A Novel Serum Inflammation Risk-Index (SIRI-RT)-Driven Nomogram for Predicting Secondary Malignancy Outcomes Post-Radiotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Data Acquisition
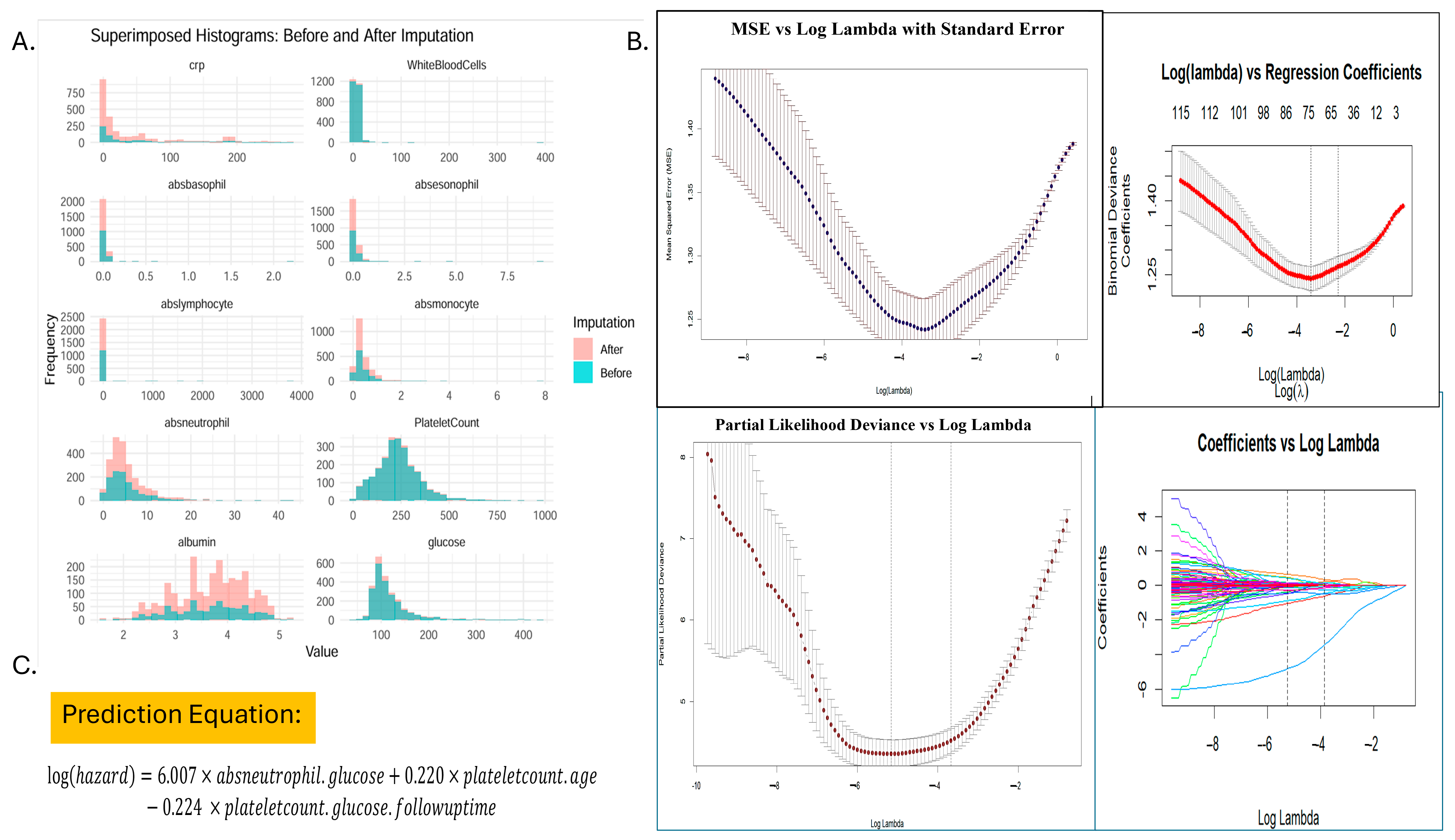
2.3. Variable Selection and Data Preprocessing
