Patient-Reported Outcomes Before and After Radiotherapy for Brain Metastases—A Prospective Cohort Study of 239 Non-Small-Cell Lung Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification
2.2. Patient Characteristics
2.3. Treatment
2.4. Patient-Reported Outcome Measures
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Patient Characteristics
3.2. Survival
3.3. PROs
3.3.1. Association Between PROs at Start of Radiotherapy and Survival
3.3.2. Patients Completing Questionnaires at M0 Only
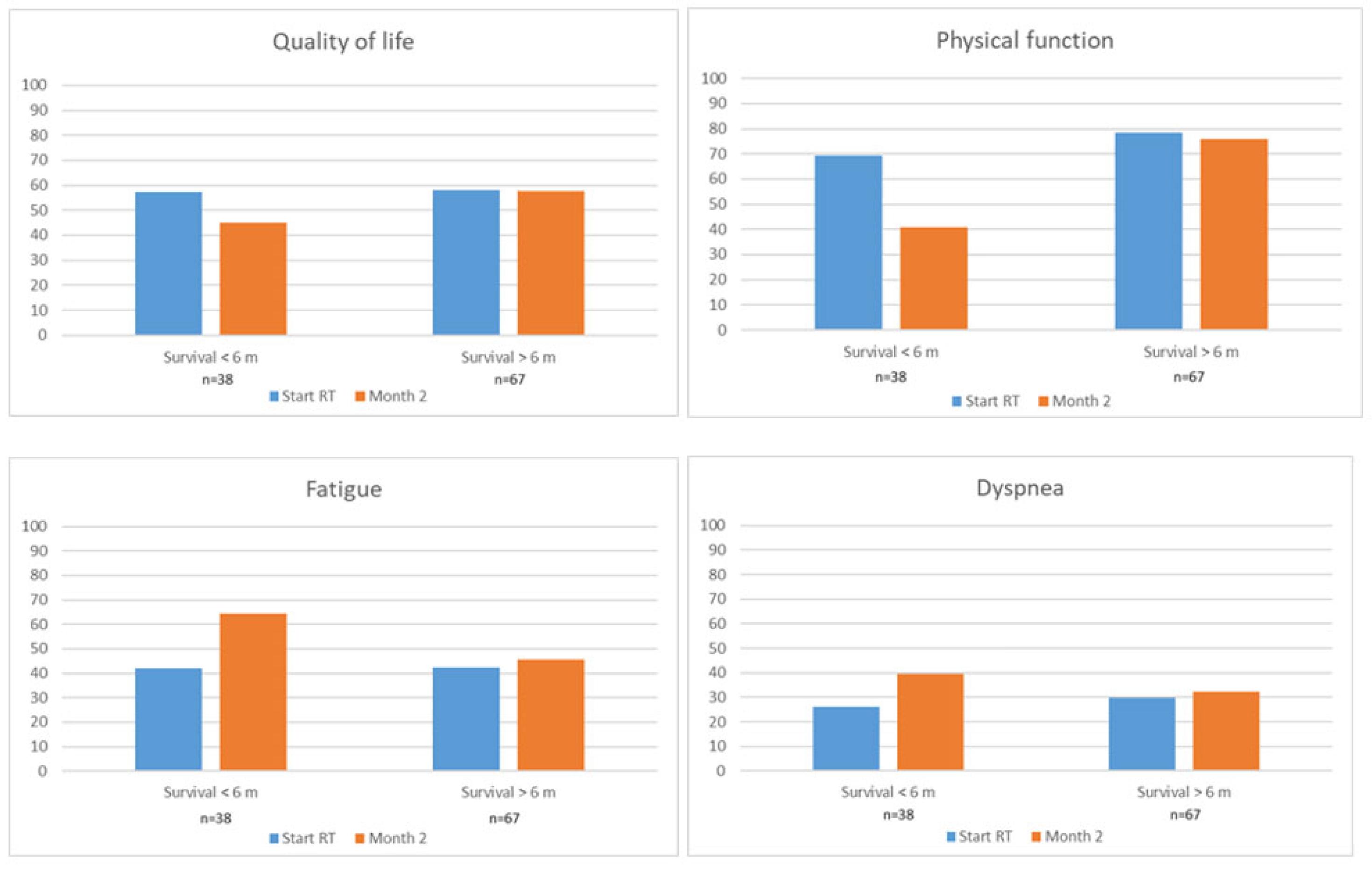
3.3.3. Patients Completing Questionnaires at Both M0 and M2
3.3.4. PROs According to ECOG Status
3.3.5. PROs According to Number of BMs
4. Discussion
4.1. Major Findings
4.2. Multivariable Analyses
4.3. PRO Scores
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berghoff, A.S.; Schur, S.; Füreder, L.M.; Gatterbauer, B.; Dieckmann, K.; Widhalm, G.; Hainfellner, J.; Zielinski, C.C.; Birner, P.; Bartsch, R.; et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open 2016, 1, e000024. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.B.; Barreto, R.B.; de Oliveira, F.C.G.; Martin, G.S.D.; Takiguchi, O.M.Y.; Chirichela, I.A.; Miranda, M.H.F.; Bodnar, D.; Alves Reis, L.A.; Pereira, G.C.B.; et al. Radiotherapy for Brain Metastases Near the End of Life: Characterizing Patients and Tumor Features. JCO Glob. Oncol. 2023, 9, e2300143. [Google Scholar] [CrossRef]
- Di Maio, M.; Basch, E.; Denis, F.; Fallowfield, L.J.; Ganz, P.A.; Howell, D.; Kowalski, C.; Perrone, F.; Stover, A.M.; Sundaresan, P.; et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann. Oncol. 2022, 33, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.J.; Temin, S.; Ghoshal, A.; Alesi, E.R.; Ali, Z.V.; Chauhan, C.; Cleary, J.F.; Epstein, A.S.; Firn, J.I.; Jones, J.A.; et al. Palliative Care for Patients With Cancer: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 2336–2357. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef]
- Steinmann, D.; Vordermark, D.; Gerstenberg, W.; Aschoff, R.; Gharbi, N.; Müller, A.; Schäfer, C.; Theodorou, M.; Wypior, H.J.; Geinitz, H. Quality of life in patients with limited (1-3) brain metastases undergoing stereotactic or whole brain radiotherapy: A prospective study of the DEGRO QoL working group. Strahlenther. Onkol. 2020, 196, 48–57. [Google Scholar] [CrossRef]
- Peters, S.; Bexelius, C.; Munk, V.; Leighl, N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat. Rev. 2016, 45, 139–162. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 2016, 388, 2004–2014. [Google Scholar] [CrossRef]
