Advances in the Management, Treatment, and Surveillance of Anal Squamous Cell Cancer
Simple Summary
Abstract
1. Introduction
1.1. Anal Cancer
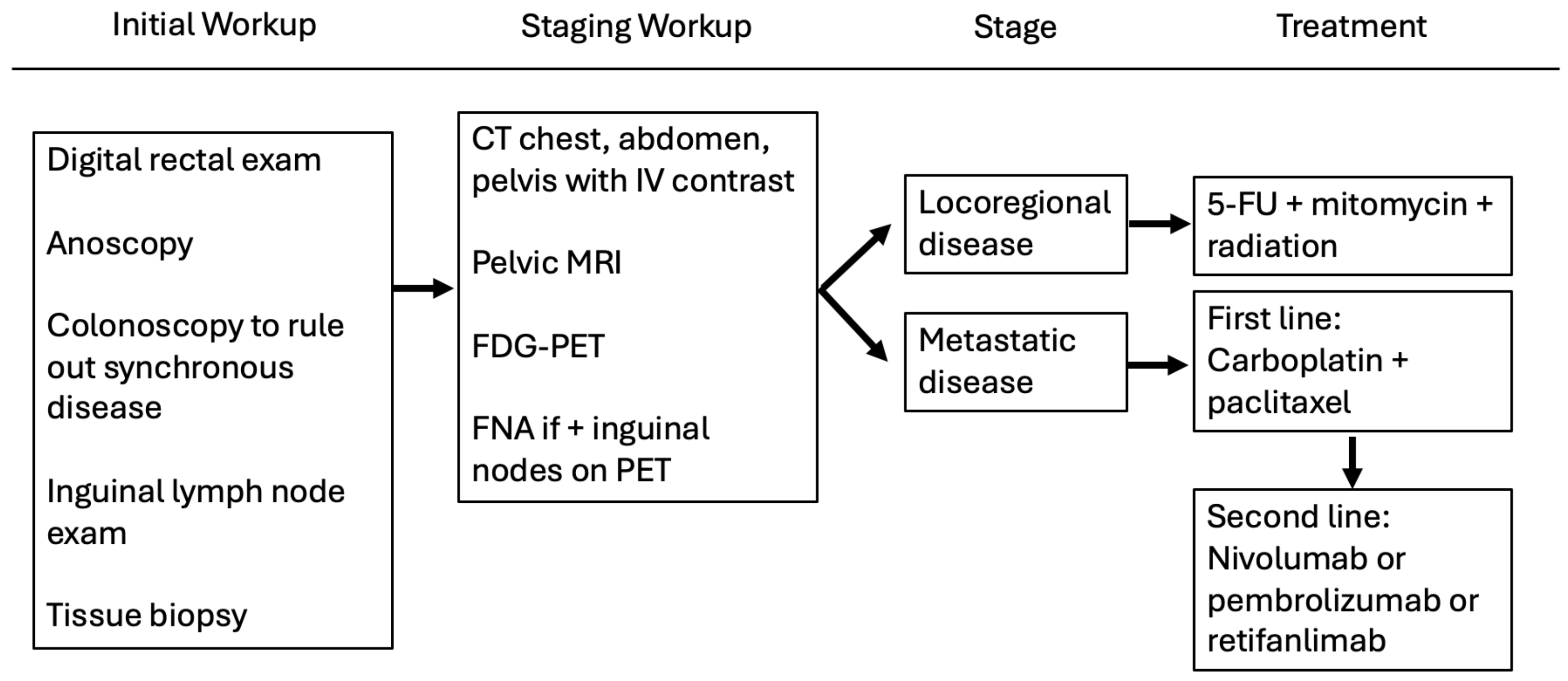
1.2. Diagnosing and Staging Anal Cancer
1.3. Nigro Protocol and Current NCCN Guidelines
1.4. Precursors to Anal Cancer
2. Clinical Trials: Screening and Treatment of HSIL
2.1. ANCHOR Study
2.2. Clinical Trials for Treatment of HSIL
3. Clinical Trials for Treatment of Anal Cancer
3.1. Locoregional Disease
3.1.1. ACT I
3.1.2. RTOG 98-11
3.1.3. ACCORD 3
3.1.4. ACT II
3.1.5. RTOG 0529
3.1.6. Summary of Clinical Trials for Locoregional Disease
3.2. Metastatic Disease
InterAAct
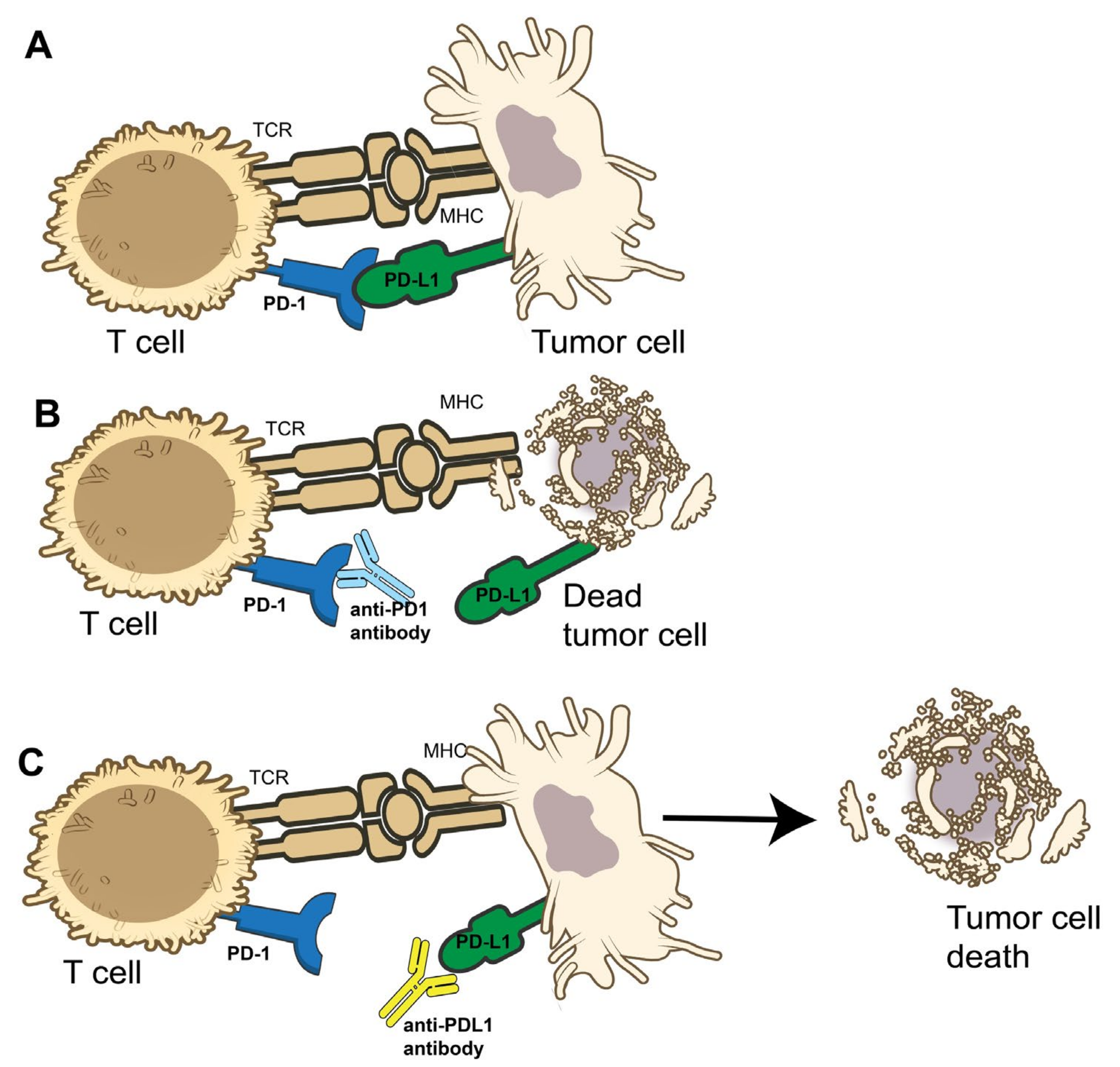
4. Clinical Trials for Immunotherapy
4.1. NCI9673
4.2. KEYNOTE 28
4.3. KEYNOTE 158
4.4. POD1UM-202
4.5. CARACAS
4.6. Other Immunotherapy
5. Surveillance
5.1. Surveillance Guidelines
5.2. Biomarkers
5.2.1. Circulating Tumor DNA
5.2.2. Tumor-Infiltrating Lymphocytes
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPV | Human papilloma Virus |
| HIV | Human immunodeficiency virus |
| SIL | Squamous intra-epithelial lesion |
| AIN | Anal intra-epithelial neoplasia |
| HSIL | High-grade intraepithelial lesion |
| LSIL | Low-grade intraepithelial lesions |
| SCCA | Squamous cell carcinoma of anus |
| SISCCA | Superficially invasive squamous cell carcinoma |
| 5-FU | 5-Fluorouracil |
| APR | Abdominoperineal resection |
| NCCN | National Comprehensive Cancer Network |
| ANCHOR | Anal-Cancer HSIL Outcomes Research |
| HRA | High Resolution Anoscopy |
| ACT | Anal Cancer Trial |
| OS | Overall survival |
| PFS | Progression-free survival |
| CCR | Complete clinical response |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed dealth-ligand 1 |
| ACT | Anal Cancer Trial |
| ctDNA | Circulating tumor DNA |
| TIL | Tumor-infiltrating lymphocytes |
References
- Institute, N.C. Cancer Stat Facts: Anal Cancer. Available online: https://seer.cancer.gov/statfacts/html/anus.html (accessed on 5 January 2025).
