Rising Incidence and Mortality of Early-Onset Colorectal Cancer in Young Cohorts Associated with Delayed Diagnosis †
Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. USCS Database
2.2.2. NCHS Database
2.2.3. SEER Database
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Incidence Rates and Time Trends of EO-CRC
3.2. Age-Specific Incidence Rates and Time Trends of EO-CRC in Different Anatomical Locations
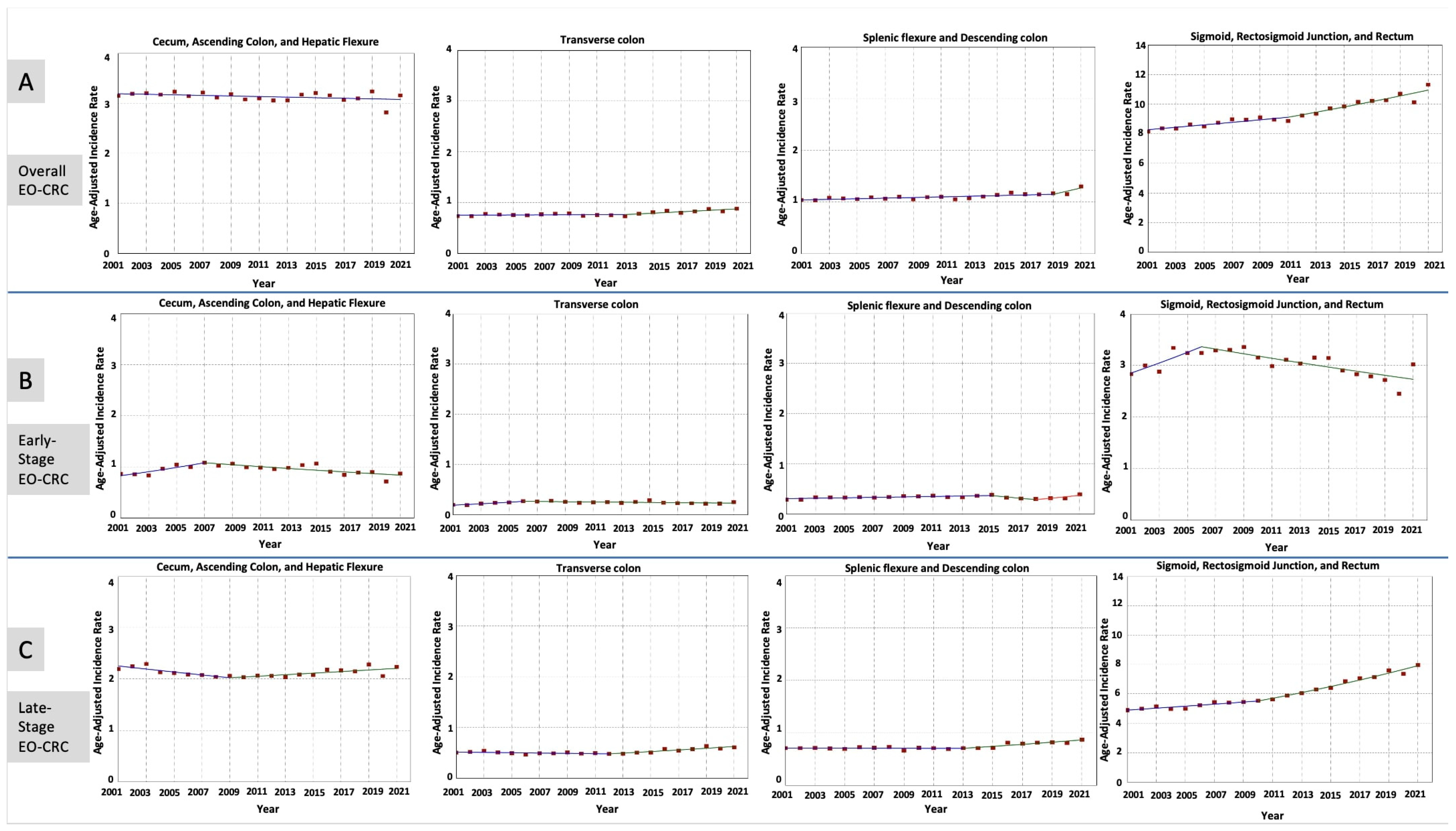
3.3. Stage-Specific Incidence Rates and Time Trends of EO-CRC in Different Anatomical Locations
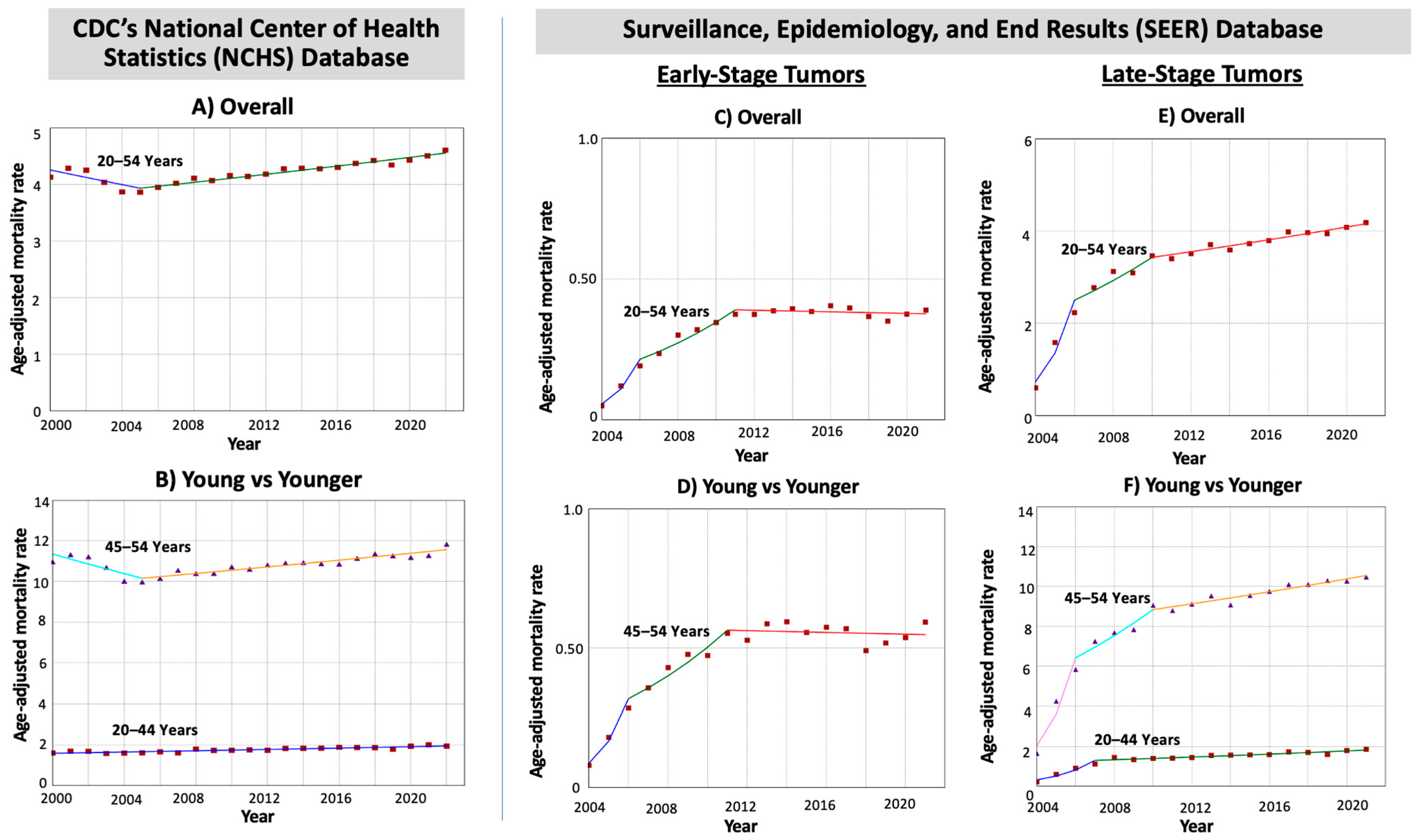
3.4. Mortality Rates and Time Trends of EO-CRC
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Abboud, Y.; Fraser, M.; Qureshi, I.; Hajifathalian, K. Early-Onset Colorectal Cancer: Are Neuroendocrine Tumors or Adenocarcinomas the Culprit? Analysis of the Largest U.S. Cancer Incidence Database, 2001–2020. J. Clin. Med. 2024, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.P.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.S.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Colorectal Cancer Screening: An Updated Decision Analysis for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2021. [Google Scholar]
- Nawras, Y.; Merza, N.; Beier, K.; Dakroub, A.; Al-Obaidi, H.; Al-Obaidi, A.D.; Amatul-Raheem, H.; Bahbah, E.; Varughese, T.; Hosny, J.; et al. Temporal Trends in Racial and Gender Disparities of Early Onset Colorectal Cancer in the United States: An Analysis of the CDC WONDER Database. J. Gastrointest. Cancer 2024, 55, 1511–1519. [Google Scholar] [CrossRef]
- Kearney, D.E.; Cauley, C.E.; Aiello, A.; Kalady, M.F.; Church, J.M.; Steele, S.R.; Valente, M.A. Increasing Incidence of Left-Sided Colorectal Cancer in the Young: Age Is Not the Only Factor. J. Gastrointest. Surg. 2020, 24, 2416–2422. [Google Scholar] [CrossRef]