- Kaasa, S.; Loge, J.H.; Aapro, M.; Albreht, T.; Anderson, R.; Bruera, E.; Brunelli, C.; Caraceni, A.; Cervantes, A.; Currow, D.C.; et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018, 19, e588–e653. [Google Scholar] [CrossRef] [PubMed]
- Caissie, A.; Nguyen, J.; Chen, E.; Zhang, L.; Sahgal, A.; Clemons, M.; Kerba, M.; Arnalot, P.F.; Danjoux, C.; Tsao, M.; et al. Quality of life in patients with brain metastases using the EORTC QLQ-BN20+2 and QLQ-C15-PAL. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Donovan, L.; Sheehan, J. Health related quality of life trajectories after stereotactic radiosurgery for brain metastases: A systematic review. J. Neurooncol. 2022, 159, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Mehta, M.P.; Smart, D.K.; Steeg, P.S.; Hong, J.A.; Espey, M.G.; Prasanna, P.G.; Crandon, L.; Hodgdon, C.; Kozak, N.; et al. National Cancer Institute Collaborative Workshop on Shaping the Landscape of Brain Metastases Research: Challenges and recommended priorities. Lancet Oncol. 2023, 24, e344–e354. [Google Scholar] [CrossRef]
- Yri, O.E.; Astrup, G.L.; Karlsson, A.T.; van Helvoirt, R.; Hjermstad, M.J.; Husby, K.M.; Loge, J.H.; Lund, J.; Lundeby, T.; Paulsen, Ø.; et al. Survival and quality of life after first-time diagnosis of brain metastases: A multicenter, prospective, observational study. Lancet Reg. Health Eur. 2025, 49, 101181. [Google Scholar] [CrossRef]
- Karlsson, A.T.; Hjermstad, M.J.; Aass, N.; Skovlund, E.; Kaasa, S.; Yri, O.E. Overall Survival after Radiotherapy for Brain Metastases According to ECOG Status—A Prospective Study of 294 NSCLC Patients. Cancers 2024, 16, 1486. [Google Scholar] [CrossRef]
- Sperduto, P.W.; De, B.; Li, J.; Carpenter, D.; Kirkpatrick, J.; Milligan, M.; Shih, H.A.; Kutuk, T.; Kotecha, R.; Higaki, H.; et al. Graded Prognostic Assessment (GPA) for Patients With Lung Cancer and Brain Metastases: Initial Report of the Small Cell Lung Cancer GPA and Update of the Non-Small Cell Lung Cancer GPA Including the Effect of Programmed Death Ligand 1 and Other Prognostic Factors. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 60–74. [Google Scholar] [CrossRef]
- Groenvold, M.; Petersen, M.A.; Aaronson, N.K.; Arraras, J.I.; Blazeby, J.M.; Bottomley, A.; Fayers, P.M.; de Graeff, A.; Hammerlid, E.; Kaasa, S.; et al. EORTC QLQ-C15-PAL: The new standard in the assessment of health-related quality of life in advanced cancer? Palliat. Med. 2006, 20, 59–61. [Google Scholar] [CrossRef]