- Quan, S.H.Q. Anal cancers: Squamous and melanoma. Cancer 1992, 70, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.B.; Gaertner, W.B.; Glasgow, S.C.; Herzig, D.O.; Feingold, D.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (Revised 2018). Dis. Colon Rectum 2018, 61, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Gondal, T.A.; Chaudhary, N.; Bajwa, H.; Rauf, A.; Le, D.; Ahmed, S. Anal Cancer: The Past, Present and Future. Curr. Oncol. 2023, 30, 3232–3250. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, L.M.; Faski, J.; Nelson, H.; Gollub, M.J.; Eng, C.; Brierley, J.D.; Palefsky, J.M.; Goldberg, R.M.; Washington, M.K.; Asare, E.A.; et al. Survival outcomes used to generate version 9 American Joint Committee on Cancer staging system for anal cancer. CA A Cancer J. Clin. 2023, 73, 516–523. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and Consensus Recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch. Pathol. Lab. Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef]
- Nigro, N.D.; Vaitkevicius, V.K.; Considine, B., Jr. Combined therapy for cancer of the anal canal: A preliminary report. Dis. Colon Rectum 1974, 17, 354–356. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Anal Carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 653–677. [Google Scholar] [CrossRef]
- Arana, R.; Fléjou, J.; Si-Mohamed, A.; Bauer, P.; Etienney, I. Clinicopathological and virological characteristics of superficially invasive squamous-cell carcinoma of the anus. Color. Dis. 2015, 17, 965–972. [Google Scholar] [CrossRef]
- Ko, G.; Sarkaria, A.; Merchant, S.J.; Booth, C.M.; Patel, S.V. A systematic review of outcomes after salvage abdominoperineal resection for persistent or recurrent anal squamous cell cancer. Color. Dis. 2019, 21, 632–650. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Nilsson, P.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Arnold, D. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 1165–1176. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Lee, J.Y.; Jay, N.; Goldstone, S.E.; Darragh, T.M.; Dunlevy, H.A.; Rosa-Cunha, I.; Arons, A.; Pugliese, J.C.; Vena, D.; et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N Engl. J. Med. 2022, 386, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Richel, O.; de Vries, H.J.; van Noesel, C.J.; Dijkgraaf, M.G.; Prins, J.M. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: An open-label, randomised controlled trial. Lancet Oncol. 