- Lu, P.; Fields, A.C.; Vise, A.S.; Shabat, G.; Irani, J.L.; Bleday, R.; Goldberg, J.E.; Melnitchouk, N. Anatomic Distribution of Colorectal Adenocarcinoma in Young Patients. Dis. Colon Rectum 2019, 62, 920–924. [Google Scholar] [CrossRef]
- Lee, M.H.; Hinshaw, J.L.; Kim, D.H.; Pickhardt, P.J. Symptomatic Versus Asymptomatic Colorectal Cancer: Predictive Features at CT Colonography. Acad. Radiol. 2016, 23, 712–717. [Google Scholar] [CrossRef]
- Naveed, M.; Jamil, L.H.; Fujii-Lau, L.L.; Al-Haddad, M.; Buxbaum, J.L.; Fishman, D.S.; Jue, T.L.; Law, J.K.; Lee, J.K.; Qumseya, B.J.; et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of acute colonic pseudo-obstruction and colonic volvulus. Gastrointest. Endosc. 2020, 91, 228–235. [Google Scholar] [CrossRef]
- National Program of Cancer Registries and Surveillance. United States Cancer Statistics. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, Volume 2023. Available online: https://www.cdc.gov/national-program-cancer-registries/index.html (accessed on 1 January 2025).
- National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program. SEER*Stat Database: NPCR and SEER Incidence–U.S. Cancer Statistics 2001–2020 Public Use Research Database, 2022 Submission (2001–2020), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2023. Available online: https://www.cdc.gov/united-states-cancer-statistics/public-use/?CDC_AAref_Val=https://www.cdc.gov/cancer/uscs/public-use/ (accessed on 1 January 2025).
- Surveillance Epidemiology aERSPwscgSSDM-AC, Aggregated with State, Total U.S. (1990–2020) Katrina/Rita Population Adjustment, National Cancer Institute, DCCPS, Surveillance Research Program, Released June 2022. Underlying Mortality Data Provided By NCHS. Available online: https://www.cdc.gov/nchs/ (accessed on 1 January 2025).
