Management of Squamous Cell Carcinomas of the Anal Canal and Anal Margin After Failure of Chemoradiotherapy Treatment: A Narrative Review
Simple Summary
Abstract
1. Introduction
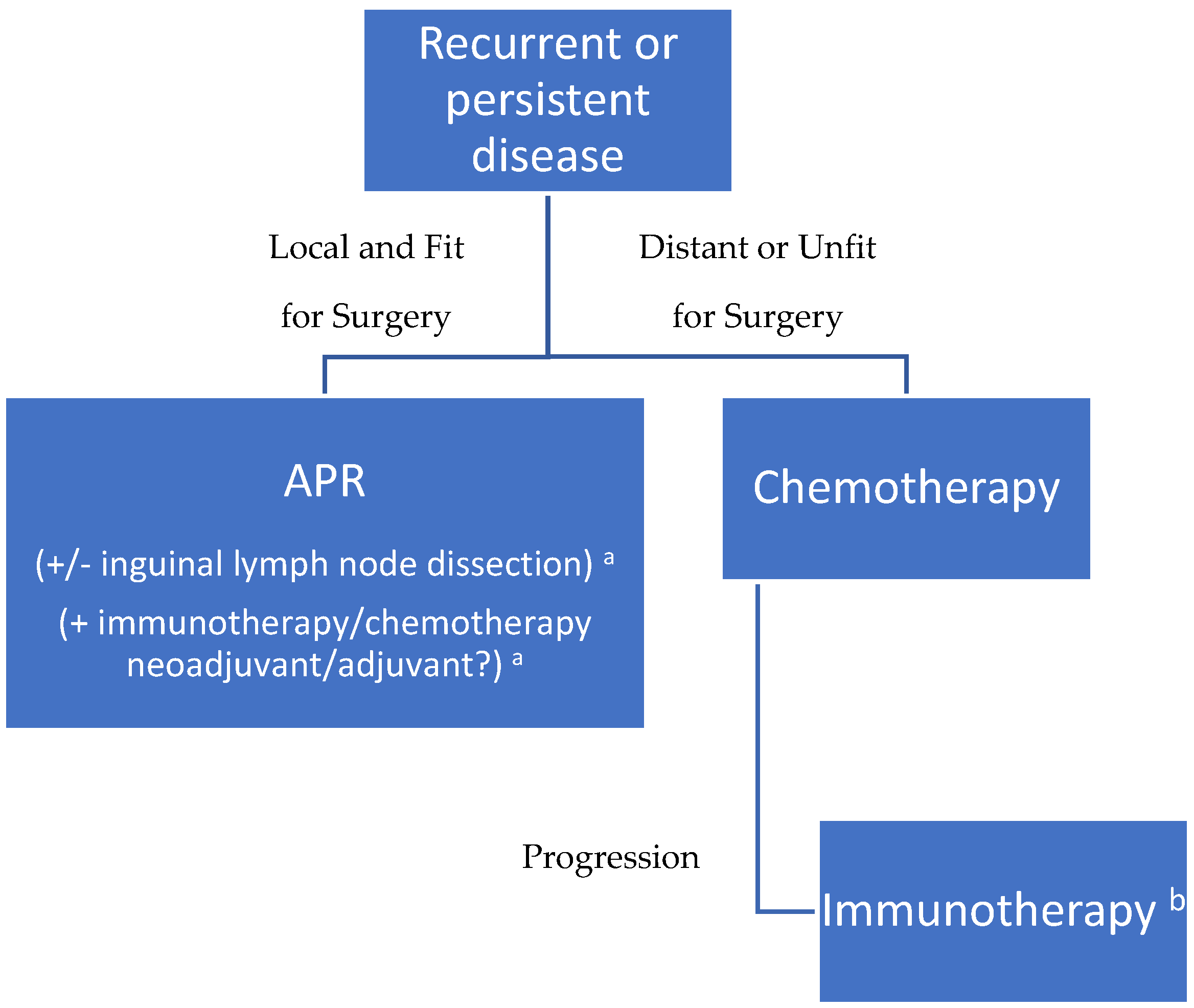
2. Management Strategies in Local/Loco-Regional Disease
2.1. Salvage Surgery
2.2. Chemotherapy
2.3. Radiotherapy
2.4. Immunotherapy
2.5. Cryotherapy
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, S.; Guren, M.G.; Khan, K.; Brown, G.; Renehan, A.G.; Steigen, S.E.; Deutsch, E.; Martinelli, E.; Arnold, D.; ESMO Guidelines Committee. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Gondal, T.A.; Chaudhary, N.; Bajwa, H.; Rauf, A.; Le, D.; Ahmed, S. Anal Cancer: The Past, Present and Future. Curr. Oncol. 2023, 30, 3232–3250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartelink, H.; Roelofsen, F.; Eschwege, F.; Rougier, P.; Bosset, J.F.; Gonzalez, D.G.; Peiffert, D.; van Glabbeke, M.; Pierart, M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J. Clin. Oncol. 1997, 15, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Northover, J.; Glynne-Jones, R.; Sebag-Montefiore, D.; James, R.; Meadows, H.; Wan, S.; Jitlal, M.; Ledermann, J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year followup of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 2010, 102, 1123–1128. [Google Scholar] [CrossRef]
- Peiffert, D.; Tournier-Rangeard, L.; Gérard, J.P.; Lemanski, C.; François, E.; Giovannini, M.; Cvitkovic, F.; Mirabel, X.; Bouché, O.; Luporsi, E.; et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: Final analysis of the randomized UNICANCER ACCORD 03 trial. J. Clin. Oncol. 2012, 30, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Myint, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C. Fluorouracil, mitomycin, and radiotherapy vs. fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA 2008, 299, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- UKCCCR Anal Cancer Trial Working Party. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996, 348, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.; Sarkaria, A.; Merchant, S.J.; Booth, C.M.; Patel, S.V. A systematic review of outcomes after salvage abdominoperineal resection for persistent or recurrent anal squamous cell cancer. Color. Dis. 2019, 21, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, D.; Tsukada, Y.; Ito, M.; Horasawa, S.; Bando, H.; Yoshino, T.; Yamada, K.; Ajioka, Y.; Sugihara, K. Survival outcomes following salvage abdominoperineal resection for recurrent and persistent anal squamous cell carcinoma. Eur. J. Surg. Oncol. 2023, 49, 106929. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Yuan, Y.; Wang, S.; Liao, Z.; Cai, P.; Chen, B.; Zhang, R.; Wang, F.; Zeng, Z.; Gao, Y. Neoadjuvant PD-1 Blockade Combined With Chemotherapy Followed by Concurrent Immunoradiotherapy in Locally Advanced Anal Canal Squamous Cell Carcinoma Patients: Antitumor Efficacy, Safety and Biomarker Analysis. Front. Immunol. 