardiac Hemangioma in the Left Ventricular Septum. Thorac. Cardiovasc. Surg. Rep. 2024, 13, e4–e7. [Google Scholar] [CrossRef]
- Kobayashi, T.; Numata, S.; Hohri, Y.; Kawajiri, H.; Yaku, H. Cardiac Cavernous Hemangioma with High Fluorodeoxyglucose Uptake on Preoperative Positron Emission Tomography/Computed Tomography: A Case Report. Gen. Thorac. Cardiovasc. Surg. Cases 2023, 2, 39. [Google Scholar] [CrossRef]
- Fan, J.; Guo, L.; Teng, P.; Dai, X.; Zheng, Q.; Wu, S.; Ni, Y. Diagnostic Mystery—A Rare Right Ventricular Cardiac Hemangioma: A Case Report. J. Cardiothorac. Surg. 2021, 16, 362. [Google Scholar] [CrossRef]
- Shashikanth, M.; Nicola, S.; Yi, C.; Julian, S. Right Atrial Cavernous Hemangioma. Ann. Card. Anaesth. 2020, 23, 335–337. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Hu, P.; Ma, X.J.; Xie, J. Rare Gourd-Shaped Cardiac Hemangioma: Computed Tomography Imaging Characteristics and Clinical Management. Anatol. J. Cardiol. 2025, 29, E3–E4. [Google Scholar] [CrossRef]
- Anbardar, M.H.; Soleimani, N.; Mohammadzadeh, S. Two Cases of Cardiac Hemangioma in Different Anatomical Locations Presenting with Chest Pain and Palpitation. Clin. Case Rep. 2022, 10, e05495. [Google Scholar] [CrossRef] [PubMed]
- Thilak, R.; Sivanesan, A.; Munuswamy, H.; Toi, P.C. A Giant Right Atrial Hemangioma—Case Report. Cureus 2022, 14, e24622. [Google Scholar] [CrossRef]
- Cattapan, C.; Testolin, L.; Caprioglio, F.; Cerrito, L.F.; Basso, C.; Rizzo, S.; Gerosa, G. Preoperative Transcatheter Diagnosis of Right Atrial Hemangioma. JACC Case Rep. 2023, 15, 101857. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ma, H.; Guo, Y. Giant Cavernous Hemangioma in the Aortic Root and Right Atrioventricular Groove. J. Thorac. Cardiovasc. Surg. 2020, 159, e291–e293. [Google Scholar] [CrossRef]
- Toscano, M.; Alves, A.R.; Matias, C.; Carvalho, M.; Marques, M. Hemangioma of the Mitral Valve: Following the Murmur. Rev. Port. Cardiol. 2022, 41, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Kaewboonlert, N.; Chunharas, P.; Pluthikarmpae, N.; Poontananggul, J.; Wongthep, A.; Pongsuwan, N.; Lerssuttipon, U. Right Ventricular Outflow Tract Obstruction by Cardiac Hemangioma in Asymptomatic Patient. J. Surg. Case Rep. 2024, 2024, rjae321. [Google Scholar] [CrossRef] [PubMed]
- Kesieme, E.B.; Buchan, K.G. Multiple Right Ventricular Haemangiomas. Cureus 2023, 15, e36570. [Google Scholar] [CrossRef]
- Liu, F.; Dong, M.; Li, Q. Lobulated Hemangioma as a Rare Cause of Tricuspid Regurgitation. Clin. Med. Insights Case Rep. 2024, 17, 11795476241274699. [Google Scholar] [CrossRef]
- Vu, T.T.; Nguyen, V.T.; Tran, Q.T.; Ngo Thi, M.H.; Do, T.H.; Le, H.S.; Nguyen, N.T.; Nguyen, V.T.; Lam, K. A Case of a Small-Sized Cavernous Hemangioma in the Right Ventricle—An Incidental Finding. Radiol. Case Rep. 2022, 17, 856–862. [Google Scholar] [CrossRef]
- Dobritoiu, F.; Moldovan, H.; Oncica, R.; Vasile, G.; Nechifor, E.; Copaescu, C. Giant Cavernous Hemangioma of the Right Atrium—A Rare Case and Literature Review. Chirurgia 2020, 115, 267–273. [Google Scholar] [CrossRef]
- Rocco, R.; Daly, R.; Arghami, A. Robotic-Assisted Resection of Rare Mitral Valve Hemangioma. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 249–252. [Google Scholar] [CrossRef]
- Dursun, A.; Hakgor, A.; Kenger, M.Z.; Karaca, O. Concomitant Right Atrial Hemangioma Resection with LVAD Implantation: First-in-Human Experience. JACC Case Rep. 2024, 29, 102860. [Google Scholar] [CrossRef] [PubMed]
