Café-Au-Lait Macules in Neurofibromatosis Type 1: Birthmark or Biomarker?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Cohort Description
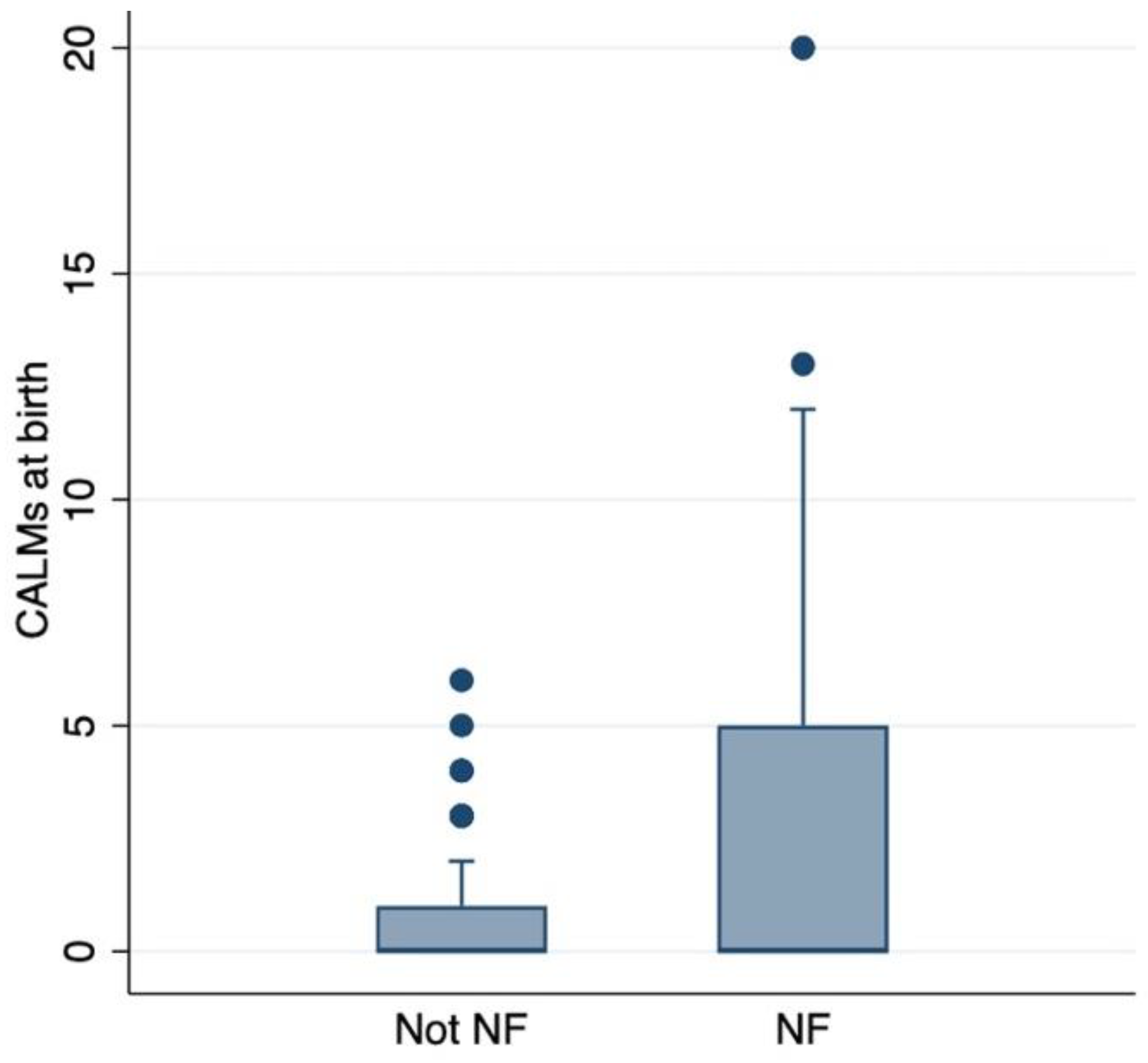
3.2. CALMs’ Correlation with NF1 Diagnosis and pNF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scala, M.; Schiavetti, I.; Madia, F.; Chelleri, C.; Piccolo, G.; Accogli, A.; Riva, A.; Salpietro, V.; Bocciardi, R.; Morcaldi, G.; et al. Genotype-Phenotype Correlations in Neurofibromatosis Type 1: A Single-Center Cohort Study. Cancers 2021, 13, 1879. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet. Med. 2021, 23, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Chamberlain, R.S. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist 2012, 17, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.K.; Perrin, R.; Guha, A. Peripheral nerve tumors: Management strategies and molecular insights. J. Neurooncol. 2004, 69, 335–349. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Friedman, J.M. NF1 gene and neurofibromatosis 1. Am. J. Epidemiol. 2000, 151, 33–40. [Google Scholar] [CrossRef]
- Tucker, T.; Wolkenstein, P.; Revuz, J.; Zeller, J.; Friedman, J.M. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005, 65, 205–211. [Google Scholar] [CrossRef]
- Carli, M.; Ferrari, A.; Mattke, A.; Zanetti, I.; Casanova, M.; Bisogno, G.; Cecchetto, G.; Alaggio, R.; De Sio, L.; Koscielniak, E.; et al. Pediatric malignant peripheral nerve sheath tumor: The Italian and German soft tissue sarcoma cooperative group. J. Clin. Oncol. 2005, 23, 8422–8430. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, X.; Liang, H.; Yang, R.; Yan, T.; Guo, W. Prognosis and risk factors for malignant peripheral nerve sheath tumor: A systematic review and meta-analysis. World J. Surg. Oncol. 2020, 18, 257. [Google Scholar] [CrossRef]
- Jansma, C.Y.M.N.; Acem, I.; Grünhagen, D.J.; Verhoef, C.; Martin, E.; MONACO Collaborators. Local recurrence in malignant peripheral nerve sheath tumours: Multicentre cohort study. BJS Open 2024, 8, zrae024. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, J.; Ge, S.; Jia, R.; Song, X.; Wang, Y.; Fan, X. An Overview of Optic Pathway Glioma With Neurofibromatosis Type 1: Pathogenesis, Risk Factors, and Therapeutic Strategies. Investig. Ophthalmol. Vis. Sci. 2024, 65, 8. [Google Scholar] [CrossRef]
- Bernier, A.; Larbrisseau, A.; Perreault, S. Café-au-lait Macules and Neurofibromatosis Type 1: A Review of the Literature. Pediatr. Neurol. 2016, 60, 24–29.e1. [Google Scholar] [CrossRef]