- EORTC QLQ-C30 Scoring Manual Brussels, Belgium: EORTC. Third. 2001. Available online: https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf (accessed on 11 November 2024).
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Aamdal, E.; Skovlund, E.; Jacobsen, K.D.; Straume, O.; Kersten, C.; Herlofsen, O.; Karlsen, J.; Hussain, I.; Amundsen, A.; Dalhaug, A.; et al. Health-related quality of life in patients with advanced melanoma treated with ipilimumab: Prognostic implications and changes during treatment. ESMO Open 2022, 7, 100588. [Google Scholar] [CrossRef]
- Otto-Vollaard, L.; Quint, S.; de Pree, I.M.N.; Steinvoort, I.N.; Tims, O.J.L.; Nuyttens, J.J. Brain Metastases: Patient-Reported Outcome and Quality of Life after Whole-Brain Radiotherapy. J. Palliat. Med. 2022, 25, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.Q.; Sheehan, D.E.; Sheehan, K.A.; Katsos, K.; Fadul, C.E. Quality of life after stereotactic radiosurgery for brain metastasis: An assessment from a prospective national registry. J. Neurooncol. 2024, 171, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ilie, G.; Bradfield, J.; Moodie, L.; Lawen, T.; Ilie, A.; Lawen, Z.; Blackman, C.; Gainer, R.; Rutledge, R.D.H. The Role of Response-Shift in Studies Assessing Quality of Life Outcomes Among Cancer Patients: A Systematic Review. Front. Oncol. 2019, 9, 783. [Google Scholar] [CrossRef]
- Schnurman, Z.; Mashiach, E.; Link, K.E.; Donahue, B.; Sulman, E.; Silverman, J.; Golfinos, J.G.; Oermann, E.K.; Kondziolka, D. Causes of Death in Patients With Brain Metastases. Neurosurgery 2023, 93, 986–993. [Google Scholar] [CrossRef]
- Vermassen, T.; Van Parijs, C.; De Keukeleire, S.; Vandecasteele, K.; Rottey, S. Prognostication of Brain-Metastasized Patients Receiving Subsequent Systemic Therapy: A Single-Center Long-Term Follow-Up. Curr. Oncol. 2025, 32, 74. [Google Scholar] [CrossRef]
- Steinmann, D.; Paelecke-Habermann, Y.; Geinitz, H.; Aschoff, R.; Bayerl, A.; Bölling, T.; Bosch, E.; Bruns, F.; Eichenseder-Seiss, U.; Gerstein, J.; et al. Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC Cancer 2012, 12, 283. [Google Scholar] [CrossRef]
- Komosinska, K.; Kepka, L.; Niwinska, A.; Pietrzak, L.; Wierzchowski, M.; Tyc-Szczepaniak, D.; Kaczmarczyk, A.; Bujko, K. Prospective evaluation of the palliative effect of whole-brain radiotherapy in patients with brain metastases and poor performance status. Acta Oncol. 2010, 49, 382–388. [Google Scholar] [CrossRef]