- The Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-Based Mortality–SEER Research Limited-Field Data, 22 Registries (excl IL and MA), Nov 2023 Sub (2000–2021)–Linked To County Attributes–Time Dependent (1990–2022) Income/Rurality, 1969–2022 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2024, Based on the November 2023 Submission. Available online: https://seer.cancer.gov/ (accessed on 1 January 2025).
- CDC USCS Files. Available online: https://www.cdc.gov/united-states-cancer-statistics/technical-notes/pdf/uscs-data-visualizations-tool-technical-notes-2021-june-508.pdf (accessed on 21 April 2025).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. JAMA 2017, 318, 572–574. [Google Scholar] [CrossRef]
- Montminy, E.M.; Zhou, M.; Maniscalco, L.; Abualkhair, W.; Kim, M.K.; Siegel, R.L.; Wu, X.C.; Itzkowitz, S.H.; Karlitz, J.J. Contributions of Adenocarcinoma and Carcinoid Tumors to Early-Onset Colorectal Cancer Incidence Rates in the United States. Ann. Intern. Med. 2021, 174, 157–166. [Google Scholar] [CrossRef]
- Tiwari, R.C.; Clegg, L.X.; Zou, Z. Efficient interval estimation for age-adjusted cancer rates. Stat. Methods Med. Res. 2006, 15, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yu, B.; Feuer, E.J. Selecting The Number Of Change-Points In Segmented Line Regression. Stat. Sin. 2009, 19, 597–609. [Google Scholar] [PubMed]
- Kim, J.; Kim, H.J. Consistent Model Selection in Segmented Line Regression. J. Stat. Plan. Inference 2016, 170, 106–116. [Google Scholar] [CrossRef]
- Joinpoint Regression Program, Version 5.4.0.0-April 2025; Statistical Research and Applications Branch, Surveillance Research Program, National Cancer Institute. Available online: https://surveillance.cancer.gov/help/joinpoint (accessed on 1 January 2025).