2013, 14, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, S.E.; Lensing, S.Y.; Stier, E.A.; Darragh, T.; Lee, J.Y.; van Zante, A.; Jay, N.; Berry-Lawhorn, J.M.; Cranston, R.D.; Mitsuyasu, R.; et al. A Randomized Clinical Trial of Infrared Coagulation Ablation Versus Active Monitoring of Intra-anal High-grade Dysplasia in Adults With Human Immunodeficiency Virus Infection: An AIDS Malignancy Consortium Trial. Clin. Infect. Dis. 2018, 68, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996, 348, 1049–1054. [Google Scholar] [CrossRef]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson , A.B., 3rd; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. J. Am. Med. Assoc. 2008, 299, 1914–1921. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B.; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-Term Update of US GI Intergroup RTOG 98-11 Phase III Trial for Anal Carcinoma: Survival, Relapse, and Colostomy Failure With Concurrent Chemoradiation Involving Fluorouracil/Mitomycin Versus Fluorouracil/Cisplatin. J. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Peiffert, D.; Tournier-Rangeard, L.; Gérard, J.-P.; Lemanski, C.; François, E.; Giovannini, M.; Cvitkovic, F.; Mirabel, X.; Bouché, O.; Luporsi, E.; et al. Induction Chemotherapy and Dose Intensification of the Radiation Boost in Locally Advanced Anal Canal Carcinoma: Final Analysis of the Randomized UNICANCER ACCORD 03 Trial. J. Clin. Oncol. 2012, 30, 1941–1948. [Google Scholar] [CrossRef]
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Myint, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Willins, J.; Esthappan, J.; Haddock, M.G.; Rotman, M.; Parikh, P.J.; Safran, H.; et al. RTOG 0529: A Phase 2 Evaluation of Dose-Painted Intensity Modulated Radiation Therapy in Combination With 5-Fluorouracil and Mitomycin-C for the Reduction of Acute Morbidity in Carcinoma of the Anal Canal. Int. J. Radiat. Oncol. 2013, 86, 27–33. [Google Scholar] [CrossRef]
- Cummings, B.J. Metastatic Anal Cancer: The Search for Cure. Oncol. Res. Treat. 2006, 29, 5–6. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Zhao, N.; Wang, J.; Lin, C.; Izaguirre, E.W.; Farmer, M.; Tian, G.; Somer, B.; Dubal, N.; et al. Definitive Pelvic Radiotherapy and Survival of Patients With Newly Diagnosed Metastatic Anal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sclafani, F.; Eng, C.; Adams, R.A.; Guren, M.G.; Sebag-Montefiore, D.; Benson, A.; Bryant, A.; Peckitt, C.; Segelov, E.; et al. International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. J. Clin. Oncol. 2020, 38, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Salem, M.E.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- T Cell. 10 July 2024. Available online: https://bioart.niaid.nih.gov/bioart/508 (accessed on 3 March 2025).