2022, 12, 798451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upadhyay, L.; Hartzell, M.; Parikh, A.R.; Strickland, M.R.; Klempner, S.; Malla, M. Recent Advances in the Management of Anal Cancer. Healthcare 2023, 11, 3010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedersen, T.B.; Gocht-Jensen, P.; Klein, M.F. 30-day and long-term outcome following salvage surgery for squamous cell carcinoma of the anus. Eur. J. Surg. Oncol. 2018, 44, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Dzik-Jurasz, A.; O’Neill, B.; Tait, D.; Husband, J.E.; Brown, G. Pelvic phased-array MR imaging of anal carcinoma before and after chemoradiation. Br. J. Radiol. 2008, 81, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.B.; Juvik, A.F.; Koren, S.F.; Gocht-Jensen, P.; Klein, M.F. Quality of life following salvage surgery for squamous cell carcinoma of the anus. Eur. J. Surg. Oncol. 2019, 45, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.K.; Pal, S.; Lee, P.J.; Rodwell, L.; Solomon, M.J. Pelvic exenteration for recurrent squamous cell carcinoma of the pelvic organs arising from the cloaca—A single institution’s experience over 16 years. Color. Dis. 2013, 15, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Temperley, H.C.; Shokuhi, P.; O’Sullivan, N.J.; Mac Curtain, B.; Waters, C.; Murray, A.; Buckley, C.E.; O’Neill, M.; Mehigan, B.; McCormick, P.H.; et al. Primary closure versus vertical rectus abdominis myocutaneous (VRAM) flap closure of perineal wound following abdominoperineal resection-a systematic review and meta-analysis. Ir. J. Med. Sci. 2024, 193, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lefevre, J.H.; Parc, Y.; Kernéis, S.; Shields, C.; Touboul, E.; Chaouat, M.; Tiret, E. Abdomino-perineal resection for anal cancer: Impact of a vertical rectus abdominis myocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann. Surg. 2009, 250, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Quezada-Diaz, F.F.; Gönen, M.; Karagkounis, G.; Widmar, M.; Wei, I.H.; Smith, J.J.; Nash, G.M.; Weiser, M.R.; Paty, P.B.; et al. Oncologic Outcomes of Salvage Abdominoperineal Resection for Anal Squamous Cell Carcinoma Initially Managed with Chemoradiation. J. Clin. Med. 2024, 13, 2156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hagemans, J.A.W.; Blinde, S.E.; Nuyttens, J.J.; Morshuis, W.G.; Mureau, M.A.M.; Rothbarth, J.; Verhoef, C.; Burger, J.W.A. Salvage Abdominoperineal Resection for Squamous Cell Anal Cancer: A 30-Year Single-Institution Experience. Ann. Surg. Oncol. 2018, 25, 1970–1979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fields, A.C.; Melnitchouk, N.; Senturk, J.; Irani, J.; Bleday, R.; Goldberg, J. Early versus late salvage abdominoperineal resection for anal squamous cell carcinoma: Is there a difference in survival? J. Surg. Oncol. 