2.4. Ratio Calculation and Feature Engineering
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
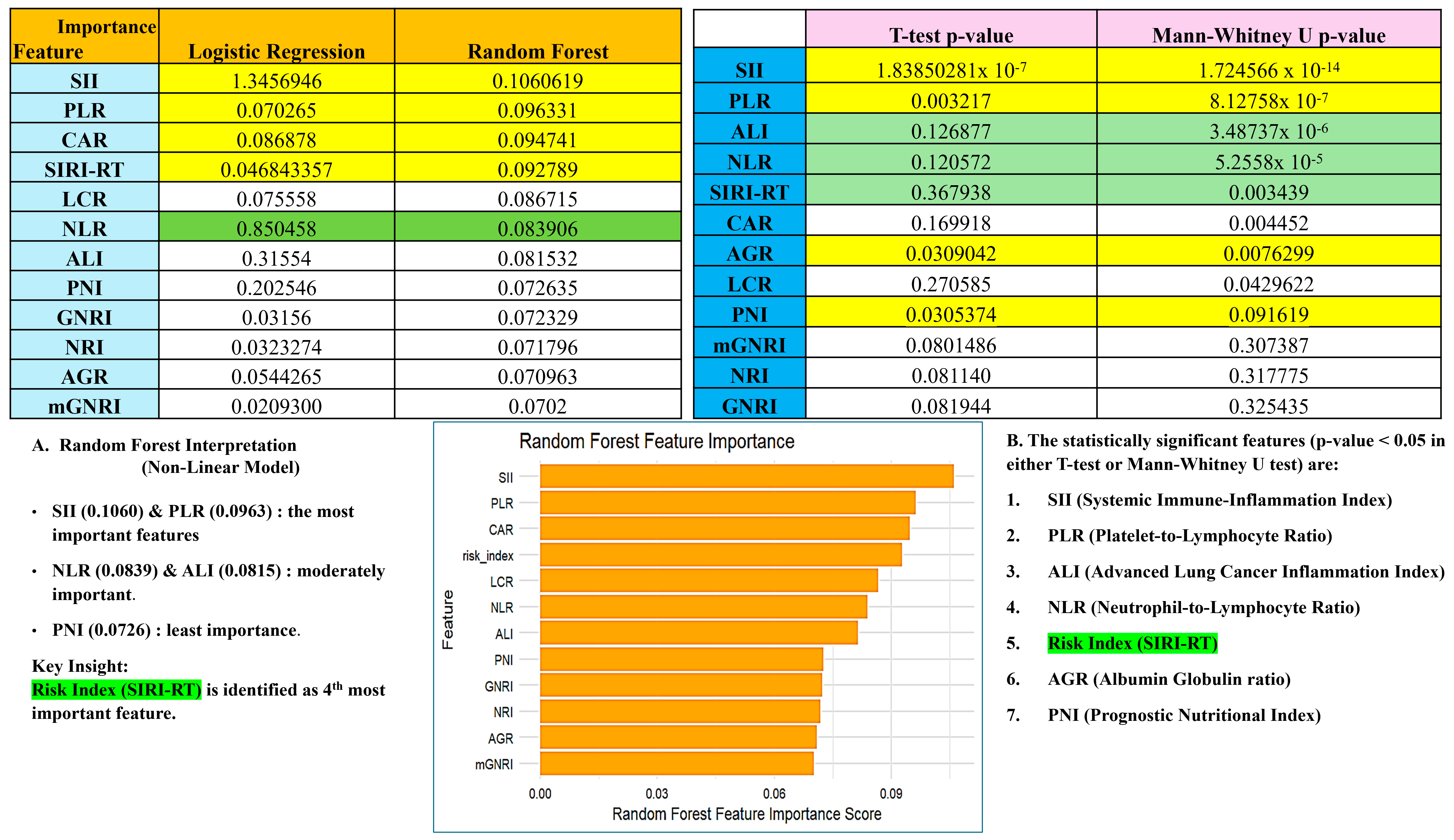
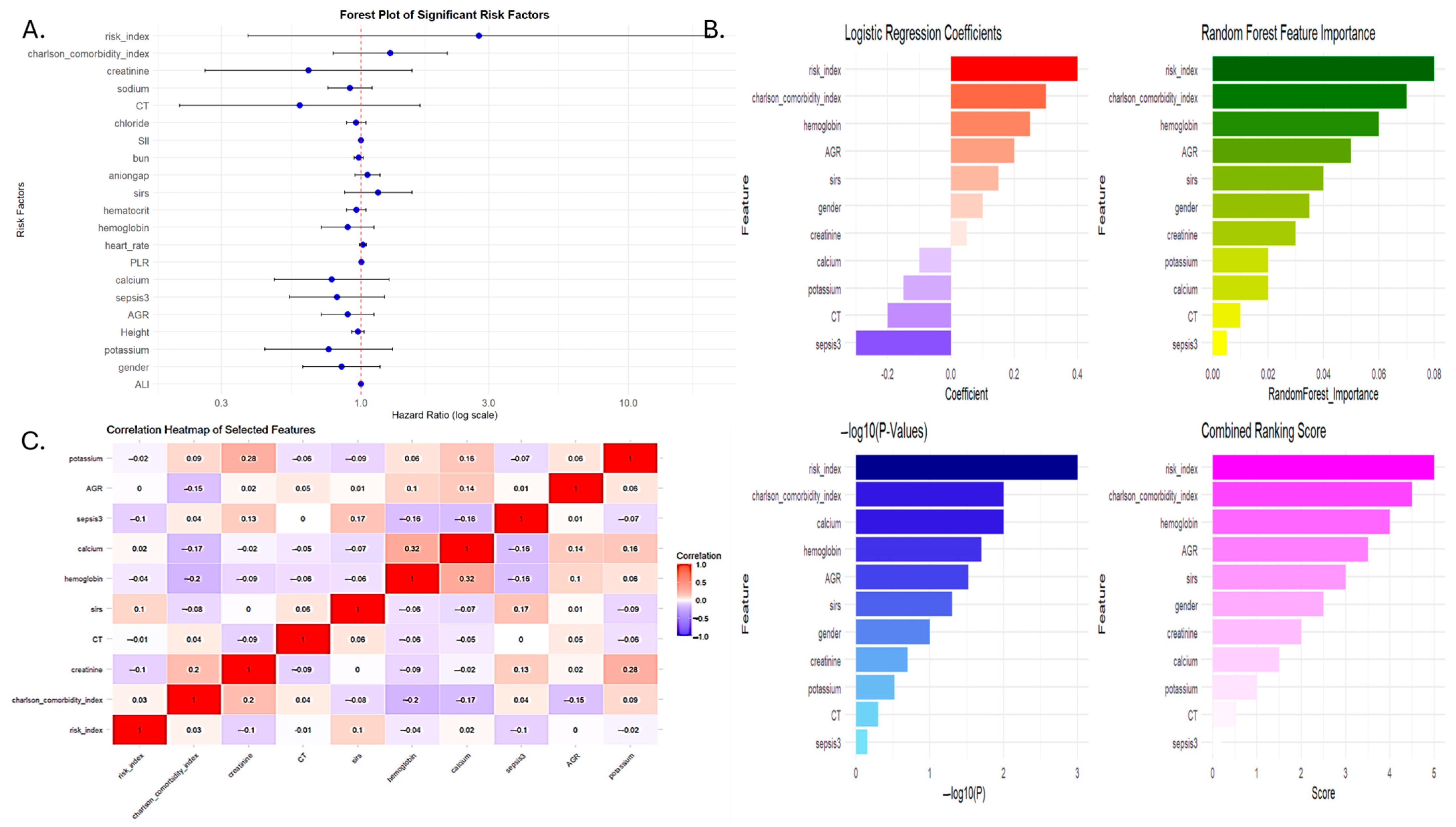
3.2. Development of the Serum Inflammation-Based Risk Index (SIRI-RT) as Prognostic Score