- Kim, H.J.; Fay, M.P.; Yu, B.; Barrett, M.J.; Feuer, E.J. Comparability of segmented line regression models. Biometrics 2004, 60, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Millien, V.O.; da Costa, W.L., Jr.; Oluyomi, A.O.; Gould Suarez, M.; Thrift, A.P. Trends in the incidence of early-onset colorectal cancer in all 50 United States from 2001 through 2017. Cancer 2022, 128, 299–310. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Williams, J.L.; Holub, J.L.; Morris, C.D.; Logan, J.R.; Eisen, G.M.; Carney, P. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest. Endosc. 2014, 80, 133–143. [Google Scholar] [CrossRef]
- Murphy, C.C.; Lund, J.L.; Sandler, R.S. Young-Onset Colorectal Cancer: Earlier Diagnoses or Increasing Disease Burden? Gastroenterology 2017, 152, 1809–1812.e3. [Google Scholar] [CrossRef]
- Shih, Y.C.; Zhao, L.; Elting, L.S. Does Medicare coverage of colonoscopy reduce racial/ethnic disparities in cancer screening among the elderly? Health Aff. 2006, 25, 1153–1162. [Google Scholar] [CrossRef]
- Rex, D.K.; Johnson, D.A.; Lieberman, D.A.; Burt, R.W.; Sonnenberg, A. Colorectal cancer prevention 2000: Screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am. J. Gastroenterol. 2000, 95, 868–877. [Google Scholar] [CrossRef]
- Karahalios, A.; English, D.R.; Simpson, J.A. Weight change and risk of colorectal cancer: A systematic review and meta-analysis. Am. J. Epidemiol. 2015, 181, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; De, P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Exarchakou, A.; Donaldson, L.J.; Girardi, F.; Coleman, M.P. Colorectal cancer incidence among young adults in England: Trends by anatomical sub-site and deprivation. PLoS ONE 2019, 14, e0225547. [Google Scholar] [CrossRef]
- Lawler, T.; Parlato, L.; Warren Andersen, S. The histological and molecular characteristics of early-onset colorectal cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1349572. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of Inflammatory Bowel Diseases: A Population Study in a Healthcare District of North-West Italy. J. Clin. Med. 2023, 12, 641. [Google Scholar] [CrossRef]
- Birch, R.J.; Burr, N.; Subramanian, V.; Tiernan, J.P.; Hull, M.A.; Finan, P.; Rose, A.; Rutter, M.; Valori, R.; Downing, A.; et al. Inflammatory Bowel Disease-Associated Colorectal Cancer Epidemiology and Outcomes: An English Population-Based Study. Am. J. Gastroenterol. 2022, 117, 1858–1870. [Google Scholar] [CrossRef]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. [Google Scholar] [CrossRef]
- Gottschalk, Z.; Redman, M.W.; Baker, K.K.; Ulrich, C.M.; Siegel, E.M.; Figueiredo, J.C.; Shibata, D.; Toriola, A.T.; Gigic, B.; Ose, J.; et al. Comparison of the disease presentation of early- vs. later-onset colorectal cancer within the prospective ColoCare study. J. Clin. Oncol. 2024, 42, 91. [Google Scholar] [CrossRef]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. 2012, 25, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Pilozzi, E.; Lorenzon, L.; Lo Baido, S.; Ferri, M.; Duranti, E.; Fochetti, F.; Mercantini, P.; Ramacciato, G.; Balducci, G.; Ruco, L. Left-sided early onset colorectal carcinomas: A sporadic neoplasm with aggressive behavior. Am. J. Surg. 2017, 214, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin. Gastroenterol. Hepatol. 2017, 15, 903–909.e6. [Google Scholar] [CrossRef]