2019, 120, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.C.H.; Hakim, A.; Kellish, A.S.; Singh, P.; Wozniak, M.; Kwiatt, M.; Gaughan, J.; Hong, Y.K. Inguinal Lymph Node Dissection Does Not Improve Overall Survival in Anal Cancer Nodal Disease. J. Surg. Res. 2020, 255, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sclafani, F.; Eng, C.; Adams, R.A.; Guren, M.G.; Sebag-Montefiore, D.; Benson, A.; Bryant, A.; Peckitt, C.; Segelov, E.; et al. International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. J. Clin. Oncol. 2020, 38, 2510–2518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.; François, E.; André, T.; Samalin, E.; Jary, M.; El Hajbi, F.; Baba-Hamed, N.; Pernot, S.; Kaminsky, M.C.; Bouché, O.; et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Damron, E.P.; McDonald, J.; Rooney, M.K.; Das, P.; Ludmir, E.B.; Minsky, B.D.; Messick, C.; Chang, G.J.; Morris, V.K.; Holliday, E.B. Salvage Treatment of Recurrent or Persistent Anal Squamous Cell Carcinoma: The Role of Multi-modality Therapy. Clin. Color. Cancer 2024, 23, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hallemeier, C.L.; You, Y.N.; Larson, D.W.; Dozois, E.J.; Nelson, H.; Klein, K.A.; Miller, R.C.; Haddock, M.G. Multimodality therapy including salvage surgical resection and intraoperative radiotherapy for patients with squamous-cell carcinoma of the anus with residual or recurrent disease after primary chemoradiotherapy. Dis. Colon. Rectum. 2014, 57, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Vaios, E.J.; Wo, J.Y. Proton beam radiotherapy for anal and rectal cancers. J. Gastrointest. Oncol. 2020, 11, 176–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marabelle, A.; Cassier, P.A.; Fakih, M.; Kao, S.; Nielsen, D.; Italiano, A.; Guren, T.K.; van Dongen, M.G.J.; Spencer, K.; Bariani, G.M.; et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: Results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol. Hepatol. 2022, 7, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ghiringhelli, F.; de la Fouchardière, C.; Evesque, L.; Smith, D.; Badet, N.; Samalin, E.; Lopez-Trabada Ataz, D.; Parzy, A.; Desramé, J.; et al. Atezolizumab plus modified docetaxel, cisplatin, and fluorouracil as first-line treatment for advanced anal cancer (SCARCE C17-02 PRODIGE 60): A randomised, non-comparative, phase 2 study. Lancet Oncol. 2024, 25, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ji, Z.; Lei, X.; He, Y.; Yuan, F. Cryotherapy for low rectal and anal cancer: Recommendation and indications. Front. Oncol. 2023, 13, 984145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| First Author (Year) | Type of Study | Sample Size (Number of Patients) | Median Follow-Up (Months) | ||
|---|---|---|---|---|---|
| Kitaguchi (2023) [12] | Multicentric retrospective cohort study | 152 | 49 | ||