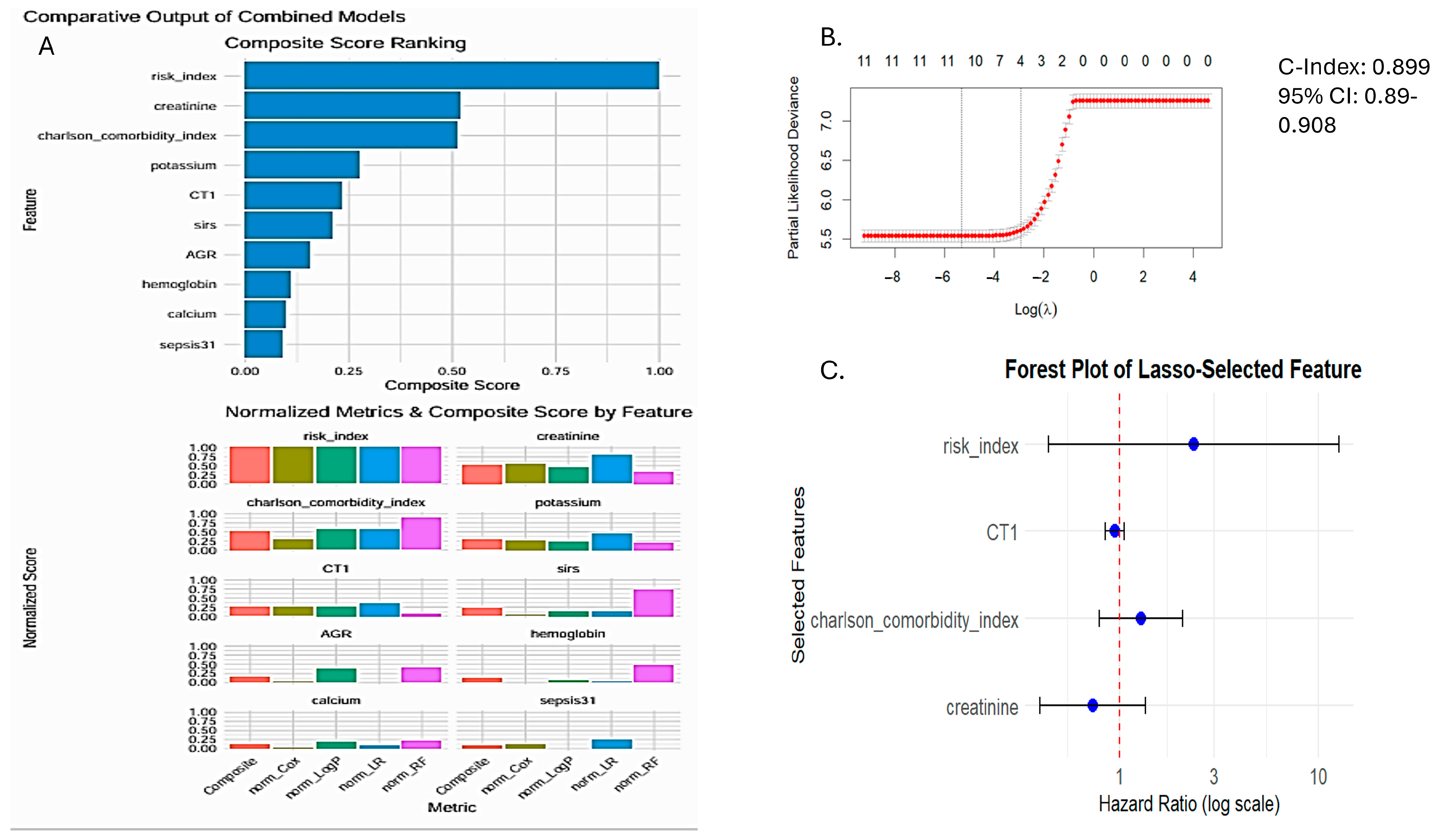
3.3. Prognostic Performance of SIRI-RT—SIRI for Radiotherapy-Induced Secondary Tumors
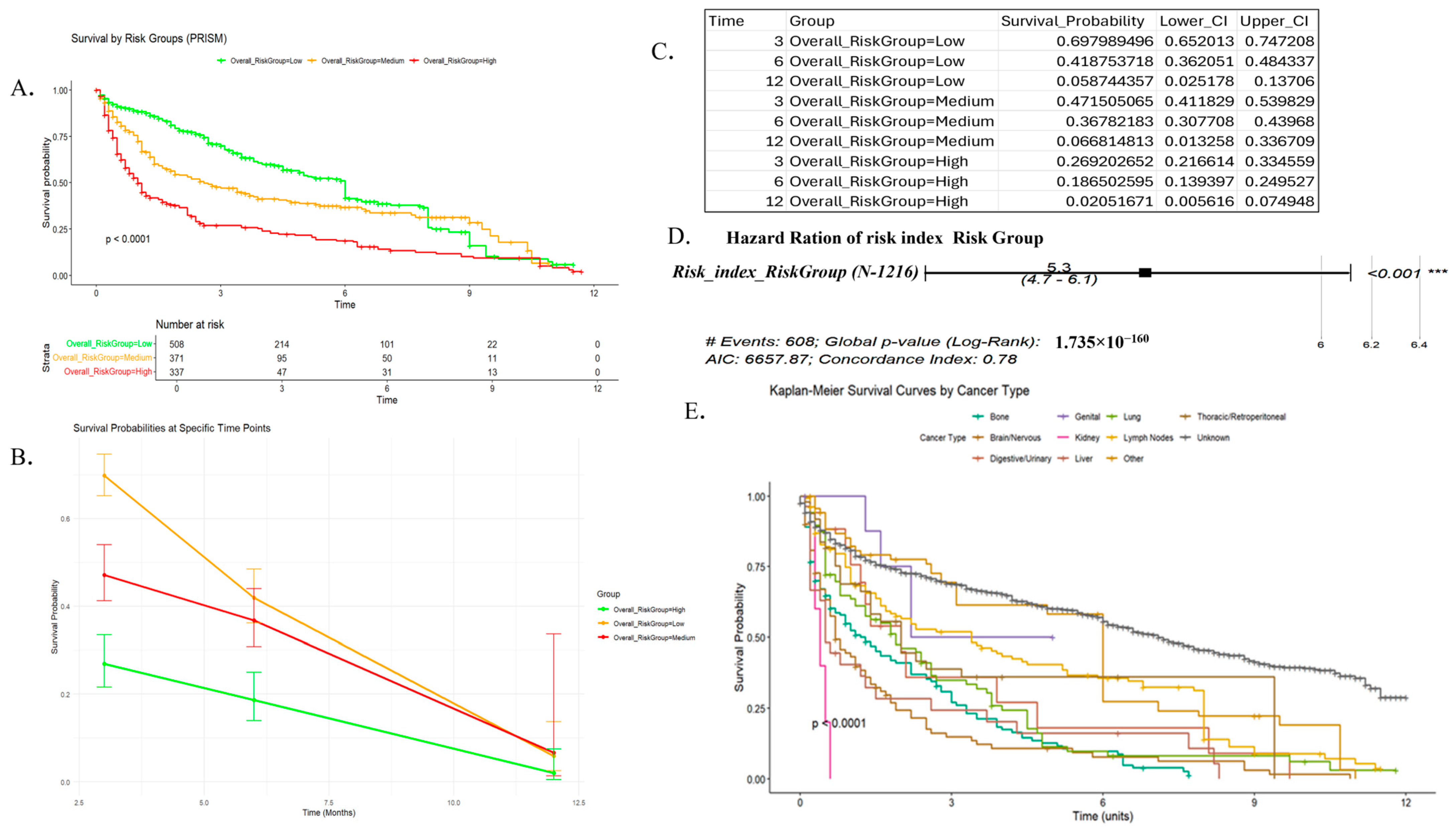
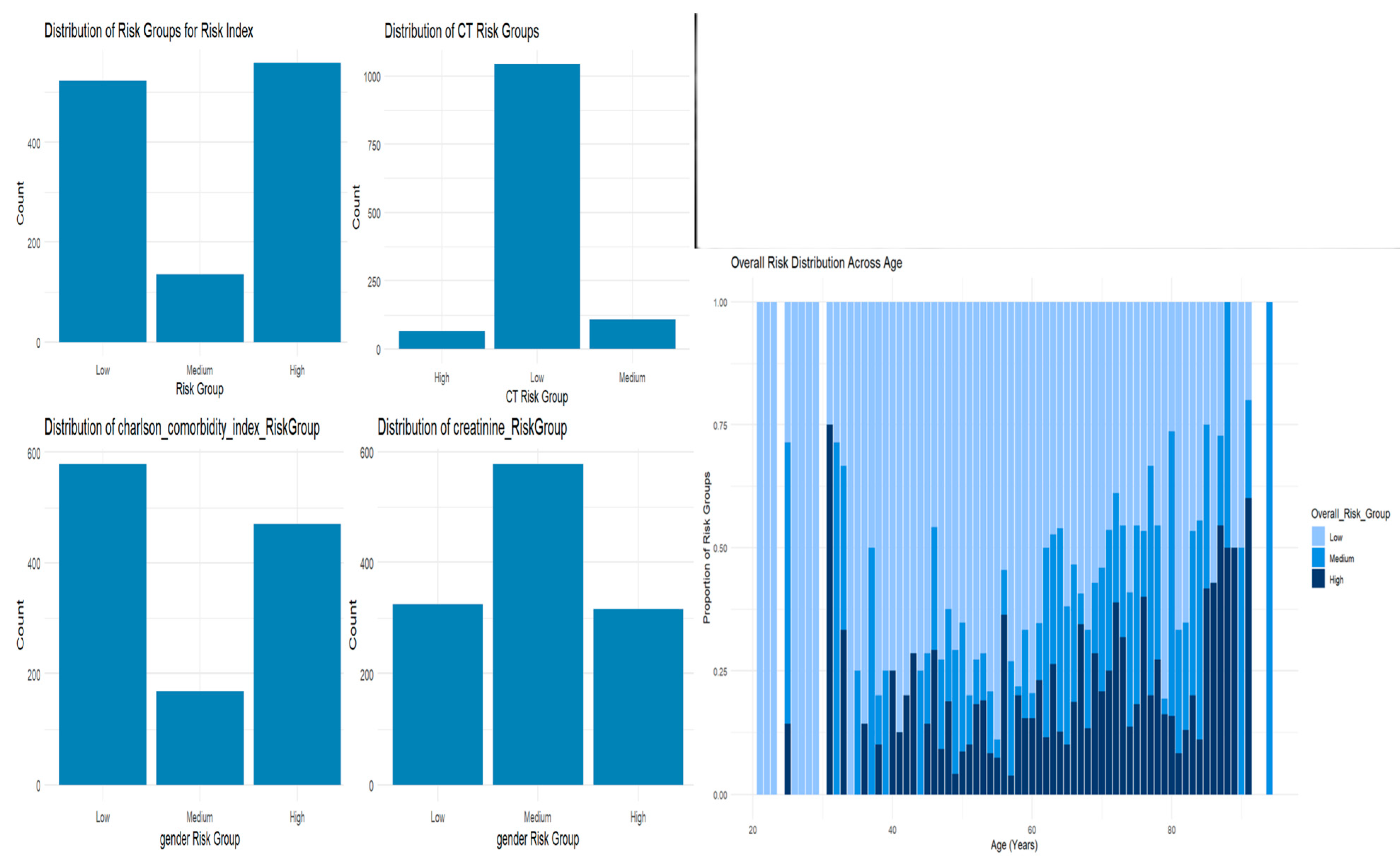
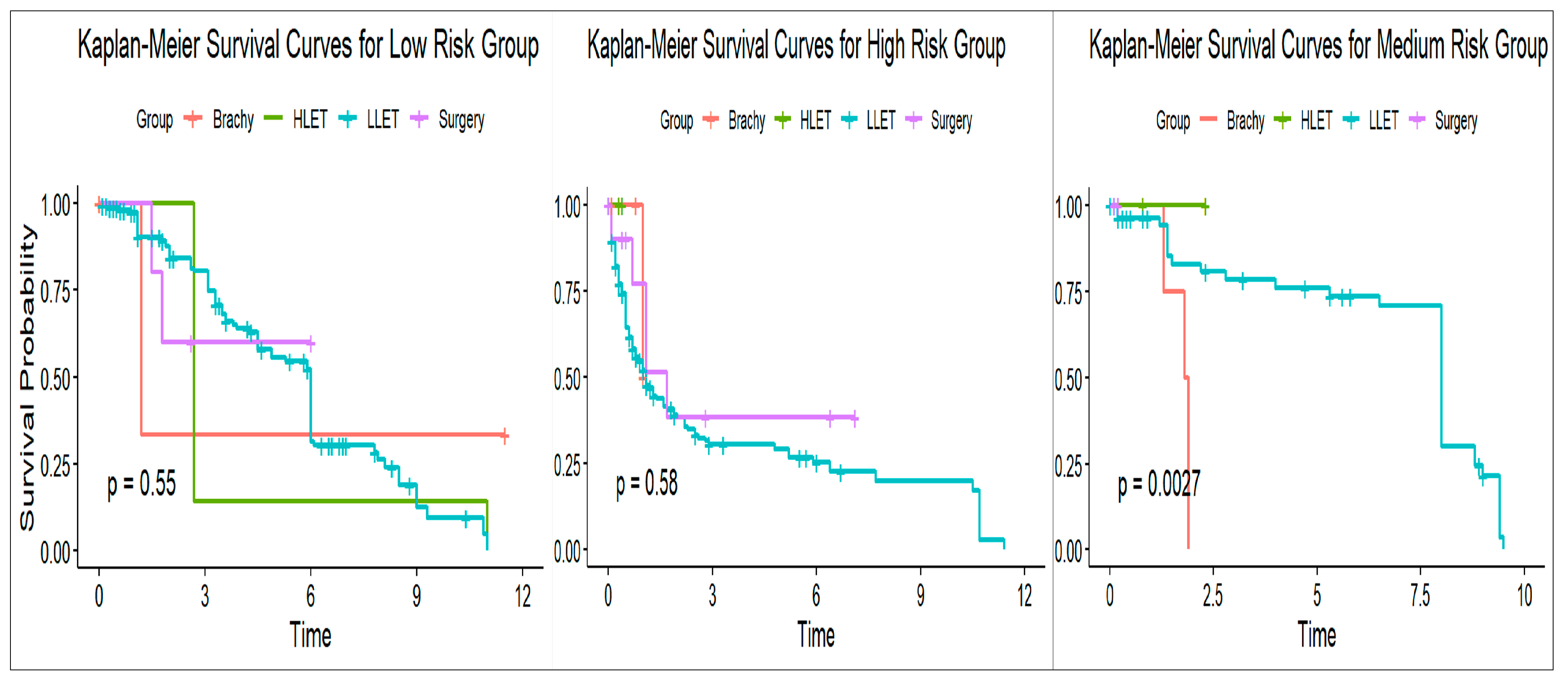