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Morton, D.; Seymour, M.; Magill, L.; Handley, K.; Glasbey, J.; Glimelius, B.; Palmer, A.; Seligmann, J.; Laurberg, S.; Murakami, K.; et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 1541–1552. [Google Scholar] [CrossRef]
- Audisio, A.; Fazio, R.; Daprà, V.; Assaf, I.; Hendlisz, A.; Sclafani, F. Neoadjuvant chemotherapy for early-stage colon cancer. Cancer Treat. Rev. 2024, 123, 102676. [Google Scholar] [CrossRef]
- Cheng, E.; Blackburn, H.N.; Ng, K.; Spiegelman, D.; Irwin, M.L.; Ma, X.; Gross, C.P.; Tabung, F.K.; Giovannucci, E.L.; Kunz, P.L.; et al. Analysis of Survival Among Adults With Early-Onset Colorectal Cancer in the National Cancer Database. JAMA Netw. Open 2021, 4, e2112539. [Google Scholar] [CrossRef]
- Carethers, J.M. Screening for colorectal cancer in African Americans: Determinants and rationale for an earlier age to commence screening. Dig. Dis. Sci. 2015, 60, 711–721. [Google Scholar] [CrossRef]

| Age Cohort (Years) | Early-Onset CRC Number of Patients (N = 474,601) a | Trends | Age-Specific AAPC Difference (95% CI) | Pairwise Comparison p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| Time Period | APC (95% CI) | AAPC (95% CI) | Age-Specific AAPC Difference | Test of Coincidence b | Test of Parallelism c | |||
| All Anatomical Locations | ||||||||
| 20–44 years | 129,938 (27.4%) | 2001–2006 | 2.35 * (1.30 to 3.42) | 1.51 * (1.24 to 1.79) | 0.78 * (0.30 to 1.25) | 0.001 | <0.001 | 0.001 |
| 2006–2021 | 1.23 * (1.05 to 1.42) | |||||||
| 45–54 years | 344,663 (72.6%) | 2001–2011 | 0.01 (−0.59 to 0.62) | 0.73 * (0.35 to 1.13) | ||||
| 2011–2021 | 1.46 * (0.87 to 2.05) | |||||||
| Cecum, Ascending Colon, and Hepatic Flexure | ||||||||
| 20–44 years | 25,772 (5.4%) | 2001–2021 | 0.07 (−0.22 to 0.36) | 0.07 (−0.22 to 0.36) | 0.35 * (0.01 to 0.70) | 0.04 | <0.001 | 0.03 |
| 45–54 years | 74,745 (15.7%) | 2001–2021 | −0.28 * (−0.51 to −0.06) | −0.28 * (−0.51 to −0.06) | ||||
| Transverse Colon | ||||||||
| 20–44 years | 7373 (1.6%) | 2001–2021 | 1.08 * (0.73 to 1.43) | 1.08 * (0.73 to 1.43) | 0.47 (−0.19 to 1.13) | 0.16 | <0.001 | 0.05 |
| 45–54 years | 17,654 (3.7%) | 2001–2013 | −0.28 (−0.94 to 0.38) | 0.61 * (0.04 to 1.18) | ||||
| 2013–2021 | 1.95 * (0.77 to 3.16) | |||||||
| Splenic Flexure and Descending Colon | ||||||||
| 20–44 years | 10,364 (2.2%) | 2001–2021 | 1.21 * (0.85 to 1.57) | 1.21 * (0.85 to 1.57) | 0.67 (−0.01 to 1.34) | 0.05 | <0.001 | 0.004 |
| 45–54 years | 24,896 (5.2%) | 2001–2012 | −0.51 (−1.29 to 0.28) | 0.54 (−0.04 to 1.13) | ||||
| 2012–2021 | 1.84 * (0.80 to 2.88) | |||||||
| Sigmoid Colon, Rectosigmoid, and Rectum | ||||||||