- Takago, S.; Iino, K.; Yamamoto, Y.; Takemura, H. Cardiac Haemangioma Treated with Surgical Resection Involving Reconstruction of the Right Ventricle. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Gallego, B.; Hernández-Jiménez, V.; Castillo, L.; González-Davia, R.; De Antonio-Antón, N.; Reyes-Copa, G. Unexpected Diagnosis: Large Hemangioma in the Interatrial Septum. J. Cardiothorac. Surg. 2024, 19, 305. [Google Scholar] [CrossRef] [PubMed]
- Drevet, G.; Chalabreysse, L.; Gamondes, D.; Tronc, F.; Maury, J.M. Epicardial Carvernous Hemangioma: The Diagnostic Challenge of a Middle Mediastinal Mass. Thorac. Cancer 2021, 12, 2404–2406. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Pham, D.T. Totally Endoscopic Resection of Epicardial Cardiac Haemangioma under On-Pump Beating Heart. Ann. Med. Surg. 2021, 69, 102838. [Google Scholar] [CrossRef]
- Ku, L.; Chen, Y.; Wang, Y.; Liu, Z.; Ma, X. Image of the Month Multimodality Imaging for the Diagnosis of Giant Cavernous Hemangioma of the Right Ventricle. Hell. J. Cardiol. 2024. [Google Scholar] [CrossRef]
- Moreno-Pallares, E.; Vargas-Vergara, D.; Bornacelly, A.; Gutiérrez, J.; Olaya-Sánchez, A.; Velasco-Morales, M. Right Ventricular Capillary Hemangioma as a Cause of Congestive Heart Failure: Case Report and Review of the Literature. Arch. Cardiol. Mex. 2024, 94, 254–257. [Google Scholar] [CrossRef]
- Bayfield, N.; Bibo, L.; Wang, E.; Passage, J. Cavernous Haemangioma of Anterior Mitral Valve Leaflet: Diagnostic Utility of 3D Echocardiography. BMJ Case Rep. 2022, 15, e247352. [Google Scholar] [CrossRef]
- Sengupta, A.; LaRocca, G.; Goldman, M.E.; Adams, D.H.; Anyanwu, A.C. Hemangioma of the Mitral Valve and Aortomitral Curtain in an Adolescent. JACC Case Rep. 2024, 29, 102840. [Google Scholar] [CrossRef]
- Piao, M.; Zhou, X.; Yan, M. Exploring the Causes of Newly Developed Mitral Valve Regurgitation after the Resection of a Giant Left Ventricular Tumor (Hemangioma). J. Cardiothorac. Vasc. Anesth. 2024, 39, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Zhang, C.; Fan, C.; Liu, L.; Wan, J. Case Report: A Primary Right Ventricular Vascular Malformation Presenting as a Mass. Front. Cardiovasc. Med. 2021, 8, 736199. [Google Scholar] [CrossRef]
- Tang, M.; Jian, Z.; Yan, Y.; Guo, F. Right Ventricular Haemangioma as a Rare Cause of Chest Pain: A Case Report. Eur. Heart J. Case Rep. 2021, 5, ytab477. [Google Scholar] [CrossRef]
- Darbari, A.; Singh, D.; Gilbert, S.; Kumar, B.; Singh, N. Capillary Haemangioma of the Heart Presenting with Pericardial Effusion: A Case Report. J. Cardiovasc. Thorac. Res. 2021, 13, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Adomat, F.; Steffen, D.A.; Suter-Magpantay, L.; Linka, A.; Weber, L. Case Report: A Non-Invasive Approach to Diagnosis and Management of Pericardial Haemangioma. Eur. Heart J. Case Rep. 2024, 8, ytae545. [Google Scholar] [CrossRef]
- Schaeffer, T.; Glatz, K.; Eckstein, F.S.; Matt, P. Composite Haemangioendothelioma in the Heart: A Case Report. Eur. Heart J. Case Rep. 2023, 7, ytad343. [Google Scholar] [CrossRef] [PubMed]
- Karaağaç, E.; Yeşilkaya, N.; Tellioğlu, T.M.; Ünay, F.Ç.; Beşir, Y. A Rare Cardiac Tumor Presenting with Myxoma: Primary Cardiac Hemangioendothelioma. Turk. J. Thorac. Cardiovasc. Surg. 2021, 29, 110–113. [Google Scholar] [CrossRef]
- Langguth, P.; Ravesh, M.S.; Haneya, A.; Both, M. Composite Hemangioendothelioma: The First Case of a Right Atrioventricular Pericardial Tumour. Eur. Heart J. Case Rep. 2020, 4, ytaa110. [Google Scholar] [CrossRef]
- Theerasuwipakorn, N.; Peenakhon, S.; Varachotisate, P.; Srinu, W.; Promratpan, W. Giant Pericardial Hemangioma with a Large Central Fibrotic Core. CJC Open 2024, 6, 1028–1031. [Google Scholar] [CrossRef]