3.4. Risk Stratification
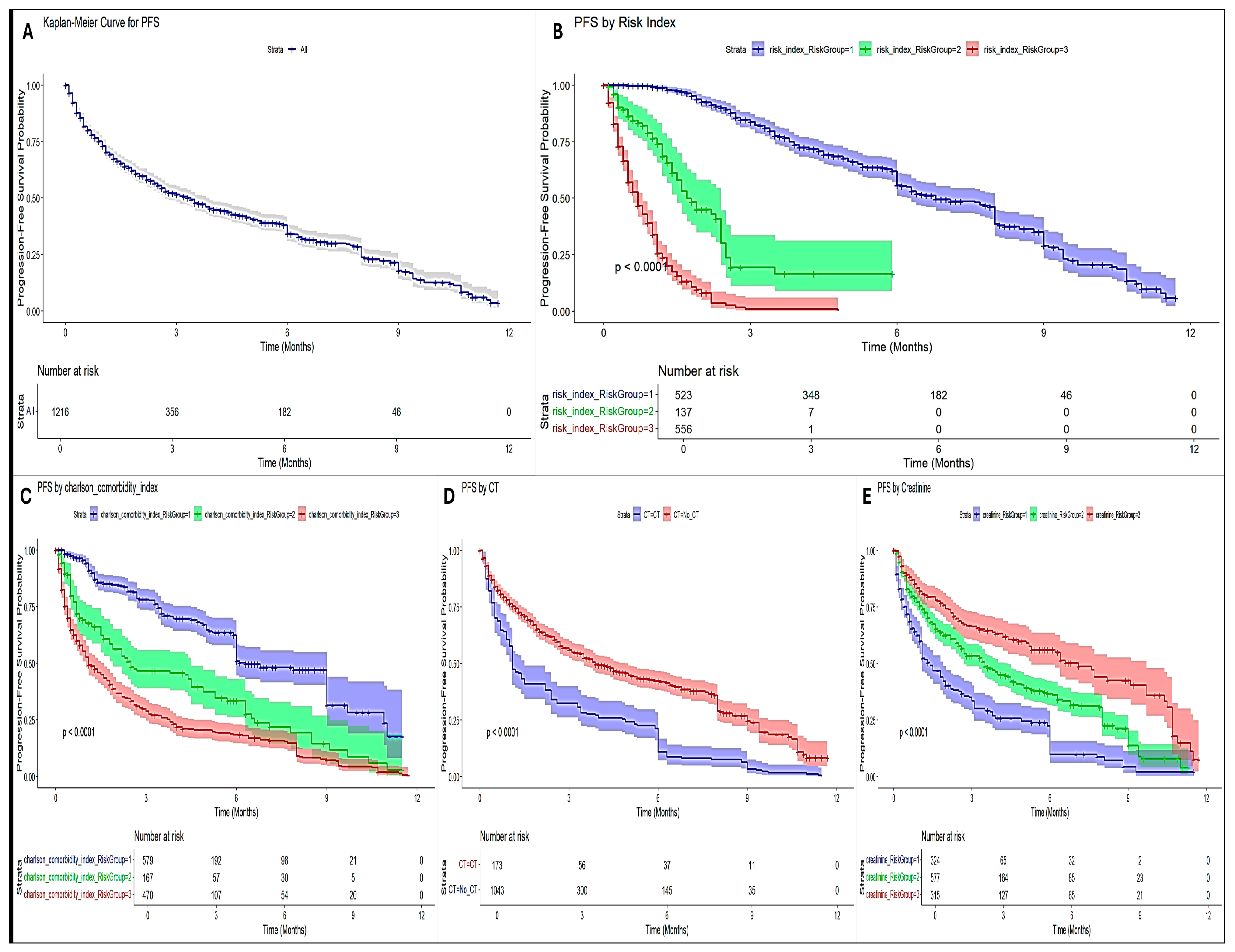
3.5. SURVIVAL Analysis and Progression-Free Survival (PFS)
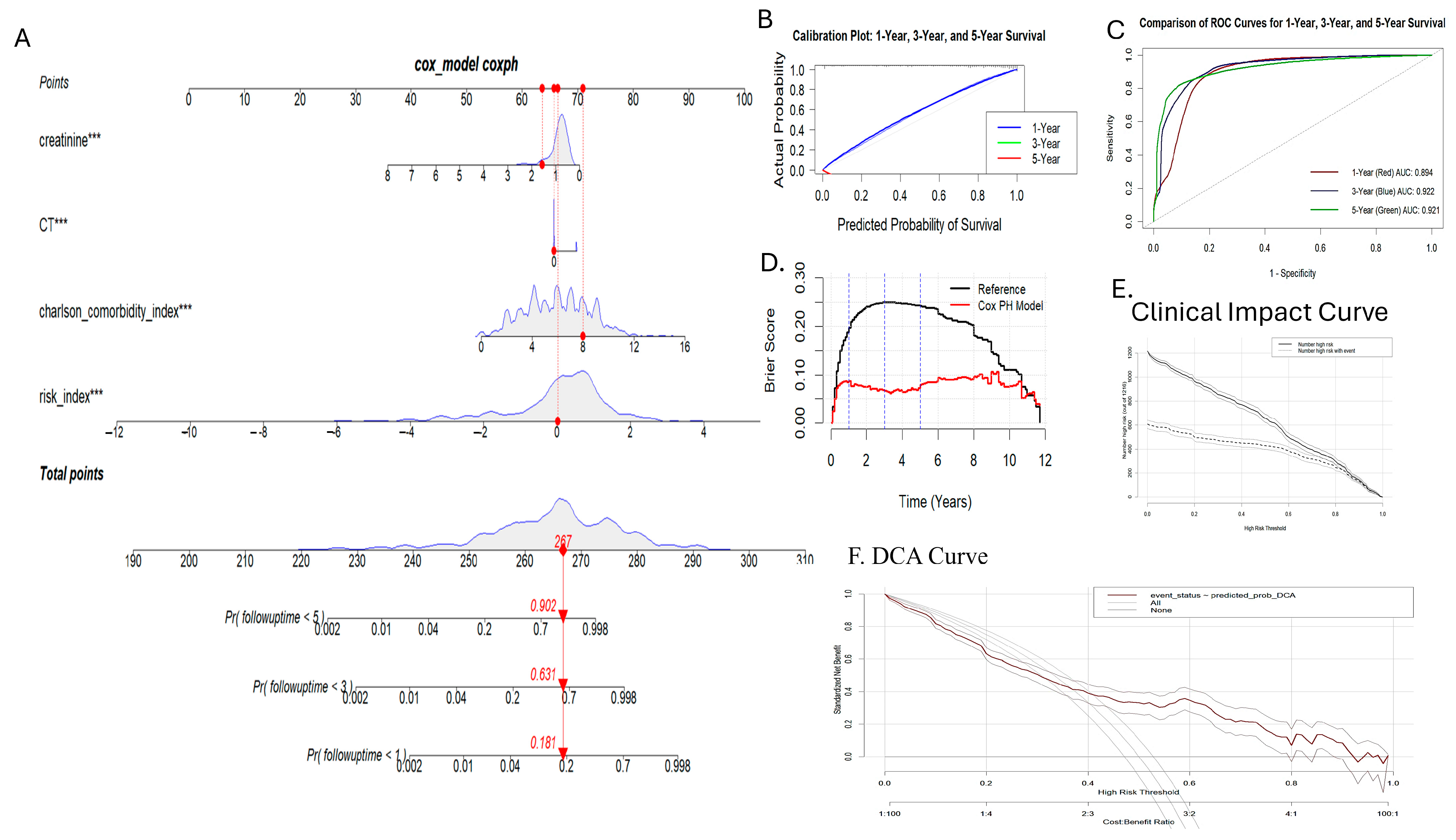
3.6. Nomogram Generation and Validation
3.7. Subgroup Analysis and Validation
4. Discussion
- Patient selection and baseline assessments:
- Initial evaluation: At the time of diagnosis and before initiating radiotherapy, patients should undergo a comprehensive evaluation including clinical, laboratory, and radiological assessments.