- Ahlner-Elmqvist, M.; Bjordal, K.; Jordhøy, M.S.; Kaasa, S.; Jannert, M. Characteristics and implications of attrition in health-related quality of life studies in palliative care. Palliat. Med. 2009, 23, 432–440. [Google Scholar] [CrossRef]
- Al-Baimani, K.; Jonker, H.; Zhang, T.; Goss, G.D.; Laurie, S.A.; Nicholas, G.; Wheatley-Price, P. Are clinical trial eligibility criteria an accurate reflection of a real-world population of advanced non-small-cell lung cancer patients? Curr. Oncol. 2018, 25, e291–e297. [Google Scholar] [CrossRef]

| Characteristic | Responders n = 239 (%) | Non-Responders n = 55 (%) | Responders at M0 Only n = 96 (%) | Responders at Both M0 and M2 n = 105 (%) | Short-Term Survivors (<6 Months) n = 138 (%) | Long-Term Survivors (>6 Months) n = 101 (%) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 122 (51) | 23 (42) | 49 (51) | 51 (49) | 72 (52) | 50 (50) |
| Age | ||||||
| <70 | 127 (53) | 25 (46) | 48 (50) | 58 (55) | 68 (49) | 59 (58) |
| ≥70 | 112 (47) | 30 (54) | 48 (50) | 47 (45) | 70 (51) | 42 (42) |
| ECOG | ||||||
| ECOG 0–1 | 131 (55) | 27 (49) | 36 (38) | 72 (69) | 53 (38) | 78 (77) |
| ECOG 2 | 70 (29) | 15 (27) | 36 (38) | 23 (22) | 54 (39) | 16 (16) |
| ECOG 3–4 | 35 (15) | 12 (22) | 23 (24) | 8 (7) | 30 (22) | 5 (5) |
| Unknown | 3 (1) | 1 (2) | 1 | 2(2) | 1 (1) | 2 (2) |
| Histology | ||||||
| Adenocarcinoma | 180 (75) | 40 (73) | 69 (72) | 85 (81) | 94 (68) | 86 (85) |
| Squamous cell carcinoma | 34 (14) | 10 (18) | 15 (16) | 12 (11) | 22 (16) | 12 (12) |
| Others * | 25 (11) | 5 (9) | 12 (12) | 8 (8) | 22 (16) | 3 (3) |
| Mutation status/PD-L1 status ** | ||||||
| Present (EGFR = 16, ALK = 4, PD-L1 = 129) | 136 (57) | 30 (55) | 50 (52) | 59 (56) | 75 (54) | 61 (60) |
| Absent | 103 (43) | 25 (45) | 46 (48) | 46 (44) | 63 (46) | 40 (40) |
| Clinical status primary tumor | ||||||
| Primary tumor controlled/removed | 69 (29) | 17 (31) | 25 (26) | 35 (33) | 36 (26) | 33 (33) |
| Uncontrolled | 170 (71) | 38 (69) | 71 (74) | 70 (67) | 102 (74) | 68 (67) |
| Extracranial metastases | ||||||
| Controlled | 97 (41) | 27 (49) | 30 (31) | 52 (50) | 41 (30) | 56 (55) |
| Uncontrolled | 142 (59) | 28 (51) | 66 (69) | 53 (50) | 97 (70) | 45 (45) |
| Number of BMs | ||||||
| 1 | 70 (29) | 19 (35) | 29 (30) | 32 (31) | 33 (24) | 37 (36) |
| 2–4 | 76 (32) | 24 (43) | 25 (26) | 38 (36) | 44 (32) | 32 (32) |
| ≥ 5 | 93 (39) | 12 (22) | 42 (44) | 35 (33) | 61 (44) | 32 (32) |
| Largest diameter of BMs | ||||||
| <3 cm | 163 (68) | 39 (71) | 62 (65) | 74 (70) | 89 (65) | 74 (73) |
| ≥3 cm | 68 (29) | 12 (22) | 31 (32) | 27 (26) | 44 (32) | 24 (24) |
| Missing | 8 (3) | 4 (7) | 3 (3) | 4 (4) | 5 (3) | 3 (3) |