| 20–44 years | 81,825 (17.2%) | 2001–2007 | 2.73 * (1.82 to 3.64) | 2.04 * (1.75 to 2.34) | 0.88 * (0.39 to 1.38) | <0.001 | <0.001 | <0.001 |
| 2007–2021 | 1.75 * (1.53 to 1.98) | |||||||
| 45–54 years | 216,592 (45.6%) | 2001–2011 | 0.43 (−0.19 to 1.06) | 1.16 * (0.76 to 1.56) | ||||
| 2011–2021 | 1.90 * (1.31 to 2.49) | |||||||
| Tumor Anatomical Location | Early-Onset CRC Number of Patients (N = 474,601) a | Trends | ||||
|---|---|---|---|---|---|---|
| Time Period | APC (95% CI) | p-Value | AAPC (95% CI) | p-Value | ||
| All Stages Combined | ||||||
| Cecum, Ascending Colon, and Hepatic Flexure | 100,517 (21.2%) | 2001–2021 | −0.18 (−0.44 to 0.07) | 0.13 | −0.18 (−0.44 to 0.07) | 0.13 |
| Transverse Colon | 25,027 (5.3%) | 2001–2013 | 0.13 (−2.78 to 0.98) | 0.93 | 0.76 * (0.36 to 1.15) | 0.001 |
| 2013–2021 | 1.72 * (0.57 to 5.81) | 0.03 | ||||
| Splenic Flexure and Descending Colon | 35,260 (7.4%) | 2001–2019 | 0.55 (−0.33 to 0.86) | 0.08 | 1.02 * (0.60 to 1.24) | <0.001 |
| 2019–2021 | 5.27 * (0.79 to 7.68) | <0.001 | ||||
| Sigmoid, Rectosigmoid, and Rectum | 298,417 (62.9%) | 2001–2011 | 0.97 (−1.24 to 1.58) | 0.20 | 1.41 * (1.18 to 1.65) | <0.001 |
| 2011–2021 | 1.85 * (1.35 to 3.86) | 0.02 | ||||
| Early-Stage Tumors | ||||||
| Cecum, Ascending Colon, and Hepatic Flexure | 30,838 (6.5%) | 2001–2007 | 4.53 * (1.85 to 11.62) | <0.001 | 0.06 (−0.59 to 0.88) | 0.85 |
| 2007–2021 | −1.79 * (−2.97 to −0.96) | <0.001 | ||||
| Transverse Colon | 7905 (1.7%) | 2001–2006 | 6.84 * (2.85 to 18.52) | <0.001 | 0.95 * (0.16 to 1.89) | 0.02 |
| 2006–2021 | −0.94 * (−2.19 to −0.17) | 0.02 | ||||
| Splenic Flexure and Descending Colon | 10,932 (2.3%) | 2001–2015 | 1.31 * (0.68 to 2.28) | 0.01 | 0.98 * (0.37 to 1.55) | 0.009 |
| 2015–2018 | −7.91 * (−11.46 to −2.27) | 0.01 | ||||
| 2018–2021 | 9.04 * (2.86 to 19.14) | 0.01 | ||||
| Sigmoid, Rectosigmoid, and Rectum | 98,581 (20.8%) | 2001–2006 | 3.35 * (0.75 to 10.42) | 0.01 | −0.22 (−0.76 to 0.42) | 0.42 |
| 2006–2021 | −1.38 * (−2.29 to −0.83) | <0.001 | ||||
| Late-Stage Tumors | ||||||
| Cecum, Ascending Colon, and Hepatic Flexure | 67,754 (14.3%) | 2001–2009 | −1.34 * (−3.23 to −0.56) | <0.001 | −0.10 (−0.38 to 0.17) | 0.42 |
| 2009–2021 | 0.73 * (0.28 to 1.60) | 0.001 | ||||
| Transverse Colon | 16,561 (3.5%) | 2001–2012 | −0.64 (−1.88 to 0.13) | 0.10 | 0.97 * (0.58 to 1.37) | <0.001 |
| 2012–2021 | 2.97 * (1.98 to 4.79) | <0.001 | ||||
| Splenic Flexure and Descending Colon | 23,463 (4.9%) | 2001–2013 | −0.06 (−0.78 to 0.42) | 0.76 | 0.99 * (0.72 to 1.27) | <0.001 |
| 2013–2021 | 2.59 * (1.77 to 3.98) | <0.001 | ||||
| Sigmoid, Rectosigmoid, and Rectum | 189,847 (40.0%) | 2001–2010 | 1.34 * (0.55 to 1.88) | 0.007 | 2.44 * (2.26 to 2.64) | <0.001 |
| 2010–2021 | 3.35 * (3.01 to 3.87) | <0.001 | ||||
| Age Cohort (Years) | Early-Onset CRC Deaths: NCHS (N = 147,026) a SEER (N = 47,308) a | Trends | Age-Specific AAPC Difference (95% CI) | Pairwise Comparison p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| Time Period | APC (95% CI) | AAPC (95% CI) | Age-Specific AAPC Difference | Test of Coincidence b | Test of Parallelism c | |||