- SIRI-RT calculation: Incorporate SIRI-RT, which uses routinely collected clinical parameters, to generate a baseline risk index.
- Risk stratification:
- Classification into Risk Groups: Based on the SIRI-RT score, patients are stratified into low-, medium-, and high-risk groups for adverse outcomes such as secondary malignancies.
- Multidisciplinary Review: During tumor board meetings, specialists such as oncologists and radiologists conduct a multidisciplinary review, where they assess risk stratification by combining these insights with other clinical information.
- Tailored treatment planning:
- High-risk patients: For individuals classified as high-risk, consider implementing changes such as the following:
- More frequent monitoring and follow-up appointments;
- Alterations in the dosage or methods of radiotherapy delivery;
- Incorporation of additional systemic treatments to reduce risk.
- Low-risk patients: Standard radiotherapy protocols may be maintained with routine follow-up.
- Intermediate-risk patients: Use a balanced approach, potentially incorporating additional diagnostic imaging or biomarkers to further refine risk assessment.
- Ongoing monitoring and reassessment:
- Dynamic risk evaluation: Re-assess SIRI-RT at defined intervals during and after radiotherapy to monitor changes in risk profile.
- Adjusting follow-up protocols: Based on changes in SIRI-RT scores, adjust follow-up intensity and imaging schedules to promptly detect any treatment-related adverse effects.
- Integration into clinical decision support systems:
- Electronic Health Records (EHR) integration: Embed SIRI-RT within the EHR to automatically calculate risk scores and flag high-risk patients.
- Decision support alerts: Provide real-time decision support for clinicians, prompting re-assessment or intervention when significant changes in SIRI-RT are detected.
- Continuous quality improvement:
- Outcome tracking: Registries can be utilized to track patient outcomes relative to their SIRI-RT scores.
- Model refinement: The risk model based on real-world data and outcomes to improve its predictive accuracy and clinical utility can be periodically reviewed and updated.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RISM | Radiation-Induced Secondary Malignancy |
| AG | anion gap |
| AGR | Albumin–Globulin ratio |
| ALI | advanced lung cancer inflammation index |
| APSIII | acute physiology score III |
| AUC | area under the curve |
| BUN | blood urea nitrogen |
| CAR | CRP_Albumin ratio |
| CI | confidence interval |
| CIC | clinical impact curve |
| CNS | central nervous system |
| CRP | C-reactive protein |
| DCA | decision curve analysis |
| DFS | disease-free survival |
| GCS score | Glasgow Coma Scale |
| GNRI | Geriatric Nutrition Risk Index |
| HLET | high linear energy transfer |
| HR | hazard ratio |
| IARC | International Agency for Research on Cancer |
| ICD | International Classification of Diseases |
| INR | international normalized ratio |
| KM | Kaplan–Meier |
| LASSO | least absolute shrinkage and selection operator |
| LCR | Lumphocyte to CRP ratio |
| LLET | low linear energy transfer |
| mbp | mean blood pressure |
| MDS | myelodysplastic syndrome |
| mGNRI | Modified Geriatric Nutrition Risk Index |
| mGPS | modified Glasgow Prognostic Score |
| MIMIC-IV | Medical Information Mart for Intensive Care |
| MSE | mean squared error |
| NF-κB | nuclear factor-kappaB |
| NGR | absneutrophil.glucose ratio |
| NLR | neutrophils to lymphocytes ratio |
| OASIS | Oxford Acute Severity of Illness Score |
| OS | overall survival |
| PAR | plateletcount.age ratio |
| PFS | progression-free survival |
| PGF | Plateletcount/glucose*followuptime |
| PLR | platelet-to-lymphocyte ratio |
| PNI | prognostic nutrition index |
| PT | prothrombin time |
| PTT | partial prothrombin time |
| ROC | receiver operator characteristic |
| RR | relative risk |
| SAPSII | Simplified Acute Physiology Score II |
| SD | standard deviation |
| SII | Systemic Immune-Inflammation Index |
| SIRI-RT | Serum Inflammation Risk Index for Radiation-Induced Secondary Malignancy |
| SIRS | Systemic Inflammatory Response Syndrome |
| SIS | systemic inflammation-immune status |
| SOFA | Sequential Organ Failure Assessment |
| SQL | Structured Query Language |
| TM | Thrombomodulin |
| VIF | variance inflation factor |
| WBC | white blood cells |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.R.; Wen, G.; Wang, S.L.; Zhang, Y.; Yu, T.; Fang, H.; Lu, N.; Chen, B.; Tang, Y.; Li, N.; et al. Systemic Inflammation-Immune Status Score Predicts the Incidence of Radiation Induced Pneumonitis in Patients with Breast Cancer After Whole Breast Irradiation: A Result From Two Medical Centers. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e226–e227. [Google Scholar] [CrossRef]
- Lee, J.W.; Wernicke, A.G. Risk and Survival Outcomes of Radiation-Induced CNS Tumors. J. Neuro-Oncol. 2016, 129, 15–22. [Google Scholar] [CrossRef]
- Diallo, I.; Haddy, N.; Adjadj, E.; Samand, A.; Quiniou, E.; Chavaudra, J.; Alziar, I.; Perret, N.; Guérin, S.; Lefkopoulos, D.; et al. Frequency Distribution of Second Solid Cancer Locations in Relation to the Irradiated Volume Among 115 Patients Treated for Childhood Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 876–883. [Google Scholar] [CrossRef]
- de Gonzalez, A.B.; Gilbert, E.; Curtis, R.; Inskip, P.; Kleinerman, R.; Morton, L.; Rajaraman, P.; Little, M.P. Second Solid Cancers After Radiation Therapy: A Systematic Review of the Epidemiologic Studies of the Radiation Dose-Response Relationship. Int. J. Radiat. Oncol. Biol. Phys. 2012, 86, 224–233. [Google Scholar] [CrossRef]