- Nunley, K.S.; Gao, F.; Albers, A.C.; Bayliss, S.J.; Gutmann, D.H. Predictive value of café au lait macules at initial consultation in the diagnosis of neurofibromatosis type 1. Arch. Dermatol. 2009, 145, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Mendez, M.D. Cafe Au Lait Macules. [Updated 2023 Jul 31]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Messiaen, L.; Yao, S.; Brems, H.; Callens, T.; Sathienkijkanchai, A.; Denayer, E.; Spencer, E.; Arn, P.; Babovic-Vuksanovic, D.; Bay, C.; et al. Clinical and mutational spectrum of neurofibromatosis type 1-like syndrome. JAMA 2009, 302, 2111–2118, [published correction appears in JAMA 2010, 303, 2477]. [Google Scholar] [CrossRef] [PubMed]
- Chelleri, C.; Brolatti, N.; De Marco, P.; Ognibene, M.; Diana, M.C.; Madia, F.; Duca, M.D.; Santangelo, A.; Capra, V.; Striano, P.; et al. Novel causative variants in Legius syndrome: SPRED1 Genotype spectrum expansion. Am. J. Med. Genet. A. 2024, 194, e63824. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Wolkenstein, P.; Valeyrie-Allanore, L.; Rodriguez, D.; Hadj-Rabia, S.; Ferkal, S.; Lacour, J.P.; Leonard, J.C.; Taillandier, L.; Sportich, S.; et al. NF-1Score: A prediction score for internal neurofibromas in neurofibromatosis-1. J. Investig. Dermatol. 2010, 130, 2173–2178. [Google Scholar] [CrossRef]
- Tong, H.X.; Li, M.; Zhang, Y.; Zhu, J.; Lu, W.Q. A novel NF1 mutation in a Chinese patient with giant café-au-lait macule in neurofibromatosis type 1 associated with a malignant peripheral nerve sheath tumor and bone abnormality. Genet. Mol. Res. 2012, 11, 2972–2978. [Google Scholar] [CrossRef]
- Boyd, K.P.; Gao, L.; Feng, R.; Beasley, M.; Messiaen, L.; Korf, B.R.; Theos, A. Phenotypic variability among café-au-lait macules in neurofibromatosis type 1. J. Am. Acad. Dermatol. 2010, 63, 440–447. [Google Scholar] [CrossRef][Green Version]
- Ben-Shachar, S.; Dubov, T.; Toledano-Alhadef, H.; Mashiah, J.; Sprecher, E.; Constantini, S.; Leshno, M.; Messiaen, L.M. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J. Am. Acad. Dermatol. 2017, 76, 1077–1083.e3. [Google Scholar] [CrossRef]
- Parrozzani, R.; Clementi, M.; Frizziero, L.; Miglionico, G.; Perrini, P.; Cavarzeran, F.; Kotsafti, O.; Comacchio, F.; Trevisson, E.; Convento, E.; et al. In Vivo Detection of Choroidal Abnormalities Related to NF1: Feasibility and Comparison With Standard NIH Diagnostic Criteria in Pediatric Patients. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6036–6042. [Google Scholar] [CrossRef]