- Marabelle, A.; Cassier, P.A.; Fakih, M.; Kao, S.; Nielsen, D.; Italiano, A.; Guren, T.K.; van Dongen, M.G.J.; Spencer, K.; Bariani, G.M.; et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: Results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol. Hepatol. 2022, 7, 446–454. [Google Scholar] [CrossRef]
- Rao, S.; Anandappa, G.; Capdevila, J.; Dahan, L.; Evesque, L.; Kim, S.; Saunders, M.; Gilbert, D.; Jensen, L.; Samalin, E.; et al. A phase II study of retifanlimab (INCMGA00012) in patients with squamous carcinoma of the anal canal who have progressed following platinum-based chemotherapy (POD1UM-202). ESMO Open 2022, 7, 100529. [Google Scholar] [CrossRef]
- Rao, S.; Samalin-Scalzi, E.; Evesque, L.; Abdelghani, M.B.; Morano, F.; Roy, A.C.; Dahan, L.; Tamberi, S.; Dhadda, A.S.; Saunders, M.P.; et al. LBA2 POD1UM-303/InterAACT 2: Phase III study of retifanlimab with carboplatin-paclitaxel (c-p) in patients (Pts) with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC) not previously treated with systemic chemotherapy (Chemo). Ann. Oncol. 2024, 35, S1217. [Google Scholar]
- Lonardi, S.; Prete, A.A.; Morano, F.; Messina, M.; Formica, V.; Corsi, D.C.; Orciuolo, C.; Frassineti, G.L.; Zampino, M.G.; Casagrande, M.; et al. Randomized phase II trial of avelumab alone or in combination with cetuximab for patients with previously treated, locally advanced, or metastatic squamous cell anal carcinoma: The CARACAS study. J. Immunother. Cancer 2021, 9, e002996. [Google Scholar] [CrossRef]
- Dhawan, N.; Afzal, M.Z.; Amin, M. Immunotherapy in Anal Cancer. Curr. Oncol. 2023, 30, 4538–4550. [Google Scholar] [CrossRef]
- Morris, V.; Ciombor, K.; Polite, B.; Mukherjee, S.; Krauss, J.; Shields, A.; Aranha, O.; Hays, J.; Kazmi, S.; Weinberg, B.; et al. O-12 NCI9673 (Part B): A multi-institutional ETCTN randomized phase II study of nivolumab with or without ipilimumab in refractory, metastatic squamous cell carcinoma of the anal canal. Ann. Oncol. 2023, 34, S185–S186. [Google Scholar] [CrossRef]