- Mitsuishi, A.; Miura, Y.; Hosogi, S.; Tsutsui, M.; Kitaoka, H. 3 Lobes of Extracardiac Hemangioma. JACC Case Rep. 2024, 29, 102406. [Google Scholar] [CrossRef]
- Sadegh Beigee, F.; Sheikhy, A.; Sheikhy, K. Reconstruction of Chest Wall by Cryopreserved Sternum Allograft After Resection of Sternal Hemangioma: A Case Report. Front. Surg. 2022, 9, 796806. [Google Scholar] [CrossRef]
- Alsaloum, M.; Lee, C.; Dudorova, E.; Szabolcs, M.J.; Shetty, M.; Navot, B.; Ravalli, S. A Right Atrial Mass Discovered Postpartum: A Diagnostic Challenge. CASE 2023, 7, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.; Hopkins, K.; Chugh, M.; Murthy, R.; Choueiter, N. Multimodality Approach to a Neonate with a Pericardial Mass and a Hemorrhagic Pericardial Effusion. JACC Case Rep. 2024, 29, 102921. [Google Scholar] [CrossRef] [PubMed]
- Stahel, H.T.; Haranal, M.; Song, L. Case Report: Right Ventricular Outflow Tract Obstruction Caused by Multicomponent Mesenchymal Tumor. Front. Cardiovasc. Med. 2022, 9, 988271. [Google Scholar]
- Ranjan, A.; Agarwal, R.; Singh, D. Capillary Haemangioma of the Tricuspid Valve Annulus: A Rare Presentation. Int. J. Surg. Case Rep. 2023, 106, 108171. [Google Scholar] [CrossRef]
- Qiao, E.; Wang, Y.; Huang, Z.; Li, F.; Wang, W. Long-Term Follow-up of Resection of Primary Benign Right Ventricular Tumours: A 10-Year Surgical Experience. Ann. R. Coll. Surg. Engl. 2021, 103, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Barros Alves, F.; Ribeiro Morgado, M.; Carvalho, A.; Vasconcelos, M.; Rodrigues-Pereira, P.; Alves, S.; Macedo, F.; Madureira, A.J. Pericardial Hemangioma—Imaging with Pathologic Correlation of an Extremely Rare Mediastinal Lesion. Rev. Port. Cardiol. 2024, 43, 49–50. [Google Scholar] [CrossRef]
- Braun, V.; Prey, S.; Gurioli, C.; Boralevi, F.; Taieb, A.; Grenier, N.; Loot, M.; Jullie, M.L.; Léauté-Labrèze, C. Congenital Haemangiomas: A Single-Centre Retrospective Review. BMJ Paediatr. Open 2020, 4, e000816. [Google Scholar] [CrossRef]
- Huang, W.; Li, L.; Gao, J.; Gao, J.B. Epithelioid Hemangioendothelioma of the Right Atrium Invaded the Superior Vena Cava: Case Report and Review of Literature. Int. J. Cardiovasc. Imaging 2021, 37, 285–290. [Google Scholar] [CrossRef]
- Jacob, D.; Pratap, T.; Kumar, A.; Rashmi, R.; Vishnu, A.K. Benign Pericardial Hemangioma-A Rare Cause of Cardiac Tamponade. Indian J. Radiol. Imaging 2021, 31, 754–757. [Google Scholar] [CrossRef]
- Xu, M.; Xiong, F.; Zhang, L.; Wang, S. Hemangioma Mimicking Left Atrial Mass in the Posterior Mediastinum: A Case Report with Literature Review. Int. Heart J. 2021, 62, 453–457. [Google Scholar] [CrossRef]
- Castaneda, A.R.; Varco, R.L. Tumors of the Heart: Surgical Considerations. Am. J. Cardiol. 1968, 21, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chapman, D.; Siddiqui, F. A Rare Cardiac Cavernous Hemangioma Treated with Radiotherapy. Case Rep. Vasc. Med. 2022, 2022, 5698475. [Google Scholar] [CrossRef] [PubMed]
- Mosby. WHO Classification of Soft Tissue Tumors, 10th ed.; Anderson’s Pathology; Mosby: Maryland Heights, MO, USA, 1996. [Google Scholar]
- Shafer, W.G.; Hine, M.K.; Levy, B.M. Chapter 2 Benign and Malignant Tumors of Oral Cavity. In Text Book of Oral Pathology; Elsevier: Amsterdam, The Netherlands, 1983; pp. 197–202. [Google Scholar]
- Jin, Y.N.; Cheng, J.L.; Zhang, Y.; Shao, X.N.; Zhang, X.P.; Zhang, W.B. An Mri Image Analysis of Primary Cardiac Neoplasms. Int. J. Gen. Med. 2021, 14, 2943–2951. [Google Scholar] [CrossRef]