- DeBella, K.; Szudek, J.; Friedman, J.M. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 2000, 105 (Pt 1), 608–614. [Google Scholar] [CrossRef]
- Marque, M.; Roubertie, A.; Jaussent, A.; Carneiro, M.; Meunier, L.; Guillot, B.; Pinson, L.; Pinson, S.; Bessis, D. Nevus anemicus in neurofibromatosis type 1: A potential new diagnostic criterion. J. Am. Acad. Dermatol. 2013, 69, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, M.H.; Moons, K.G.; Garssen, M.P.; Pasmans, N.M.; de Goede-Bolder, A.; Niermeijer, M.F.; Grobbee, D.E. Minor disease features in neurofibromatosis type 1 (NF1) and their possible value in diagnosis of NF1 in children < or = 6 years and clinically suspected of having NF1. Neurofibromatosis team of Sophia Children’s Hospital. J. Med. Genet. 1998, 35, 624–627. [Google Scholar] [PubMed]
- Nasi, L.; Alexopoulos, A.; Kokkinou, E.; Roka, K.; Tzetis, M.; Tsipi, M.; Kakourou, T.; Kanaka-Gantenbein, C.; Chrousos, G.; Kattamis, A.; et al. Characteristics of Café-au-lait Macules and their Association with the Neurofibromatosis type I Genotype in a Cohort of Greek Children. Acta Derm. Venereol. 2023, 103, adv5758. [Google Scholar] [CrossRef] [PubMed]
- Borgia, P.; Piccolo, G.; Santangelo, A.; Chelleri, C.; Viglizzo, G.; Occella, C.; Minetti, C.; Striano, P.; Diana, M.C. Dermatologic Effects of Selumetinib in Pediatric Patients with Neurofibromatosis Type 1: Clinical Challenges and Therapeutic Management. J. Clin. Med. 2024, 13, 1792. [Google Scholar] [CrossRef]
- Morello, A.; Scala, M.; Schiavetti, I.; Diana, M.C.; Severino, M.; Tortora, D.; Piatelli, G.; Pavanello, M. Surgical revascularization as a procedure to prevent neurological complications in children with moyamoya syndrome associated with neurofibromatosis I: A single institution case series. Childs Nerv. Syst. 2024, 40, 1731–1741. [Google Scholar] [CrossRef]
- Ognibene, M.; Scala, M.; Iacomino, M.; Schiavetti, I.; Madia, F.; Traverso, M.; Guerrisi, S.; Di Duca, M.; Caroli, F.; Baldassari, S.; et al. Moyamoya Vasculopathy in Neurofibromatosis Type 1 Pediatric Patients: The Role of Rare Variants of RNF213. Cancers 2023, 15, 1916. [Google Scholar] [CrossRef]
- Tortora, D.; Scavetta, C.; Rebella, G.; Bertamino, M.; Scala, M.; Giacomini, T.; Morana, G.; Pavanello, M.; Rossi, A.; Severino, M. Spatial coefficient of variation applied to arterial spin labeling MRI may contribute to predict surgical revascularization outcomes in pediatric moyamoya vasculopathy. Neuroradiology 2020, 62, 1003–1015. [Google Scholar] [CrossRef]