| Initial treatment | ||||||
| WBRT | 124 (52) | 17 (31) | 57 (59) | 44 (42) | 85 (62) | 39 (39) |
| SRT | 115 (48) | 38 (69) | 39 (41) | 61 (58) | 53 (38) | 62 (61) |
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Baseline characteristic and PROs | |||||||||
| ECOG | |||||||||
| ECOG 0–1 | 131 | 1 | 1 | 1 | 1 | ||||
| ECOG 2–4 | 105 | 3.0 (2.2–4.0) | <0.001 | 2.5 (1.8–3.6) | <0.001 | 2.8 (2.0–3.7) | <0.001 | 2.7 (2.0–3.7) | <0.001 |
| Missing | 3 | ||||||||
| ECM | |||||||||
| Controlled | 96 | 1 | 1 | 1 | 1 | ||||
| Uncontrolled | 140 | 1.9 (1.4–2.5) | <0.001 | 1.9 (1.4–2.6) | <0.001 | 2.0 (1.5–2.6) | <0.001 | 1.9 (1.4–2.5) | <0.001 |
| BMs | |||||||||
| 1–4 BMs | 145 | 1 | |||||||
| ≥ 5 | 91 | 1.5 (1.1–2.0) | 0.005 | ||||||
| Largest diameter of BMs | |||||||||
| <3 cm | 163 | 1 | |||||||
| ≥3 cm | 68 | 1.4 (1.0–1.8) | 0.044 | ||||||
| Missing | 8 | ||||||||
| Clinical status primary tumor | |||||||||
| Controlled/removed | 69 | 1 | |||||||
| Uncontrolled | 167 | 1.1 (0.8–1.5) | 0.622 | ||||||
| EORTC QLQ-C15 PAL | |||||||||
| Function scales | (>66.7/≤66.7) a | ||||||||
| Overall QoL | 54/177 c | 1.2 (0.9–1.7) | 0.279 | ||||||
| Physical function | 115/117 d | 2.1 (1.6–2.8) | <0.001 | 1.3 (1.0–1.9) | 0.073 | ||||
| Emotional function | 126/107 e | 1.2 (0.9–1.5) | 0.271 | ||||||
| Symptom scales | (<33.3/≥33.3) b | ||||||||
| Fatigue | 50/183 f | 1.3 (0.9–1.9) | 0.112 | ||||||
| Nausea/vomiting | 155/77 g | 1.2 (0.9–1.6) | 0.294 | ||||||
| Pain | 121/111 h | 1.6 (1.2–2.1) | 0.002 | ||||||
| Dyspnea | 80/153 i | 1.1 (0.8–1.5) | 0.486 | ||||||
| Sleep disturbance | 89/144 j | 0.8 (0.6–1.0) | 0.101 | ||||||
| Appetite loss | 133/99 k | 1.4 (1.1–1.9) | 0.014 | ||||||
| Constipation | 104/127 l | 1.4 (1.0–1.8) | 0.040 | ||||||
| EORTC QLQ-BN20 | |||||||||
| Headaches | 136/98 m | 0.9 (0.7–1.2) | 0.333 | ||||||
| Visual disorder | 179/55 n | 1.4 (1.0–1.9) | 0.035 | ||||||
| Seizures | 201/33 o | 1.1 (0.8–1.6) | 0.632 | ||||||
| Motor dysfunction | 133/103 | 1.7 (1.3–2.2) | <0.001 | 1.3 (1.0–1.8) | 0.053 | ||||
| Communication deficit | 189/46 p | 1.3 (1.0–1.9) | 0.093 | ||||||
| Drowsiness | 56/177 q | 1.5 (1.1–2.2) | 0.011 | ||||||
| Weakness of legs | 101/132 r | 1.8 (1.4–2.4) | <0.001 | 1.5 (1.1–2.0) | 0.008 | ||||
| EORTC Scale | Responders n = 239 | Responders at M0 Only n = 96 | Responders Both at M0 and M2 n = 105 | |
|---|---|---|---|---|
| M0 | M0 | M2 | ||
| EORTC QLQ-C15 PAL | Mean score (SD) | |||
| Overall QoL | 54.1 (25.5) | 47.7 (25.3) | 58.5 (23.3) | 53.6 (22.7) |