- Eng, C.; Fakih, M.; Amin, M.; Morris, V.; Hochster, H.S.; Boland, P.M.; Uronis, H. A phase II study of axalimogene filolisbac for patients with previously treated, unresectable, persistent/recurrent loco-regional or metastatic anal cancer. Oncotarget 2020, 11, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Sebag-Montefiore, D.; Meadows, H.M.; Cunningham, D.; Begum, R.; Adab, F.; Benstead, K.; Harte, R.J.; Stewart, J.; Beare, S.; et al. Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): A post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Xiao, W.; Lin, K.; Wong, C.W.; Wotman, M.T.; Holliday, E.B.; Huey, R.W.; Noticewala, S.S.; Ludmir, E.B.; Bent, A.H.; et al. Time dependency for human papillomavirus circulating tumor DNA detection after chemoradiation as a prognostic biomarker for localized anal cancer. Clin. Cancer Res. 2025, OF1–OF7. [Google Scholar] [CrossRef]
- Bernard-Tessier, A.; Jeannot, E.; Guenat, D.; Debernardi, A.; Michel, M.; Proudhon, C.; Vincent-Salomon, A.; Bièche, I.; Pierga, J.-Y.; Buecher, B.; et al. Clinical Validity of HPV Circulating Tumor DNA in Advanced Anal Carcinoma: An Ancillary Study to the Epitopes-HPV02 Trial. Clin. Cancer Res. 2019, 25, 2109–2115. [Google Scholar] [CrossRef]
- Gilbert, D.C.; Serup-Hansen, E.; Linnemann, D.; Høgdall, E.; Bailey, C.; Summers, J.; Havsteen, H.; Thomas, G.J. Tumour-infiltrating lymphocyte scores effectively stratify outcomes over and above p16 post chemo-radiotherapy in anal cancer. Br. J. Cancer 2016, 114, 134–137. [Google Scholar] [CrossRef]
- Lower-Dose Chemoradiation in Treating Patients With Early-Stage Anal Cancer, the DECREASE Study. 7 August 2024. Available online: https://clinicaltrials.gov/study/NCT04166318#study-overview (accessed on 16 February 2025).
- Sebag-Montefiore, D.; Adams, R.; Bell, S.; Berkman, L.; Gilbert, D.; Glynne-Jones, R.; Goh, V.; Gregory, W.; Harrison, M.; Kachnic, L.; et al. The Development of an Umbrella Trial (PLATO) to Address Radiation Therapy Dose Questions in the Locoregional Management of Squamous Cell Carcinoma of the Anus. Int. J. Radiat. Oncol. 2016, 96, E164–E165. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. HPV Vaccination. 20 August 2024. Available online: https://www.cdc.gov/hpv/vaccines/index.html (accessed on 16 February 2025).