- Multhoff, G.; Radons, J. Radiation, Inflammation, and Immune Responses in Cancer. Front. Oncol. 2012, 2, 58. [Google Scholar] [CrossRef]
- Schaue, D.; Mcbride, W.H. Links between Innate Immunity and Normal Tissue Radiobiology. Radiat. Res. 2010, 173, 406–417. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Wright, E.G.; Park, C. Radiation and the Microenvironment—Tumorigenesis and Therapy. Nat. Rev. Cancer 2005, 5, 867–875. [Google Scholar] [CrossRef]
- Kadhim, M.; Salomaa, S.; Hildebrandt, G.; Belyakov, O.V.; Wright, E.; Prise, K.M.; Little, M.P. Non-Targeted Effects of Ionising Radiation--Implications for Low Dose Risk. Mutat. Res./Rev. Mutat. Res. 2012, 752, 84–98. [Google Scholar] [CrossRef]
- Allin, K.H.; Nordestgaard, B.G. Elevated C-Reactive Protein in the Diagnosis, Prognosis, and Cause of Cancer. Crit. Rev. Clin. Lab. Sci. 2011, 48, 155–170. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; Mcmillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Abstract PR1: Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-like Transition and Promotes Metastasis. Cancer Res. 2011, 71, PR1. [Google Scholar] [CrossRef]
- Cao, Y.; Yi, Y.; Han, C.; Shi, B. NF-ΚB Signaling Pathway in Tumor Microenvironment. Front. Immunol. 2024, 15, 1476030. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Cassatt, D.R.; Winters, T.A.; Prabhudas, M. Immune Dysfunction from Radiation Exposure. Radiat. Res. 2023, 200, 396–416. [Google Scholar] [CrossRef]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of Immune Cells and Cytokines on Inflammation and Immunosuppression in the Tumor Microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef]
- García-Núñez, A.; Jiménez-Gómez, G.; Hidalgo-Molina, A.; Córdoba-Doña, J.A.; León-Jiménez, A.; Campos-Caro, A. Inflammatory Indices Obtained from Routine Blood Tests Show an Inflammatory State Associated with Disease Progression in Engineered Stone Silicosis Patients. Sci. Rep. 2022, 12, 8211. [Google Scholar] [CrossRef]
- Zhou, H.-H.; Xu, T.-H.; Cheng, B.; Tang, Y.-L. C-Reactive Protein: Structure, Function, Regulation, and Role in Clinical Diseases. Front. Immunol. 2024, 15, 1425168. [Google Scholar] [CrossRef]
- Huang, K.; Prasad, S.; Ma, S.J.; Yu, H.; Iovoli, A.J.; Farrugia, M.K.; Dexter, E.U.; Demmy, T.L.; Malik, N.K.; Singh, A.K. Association of Neutrophil–Lymphocyte Ratio with Survival in Peripheral Early-Stage Non-Small Cell Lung Cancer after Stereotactic Body Radiation Therapy. BMC Cancer 2023, 23, 254. [Google Scholar] [CrossRef]
- Iguchi, T.; Sugimachi, K.; Mano, Y.; Motomura, T.; Sugiyama, M.; Ota, M.; Ikebe, M.; Esaki, T.; Yoshizumi, T.; Morita, M.; et al. Prognostic Impact of Geriatric Nutritional Risk Index in Patients with Synchronous Colorectal Liver Metastasis. Anticancer Res. 2020, 40, 4165–4171. [Google Scholar] [CrossRef]
- Barth, D.A.; Brenner, C.; Riedl, J.M.; Prinz, F.; Klocker, E.V.; Schlick, K.; Kornprat, P.; Lackner, K.; Stöger, H.; Stotz, M.; et al. External Validation of the Prognostic Relevance of the Advanced Lung Cancer Inflammation Index (ALI) in Pancreatic Cancer Patients. Cancer Med. 2020, 9, 5473–5479. [Google Scholar] [CrossRef] [PubMed]
- Cotan, H.; Nitipir, C.; Iaciu, C. Role of the Modified Glasgow Prognostic Score (MGPS) as a Prognostic Factor in Metastatic Colorectal Cancer. Cureus 2024, 16, e64916. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.; Aitchison, M.; Lamb, G.W.A.; Mcmillan, D.C. Prospective Study of the Relationship between the Systemic Inflammatory Response, Prognostic Scoring Systems and Relapse-free and Cancer-specific Survival in Patients Undergoing Potentially Curative Resection for Renal Cancer. BJU Int. 2008, 101, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, S.; Eto, K.; Haruki, K.; Shiba, H.; Kosuge, M.; Ohkuma, M.; Ito, D.; Takeda, Y.; Sugano, H.; Sasaki, S.; et al. Signification of Systemic Immune-Inflammation Index for Prediction of Prognosis after Resecting in Patients with Colorectal Cancer. Int. J. Color. Dis. 2020, 35, 1549–1555. [Google Scholar] [CrossRef]
- Bailey-Whyte, M.; Dorsey, T.H.; Minas, T.Z.; Smith, C.J.; Loffredo, C.A.; Ambs, S. Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers 2023, 15, 1869. [Google Scholar] [CrossRef]
- Gabiatti, C.T.; Martins, M.C.; Miyazaki, D.L.; Silva, L.P.; Lascala, F.; Macedo, L.T.; Mendes, M.C.S.; Carvalheira, J.B.C. Myosteatosis in a Systemic Inflammation-Dependent Manner Predicts Favorable Survival Outcomes in Locally Advanced Esophageal Cancer. Cancer Med. 2019, 8, 6967–6976. [Google Scholar] [CrossRef]
- Nabavizadeh, R.; Bobrek, K.; Master, V.A. Risk Stratification for Bladder Cancer: Biomarkers of Inflammation and Immune Activation. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 706–712. [Google Scholar] [CrossRef]
- Tiainen, S.; Nurmela, V.; Selander, T.; Turunen, P.; Pasonen-Seppänen, S.; Kettunen, T.; Kuittinen, O.; Auvinen, P.; Rönkä, A. A Practical Prognostic Peripheral Blood-Based Risk Model for the Evaluation of the Likelihood of a Response and Survival of Metastatic Cancer Patients Treated with Immune Checkpoint Inhibitors. BMC Cancer 2023, 23, 1186. [Google Scholar] [CrossRef]
- Blazek, K.; Van Zwieten, A.; Saglimbene, V.; Teixeira-Pinto, A. A Practical Guide to Multiple Imputation of Missing Data in Nephrology. Kidney Int. 2020, 99, 68–74. [Google Scholar] [CrossRef]
- Ruan, G.T.; Yang, M.; Zhang, X.W.; Song, M.M.; Hu, C.L.; Ge, Y.Z.; Xie, H.L.; Liu, T.; Tang, M.; Zhang, Q.; et al. Association of Systemic Inflammation and Overall Survival in Elderly Patients with Cancer Cachexia—Results from a Multicenter Study. J. Inflamm. Res. 2021, 14, 5527–5540. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Okamoto, T. ASO Author Reflections: The C-Reactive Protein (CRP)-Albumin Ratio May Be Useful as the Most Prognostic Index Among the Immuno-Nutritional Parameters Using CRP and Albumin for Resected NSCLC. Ann. Surg. Oncol. 2020, 28, 3055–3056. [Google Scholar] [CrossRef]