- Chen, J.; Gong, X.; Xie, L.; Wu, Q.; Zhao, T.; Hu, S. Atrial Septal Defect with a Rare Occupying Lesion in Heart. BMC Cardiovasc. Disord. 2022, 22, 482. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Almeida, C.; Antunes-Sarmento, J.; Miranda, J.O. Refractory Fetal and Neonatal Supraventricular Tachycardia Associated with Mitral Valve Mass. Cureus 2024, 16, e63944. [Google Scholar] [CrossRef]
- Nakajima, T.; Shibata, T.; Ogura, K.; Iba, Y.; Kawaharada, N. A Case of a Giant Hemangioma of a Primary Cardiac Tumor. Cureus 2023, 15, e43818. [Google Scholar] [CrossRef]
- Zonooz, Y.A.; Alizadehasl, A.; Davani, D.N.; Jebelli, S.F.H.; Aliabadi, A.Y.; Najdaghi, S.; Meshgi, S.; Shafieeardestani, S. Cardiac Interventricular Septum Hemangioma in a Colon Cancer Patient Treated with Capecitabine: A Case Report and Review of Literature. Clin. Case Rep. 2024, 12, e9331. [Google Scholar] [CrossRef]
- Al Umairi, R.S.; Sabek, S. Left Ventricle Intramuscular Haemangioma a Case Report and Review of Literature. Sultan Qaboos Univ. Med. J. 2021, 21, e316–e319. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Kitai, T.; Yamane, T.; Sano, M.; Koyama, T.; Furukawa, Y. A Huge Cardiac Haemangioma in the Left Ventricular Wall. Eur. Heart J. Case Rep. 2020, 4, ytaa374. [Google Scholar] [CrossRef]
- Ciliberti, P.; Bordonaro, V.; Curione, D.; Perazzolo, A.; Ciancarella, P.; Santangelo, T.; Napolitano, C.; Natale, L.; Galletti, L.; Secinaro, A. Additional Value of Cardiac Magnetic Resonance Parametric Mapping in Tissue Characterization of Common Benign Paediatric Cardiac Tumours. Eur. Heart J. Cardiovasc. Imaging 2024, 26, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dasagrandhi, V.; Kumar, R.; Kumar, R.; Mittal, B.R. Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography Computed Tomography Imaging in a Case of Pericardial Cavernous Hemangioma. Indian J. Nucl. Med. 2020, 35, 360–361. [Google Scholar] [CrossRef] [PubMed]
- McAllister, H.A.; Fenoglio, J.J. Tumors of the Cardiovascular System; Hartmann: Heidenheim, Germany, 1978; Volume 2, p. 81. [Google Scholar]
- Available online: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Cardio-oncology-guidelines (accessed on 10 March 2025).


| Type | Avg_Age_m | Avg_Age_w | Count_m | Count_w | Max_Age_m | Max_Age_w | Min_Age_m | Min_Age_w |
|---|---|---|---|---|---|---|---|---|
| arteriovenous | 52 | 55 | 1 | 1 | 52 | 55 | 52 | 55 |
| capillary | 68.66 | 56.85 | 3 | 7 | 78 | 71 | 52 | 48 |
| cavernous | 48.7 | 57.23 | 10 | 13 | 71 | 87 | 14 | 38 |
| cavernous/capillary | 60.6 | 45.33 | 5 | 3 | 79 | 52 | 14 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, I.R.; Novaconi, R.C.; Merce, A.P.; Dima, C.N.; Falnita, L.S.; Manzur, A.R.; Streian, C.G.; Feier, H.B. Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes. Cancers 2025, 17, 1532. https://doi.org/10.3390/cancers17091532
Munteanu IR, Novaconi RC, Merce AP, Dima CN, Falnita LS, Manzur AR, Streian CG, Feier HB. Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes. Cancers. 2025; 17(9):1532. https://doi.org/10.3390/cancers17091532
Chicago/Turabian StyleMunteanu, Iulia Raluca, Ramona Cristina Novaconi, Adrian Petru Merce, Ciprian Nicusor Dima, Lucian Silviu Falnita, Andrei Raul Manzur, Caius Glad Streian, and Horea Bogdan Feier. 2025. "Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes" Cancers 17, no. 9: 1532. https://doi.org/10.3390/cancers17091532
APA StyleMunteanu, I. R., Novaconi, R. C., Merce, A. P., Dima, C. N., Falnita, L. S., Manzur, A. R., Streian, C. G., & Feier, H. B. (2025). Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes. Cancers, 17(9), 1532. https://doi.org/10.3390/cancers17091532