| All Stages (CDC’s NCHS Database) | ||||||||
| 20–54 years | 147,026 (100%) | 2000–2005 | −1.56 * (−3.52 to −0.56) | 0.31 * (0.17 to 0.47) | - | |||
| 2005–2022 | 0.87 * (0.70 to 1.07) | |||||||
| 20–44 years | 39,746 (27.0%) | 2000–2022 | 0.93 * (0.74 to 1.13) | 0.93 * (0.74 to 1.13) | 0.85 * (0.49 to 1.21) | <0.001 | <0.001 | <0.001 |
| 45–54 years | 107,280 (73.0%) | 2000–2005 | −2.19 * (−3.46 to −0.90) | 0.09 (−0.22 to 0.40) | ||||
| 2005–2022 | 0.76 * (0.57 to 0.96) | |||||||
| Early-Stage Tumors (SEER 22 Database) | ||||||||
| 20–54 years | 3775 (8.0%) | 2004–2006 | 96.21 * (55.58 to 149.24) | 11.88 * (10.40 to 14.88) | - | |||
| 2006–2011 | 12.65 * (7.99 to 17.87) | |||||||
| 2011–2021 | −0.36 (−2.11 to 0.88) | |||||||
| 20–44 years | 699 (1.5%) | ^ | - | |||||
| 45–54 years | 3076 (6.5%) | 2004–2006 | 90.57 * (46.28 to 165.15) | 11.38 * (9.28 to 15.57) | ||||
| 2006–2011 | 12.11 * (4.20 to 19.02) | |||||||
| 2011–2021 | −0.29 (−3.44 to 1.39) | |||||||
| Late-Stage Tumors (SEER 22 Database) | ||||||||
| 20–54 years | 37,677 (79.6%) | 2004–2006 | 83.90 * (60.38 to 109.49) | 10.69 * (9.38 to 12.16) | - | |||
| 2006–2010 | 8.21 * (3.85 to 13.65) | |||||||
| 2010–2021 | 1.76 * (0.02 to 2.53) | |||||||
| 20–44 years | 10,325 (21.8%) | 2004–2007 | 58.84 * (36.56 to 84.75) | 10.66 * (7.95 to 13.44) | 0.52 (−2.65 to 3.68) | 0.74 | <0.001 | 0.09 |
| 2007–2021 | 2.42 * (1.66 to 3.18) | |||||||
| 45–54 years | 27,352 (57.8%) | 2004–2006 | 77.50 * (57.44 to 100.12) | 10.15 * (8.58 to 11.73) | ||||
| 2006–2010 | 8.30 * (4.83 to 11.88) | |||||||
| 2010–2021 | 1.62 * (1.22 to 2.01) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abboud, Y.; Shah, A.; Fraser, M.; Montminy, E.M.; Pan, C.-W.; Hajifathalian, K.; Gaglio, P.J.; Al-Khazraji, A. Rising Incidence and Mortality of Early-Onset Colorectal Cancer in Young Cohorts Associated with Delayed Diagnosis. Cancers 2025, 17, 1500. https://doi.org/10.3390/cancers17091500
Abboud Y, Shah A, Fraser M, Montminy EM, Pan C-W, Hajifathalian K, Gaglio PJ, Al-Khazraji A. Rising Incidence and Mortality of Early-Onset Colorectal Cancer in Young Cohorts Associated with Delayed Diagnosis. Cancers. 2025; 17(9):1500. https://doi.org/10.3390/cancers17091500
Chicago/Turabian StyleAbboud, Yazan, Anand Shah, Madison Fraser, Eric M. Montminy, Chun-Wei Pan, Kaveh Hajifathalian, Paul J. Gaglio, and Ahmed Al-Khazraji. 2025. "Rising Incidence and Mortality of Early-Onset Colorectal Cancer in Young Cohorts Associated with Delayed Diagnosis" Cancers 17, no. 9: 1500. https://doi.org/10.3390/cancers17091500
APA StyleAbboud, Y., Shah, A., Fraser, M., Montminy, E. M., Pan, C.-W., Hajifathalian, K., Gaglio, P. J., & Al-Khazraji, A. (2025). Rising Incidence and Mortality of Early-Onset Colorectal Cancer in Young Cohorts Associated with Delayed Diagnosis. Cancers, 17(9), 1500. https://doi.org/10.3390/cancers17091500