| Physical function | 67.0 (27.0) | 57.2 (26.8) | 75.4 (24.3) | 63.9 (30.7) |
| Emotional function | 73.1 (27.1) | 70.3 (70.3) | 78.8 (23.8) | 78.6 (26.5) |
| Fatigue | 44.6 (26.1) | 49.5 (27.7) | 42.0 (24.5) | 51.6 (28.5) |
| Nausea/vomiting | 15.2 (24.9) | 20.0 (29.3) | 11.8 (20.8) | 22.1 (28.5) |
| Pain | 30.5 (31.0) | 38.9 (33.7) | 24.5 (27.3) | 26.9 (30.4) |
| Dyspnea | 33.6 (30.7) | 38.5 (33.3) | 28.8 (27.4) | 34.6 (30.1) |
| Sleep disturbance | 33.9 (33.0) | 34.4 (35.4) | 34.3 (31.6) | 25.6 (29.1) |
| Appetite loss | 23.1 (31.6) | 29.9 (34.4) | 19.1 (29.6) | 34.3 (36.1) |
| Constipation | 27.8 (30.9) | 34.7 (33.1) | 24.0 (30.4) | 26.9 (32.5) |
| EORTC QLQ-BN20 | ||||
| Headaches | 19.5 (26.9) | 22.5 (28.9) | 18.3 (25.4) | 15.7 (22.3) |
| Visual disorder | 15.2 (22.5) | 19.8 (27.4) | 13.6 (20.3) | 15.5 (20.1) |
| Seizures | 6.5 (17.5) | 5.9 (17.4) | 7.4 (18.0) | 7.4 (19.1) |
| Motor dysfunction | 29.1 (25.4) | 37.8 (25.4) | 22.2 (24.4) | 25.1 (26.4) |
| Communication deficit | 15.2 (21.7) | 15.9 (20.4) | 14.4 (23.6) | 13.4 (22.0) |
| Drowsiness | 38.3 (26.7) | 42.8 (29.8) | 36.2 (28.0) | 45.1 (28.5) |
| Weakness of legs | 29.0 (31.6) | 38.2 (32.2) | 22.8 (28.7) | 36.5 (33.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsson, A.T.; Hjermstad, M.J.; Aass, N.; Skovlund, E.; Astrup, G.L.; Kaasa, S.; Yri, O.E. Patient-Reported Outcomes Before and After Radiotherapy for Brain Metastases—A Prospective Cohort Study of 239 Non-Small-Cell Lung Cancer Patients. Cancers 2025, 17, 1529. https://doi.org/10.3390/cancers17091529
Karlsson AT, Hjermstad MJ, Aass N, Skovlund E, Astrup GL, Kaasa S, Yri OE. Patient-Reported Outcomes Before and After Radiotherapy for Brain Metastases—A Prospective Cohort Study of 239 Non-Small-Cell Lung Cancer Patients. Cancers. 2025; 17(9):1529. https://doi.org/10.3390/cancers17091529
Chicago/Turabian StyleKarlsson, Astrid Telhaug, Marianne Jensen Hjermstad, Nina Aass, Eva Skovlund, Guro Lindviksmoen Astrup, Stein Kaasa, and Olav Erich Yri. 2025. "Patient-Reported Outcomes Before and After Radiotherapy for Brain Metastases—A Prospective Cohort Study of 239 Non-Small-Cell Lung Cancer Patients" Cancers 17, no. 9: 1529. https://doi.org/10.3390/cancers17091529
APA StyleKarlsson, A. T., Hjermstad, M. J., Aass, N., Skovlund, E., Astrup, G. L., Kaasa, S., & Yri, O. E. (2025). Patient-Reported Outcomes Before and After Radiotherapy for Brain Metastases—A Prospective Cohort Study of 239 Non-Small-Cell Lung Cancer Patients. Cancers, 17(9), 1529. https://doi.org/10.3390/cancers17091529