- Palefsky, J.M.; Giuliano, A.R.; Goldstone, S.; Moreira, E.D.; Aranda, C.; Jessen, H.; Hillman, R.; Ferris, D.; Coutlee, F.; Stoler, M.H.; et al. HPV Vaccine against Anal HPV Infection and Anal Intraepithelial Neoplasia. N. Engl. J. Med. 2011, 365, 1576–1585. [Google Scholar] [CrossRef]
| Stage | T | N | M |
|---|---|---|---|
| I | T1 | N0 | M0 |
| IIA | T2 | N0 | M0 |
| IIB | T1-T2 | N1 | M0 |
| IIIA | T3 | N0–N1 | M0 |
| IIIB | T4 | N0 | M0 |
| IIIC | T4 | N1 | M0 |
| IV | Any T | Any N | M1 |
| Clinical Trial Name | Stage of Anal Cancer | Treatment Regimens | Results |
|---|---|---|---|
| ACCORD 03 | Non-metastatic anal cancer | Induction chemotherapy with concurrent chemoradiation and standard radiation boost (Arm A) OR induction chemotherapy with concurrent chemoradiation and high dose boost (Arm B) OR concurrent chemoradiation with standard boost (Arm C) OR concurrent chemoradiation with high dose boost (Arm D) (All chemotherapy regimens: 5-FU and cisplatin) | Colostomy free survival: Arm A: 69.6% Arm B: 82.4% Arm C: 77.1% Arm D: 72.7% |
| ACT I | Stage II-III, non, metastatic | Radiotherapy alone OR concurrent chemoradiation with 5-FU and mitomycin | Local failure rate in the concurrent chemoradiation group of 39% compared to the radiation alone group of 61% at 3 years |
| ACT II | I-III, non-metastatic disease | Radiation in combination with Cisplatin +5-FU OR mitomycin + 5-FU with or without maintenance chemotherapy | Complete response post treatment with mitomycin+ 5-FU: 90.5% vs. cisplatin+ 5-FU: 89.6% |
| CARACAS | IV or non-metastatic and failed a previous line of therapy | Avelumab monotherapy OR cetuximab + avelumab | Overall response rate: avelumab only: 10% vs. cetuximab + avelumab: 17% |
| INTERAACT | IV | Cisplatin +5-FU OR carboplatin + paclitaxel | Objective response rate: cisplatin + 5-FU 57% and carboplatin + paclitaxel 59% |
| KEYNOTE-28 | Locally advanced or metastatic, PD-L1 positive tumors | Pembrolizumab | Overall response rate: 17% Disease control rate: 58% |
| KEYNOTE-158 | Locally advanced or metastatic SCCA who had a previous failure or intolerance to standard therapy | Pembrolizumab | Objective response rate of 11% |
| POD1UM-202 | Advanced or metastatic, previously treated | Retifanlimab | Overall response rate in 13.8% and stable disease in 35.1% |
| RTOG 9811 | Stage II-III (T2-4, N0-3, M0) | Radiation in combination with Cisplatin + 5-FU OR mitomycin + 5-FU | 5-year disease free survival: mitomycin group 60% and cisplatin group 54% |
| Nigro Protocol Algorithm | |||||||
|---|---|---|---|---|---|---|---|
| Next steps | Physical exam, DRE at 8–12 weeks | ||||||
| Outcomes | CCR | Persistent disease | Progressive disease | ||||
| Next steps | Surveillance | Exam and DRE in 4 weeks | Biopsy and restage | ||||
| Outcomes | CCR * | Regression or no progression | Progressive Disease † | Local recurrence or inguinal disease | Distant Metastases | ||
| Next Steps | Exam Q3 months | APR or inguinal lymph node dissection Consider immunotherapy instead of APR. | Systemic therapy | ||||
| Outcomes | CCR * | Persistent or Progressive Disease † | |||||
| Surveillance Protocol After APR, local excision for T1 disease with adequate margins, Nigro protocol with CCR | |||||||
| Parameter | Frequency | ||||||
| DRE, inguinal lymph node exam | Every 3–6 months for 5 years | ||||||
| Anoscopy | Every 6–12 months for 3 years | ||||||
| CT C/A/P ‡ with contrast | Annually for 3 years | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araradian, C.; Walsh, M.; Standage, H.; Tsikitis, V.L. Advances in the Management, Treatment, and Surveillance of Anal Squamous Cell Cancer. Cancers 2025, 17, 1289. https://doi.org/10.3390/cancers17081289
Araradian C, Walsh M, Standage H, Tsikitis VL. Advances in the Management, Treatment, and Surveillance of Anal Squamous Cell Cancer. Cancers. 2025; 17(8):1289. https://doi.org/10.3390/cancers17081289
Chicago/Turabian StyleAraradian, Cynthia, Maura Walsh, Hayley Standage, and Vassiliki Liana Tsikitis. 2025. "Advances in the Management, Treatment, and Surveillance of Anal Squamous Cell Cancer" Cancers 17, no. 8: 1289. https://doi.org/10.3390/cancers17081289
APA StyleAraradian, C., Walsh, M., Standage, H., & Tsikitis, V. L. (2025). Advances in the Management, Treatment, and Surveillance of Anal Squamous Cell Cancer. Cancers, 17(8), 1289. https://doi.org/10.3390/cancers17081289