- Koczkowska, M.; Chen, Y.; Callens, T.; Gomes, A.; Sharp, A.; Johnson, S.; Hsiao, M.C.; Chen, Z.; Balasubramanian, M.; Barnett, C.P.; et al. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844-848. Am. J. Hum. Genet. 2018, 102, 69–87. [Google Scholar] [CrossRef]
- Kehrer-Sawatzki, H.; Mautner, V.F.; Cooper, D.N. Emerging genotype-phenotype relationships in patients with large NF1 deletions. Hum. Genet. 2017, 136, 349–376. [Google Scholar] [CrossRef]
| Number of CALMs | 0 | 1–2 | 3–4 | 5 or More |
|---|---|---|---|---|
| Non-NF1 patients | 32 (29%) | 19 (50%) | 8 (40%) | 2 (5%) |
| NF1 patients | 78 (71%) | 19 (50%) | 12 (60%) | 38 (95%) |
| Total | 110 | 38 | 20 | 40 |
| Comorbidities | CALMs at Birth | ||||
|---|---|---|---|---|---|
| 0 (78 Patients) | 1–2 (19 Patients) | 3–4 (12 Patients) | 5 or More (38 Patients) | p-Value | |
| OPG | 21 (27%) | 4 (21%) | 5 (42%) | 13 (34%) | 0.320 |
| Cutaneous/subcutaneous neurofibromas | 31 (40%) | 9 (47%) | 7 (58%) | 21 (55%) | 0.084 |
| pNFs | 25 (32%) | 6 (32%) | 6 (50%) | 24 (63%) | 0.001 |
| Other neoplasms | 22 (28%) | 3 (16%) | 4 (33%) | 14 (37%) | 0.320 |
| ADHD | 9 (12%) | 1 (5%) | 0 | 3 (8%) | 0.366 |
| ASD | 10 (13%) | 0 | 1 (8%) | 3 (8%) | 0.374 |
| Endocrinological manifestations | 30 (38%) | 9 (47%) | 6 (50%) | 20 (53%) | 0.134 |
| Allergic manifestations | 27 (35%) | 10 (53%) | 6 (50%) | 10 (26%) | 0.536 |
| CV manifestations | 25 (32%) | 5 (26%) | 5 (42%) | 15 (39%) | 0.372 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santangelo, A.; Chelleri, C.; Tomasino, M.; Pasquinucci, M.; Cappozzo, F.; Striano, P.; Diana, M.C.; Scala, M. Café-Au-Lait Macules in Neurofibromatosis Type 1: Birthmark or Biomarker? Cancers 2025, 17, 1490. https://doi.org/10.3390/cancers17091490
Santangelo A, Chelleri C, Tomasino M, Pasquinucci M, Cappozzo F, Striano P, Diana MC, Scala M. Café-Au-Lait Macules in Neurofibromatosis Type 1: Birthmark or Biomarker? Cancers. 2025; 17(9):1490. https://doi.org/10.3390/cancers17091490
Chicago/Turabian StyleSantangelo, Andrea, Cristina Chelleri, Marco Tomasino, Mattia Pasquinucci, Francesca Cappozzo, Pasquale Striano, Maria Cristina Diana, and Marcello Scala. 2025. "Café-Au-Lait Macules in Neurofibromatosis Type 1: Birthmark or Biomarker?" Cancers 17, no. 9: 1490. https://doi.org/10.3390/cancers17091490
APA StyleSantangelo, A., Chelleri, C., Tomasino, M., Pasquinucci, M., Cappozzo, F., Striano, P., Diana, M. C., & Scala, M. (2025). Café-Au-Lait Macules in Neurofibromatosis Type 1: Birthmark or Biomarker? Cancers, 17(9), 1490. https://doi.org/10.3390/cancers17091490