| Rosen (2024) [23] | Monocentric retrospective cohort study | 96 | 22 | ||
| Hagemans (2018) [24] | Monocentric retrospective cohort study | 47 | 80 | ||
| Ko (2019) [11] | Systematic review | 1018 | NR | ||
| Fields (2019) [25] | National Cancer Database (USA) retrospective review | 437 | 27.1 (early salvage); 26.6 (late salvage) 1 | ||
| First Author (Year) | Overall Survival (Persistent) | Overall Survival (Recurrent) | Overall Survival (Overall) | Disease-Free Survival (Overall) | Recurrence-Free Survival (Overall) |
| Kitaguchi (2023) [12] | 36% (5 y) | 75% (5 y) | 71% (5 y) | NR | 64% (5 y) |
| Rosen (2024) [23] | NR | NR | NR | 26.8% (3 y) | 54.5% (3 y) |
| Hagemans (2018) [24] | 40.4% (5 y) | 41.7% (5 y) | 41.6% (5 y) | NR | 51.1% (5 y) |
| Ko (2019) [11] | 45% (5 y) | 51% (5 y) | NR | 44% (5 y) | NR |
| Fields (2019) [25] | NR | NR | 48.4% (5 y, early salvage); 40.3% (5 y, late salvage) 1 | NR | NR |
| First Author (Year) | Type of Study | Sample Size (Number of Patients) | Tumor Status | Treatment |
|---|---|---|---|---|
| Ott (2017) [33] | Multicenter, open-label, phase 1b trial | 25 | PD-L1 positive | Pembrolizumab |
| Marabelle (2022) [34] | Non-randomized, multicohort, multicenter, phase 2 study | 112 | PD-L1 positive or negative | Pembrolizumab |
| Kim (2024) [35] | Randomized, non-comparative, phase 2 study | 97 | PD-L1 positive or negative | Pembrolizumab + mDCF |
| First Author (Year) | Median Progression-Free Survival (Months) | 12-Month Progression-Free Survival Rate | Median Overall Survival (Months) | 12-Month Overall Survival Rate |
| Ott (2017) [33] | 3 | 19.70% | 9.3 | 47.60% |
| Marabelle (2022) [34] | 2 | 15% | 11.9 | 49% |
| Kim (2024) [35] | 9.4 | 45% | NR | 77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racine, M.; Meurette, G.; Ris, F.; Meyer, J.; Toso, C.; Liot, E. Management of Squamous Cell Carcinomas of the Anal Canal and Anal Margin After Failure of Chemoradiotherapy Treatment: A Narrative Review. Cancers 2025, 17, 1511. https://doi.org/10.3390/cancers17091511
Racine M, Meurette G, Ris F, Meyer J, Toso C, Liot E. Management of Squamous Cell Carcinomas of the Anal Canal and Anal Margin After Failure of Chemoradiotherapy Treatment: A Narrative Review. Cancers. 2025; 17(9):1511. https://doi.org/10.3390/cancers17091511
Chicago/Turabian StyleRacine, Michaël, Guillaume Meurette, Frédéric Ris, Jeremy Meyer, Christian Toso, and Emilie Liot. 2025. "Management of Squamous Cell Carcinomas of the Anal Canal and Anal Margin After Failure of Chemoradiotherapy Treatment: A Narrative Review" Cancers 17, no. 9: 1511. https://doi.org/10.3390/cancers17091511
APA StyleRacine, M., Meurette, G., Ris, F., Meyer, J., Toso, C., & Liot, E. (2025). Management of Squamous Cell Carcinomas of the Anal Canal and Anal Margin After Failure of Chemoradiotherapy Treatment: A Narrative Review. Cancers, 17(9), 1511. https://doi.org/10.3390/cancers17091511