- Xie, H.L.; Zhang, Q.; Ruan, G.T.; Ge, Y.Z.; Hu, C.L.; Song, M.M.; Song, C.H.; Zhang, X.; Zhang, X.W.; Li, X.R.; et al. Evaluation and Validation of the Prognostic Value of Serum Albumin to Globulin Ratio in Patients with Cancer Cachexia: Results from a Large Multicenter Collaboration. Front. Oncol. 2021, 11, 707705. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Ichikawa, T.; Yin, C.; Suzuki, A.; Fujikawa, H.; Yasuda, H.; Hiro, J.; et al. Lymphocyte-to-C-Reactive Protein Ratio and Score Are Clinically Feasible Nutrition-Inflammation Markers of Outcome in Patients with Gastric Cancer. Clin. Nutr. 2019, 39, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.T.; Zhang, Q.; Zhang, X.; Tang, M.; Song, M.M.; Zhang, X.W.; Li, X.R.; Zhang, K.P.; Ge, Y.Z.; Yang, M.; et al. Geriatric Nutrition Risk Index: Prognostic Factor Related to Inflammation in Elderly Patients with Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2021, 12, 1969–1982. [Google Scholar] [CrossRef]
- Loh, W.W.; Ren, D. Data-Driven Covariate Selection for Confounding Adjustment by Focusing on the Stability of the Effect Estimator. Psychol. Methods 2024, 29, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.H.; Lubin, J.H.; Wacholder, S. Indirect Corrections for Confounding under Multiplicative and Additive Risk Models. Am. J. Ind. Med. 1988, 13, 119–130. [Google Scholar] [CrossRef]
- Sun, L.; Hu, W.; Liu, M.; Chen, Y.; Jin, B.; Xu, H.; Du, S.; Xu, Y.; Zhao, H.; Lu, X.; et al. High Systemic Inflammation Response Index (SIRI) Indicates Poor Outcome in Gallbladder Cancer Patients with Surgical Resection: A Single Institution Experience in China. Cancer Res. Treat. 2020, 52, 1199–1210. [Google Scholar]
- Xin, Y.; Yang, Y.; Zhou, X.; Chen, Y.; Li, X.; Wang, Y.; Zhang, X.; Li, Y. A Systemic Inflammation Response Index (SIRI)-Based Nomogram for Predicting the Recurrence of Early Stage Hepatocellular Carcinoma After Radiofrequency Ablation. CardioVascular Interv. Radiol. 2021, 45, 43–53. [Google Scholar] [CrossRef]
- Atasever Akkas, E.; Yucel, B.; Erdis, E. Prognostic Value of the Systemic Immune-Inflammation Index, Systemic Inflammation Response Index, and Prognostic Nutritional Index in Head and Neck Cancer. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 3821–3830. [Google Scholar] [CrossRef]
- Faria, S.S.; Giannarelli, D.; Cordeiro de Lima, V.C.; Anwar, S.L.; Casadei, C.; De Giorgi, U.; Madonna, G.; Ascierto, P.A.; Mendoza López, R.V.; Chammas, R.; et al. Development of a Prognostic Model for Early Breast Cancer Integrating Neutrophil to Lymphocyte Ratio and Clinical-Pathological Characteristics. Oncol. 2023, 29, e447–e454. [Google Scholar] [CrossRef]
- Eaton, B.R.; Macdonald, S.M.; Yock, T.I.; Tarbell, N.J. Secondary Malignancy Risk Following Proton Radiation Therapy. Front. Oncologist 2015, 5, 261. [Google Scholar] [CrossRef]
- Burt, L.M.; Ying, J.; Poppe, M.M.; Suneja, G.; Gaffney, D.K. Risk of Secondary Malignancies after Radiation Therapy for Breast Cancer: Comprehensive Results. Breast 2017, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yu, R.; Zhang, H.; Wang, W. Cytokine, Chemokine Alterations and Immune Cell Infiltration in Radiation-Induced Lung Injury: Implications for Prevention and Management. Int. Immunopharmacol. 2023, 126, 111263. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Skibińska, M.; Lesiak, A.; Narbutt, J.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D. Ultraviolet Radiation and Chronic Inflammation—Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life 2021, 11, 326. [Google Scholar] [CrossRef]
- Gandhi, S.; Chandna, S. Radiation-Induced Inflammatory Cascade and Its Reverberating Crosstalks as Potential Cause of Post-Radiotherapy Second Malignancies. Cancer Metastasis Rev. 2017, 36, 375–393. [Google Scholar] [CrossRef]
- Goktas Aydin, S.; Kutlu, Y.; Muglu, H.; Aydin, A.; Acikgoz, O.; Hamdard, J.; Karci, E.; Bilici, A.; Olmez, O.F.; Yildiz, O. Predictive Significance of Inflammatory Markers and MGPS in Metastatic Castration-Resistant Prostate Cancer Treated with Abiraterone or Enzalutamide. Cancer Chemother. Pharmacol. 2023, 93, 71–78. [Google Scholar] [CrossRef]
- Da, L.; Gu, K.; Shen, Y.; Huang, W.; Qu, Z.; Zhang, C.; Zhang, Y. Prognostic Value of Inflammatory Markers and Clinical Features for Survival in Advanced or Metastatic Esophageal Squamous Cell Carcinoma Patients Receiving Anti-Programmed Death 1 Treatment. Front. Oncol. 2023, 13, 11448725. [Google Scholar] [CrossRef]
- Cheng, M.-Y.; Honda, T.; Zhang, J.-T. Forward Variable Selection for Sparse Ultra-High Dimensional Varying Coefficient Models. J. Am. Stat. Assoc. 2016, 111, 1209–1221. [Google Scholar] [CrossRef]
- Little, J.B. Radiation Carcinogenesis. Carcinogenesis 2000, 21, 397–404. [Google Scholar] [CrossRef]
- Watson, M.J.; Maruf, M.; Kongnyuy, M.; Frye, T.P.; Valayil, S.G.; Pinto, P.A.; Muthigi, A.; George, A.K. Risk Stratification of Prostate Cancer: Integrating Multiparametric MRI, Nomograms and Biomarkers. Future Oncol. 2016, 12, 2417–2430. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Sharma, P.K.; Rai, G.; Mishra, S.; Arora, D.; Gupta, P.; Shukla, Y. Current Perspectives of Molecular Pathways Involved in Chronic Inflammation-Mediated Breast Cancer. Biochem. Biophys. Res. Commun. 2015, 472, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Bernhard, E.J.; Vikram, B.; Hodge, J.W.; Coleman, C.N.; Guha, C. Harnessing the Potential of Radiation-Induced Immune Modulation for Cancer Therapy. Cancer Immunol. Res. 2013, 1, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.; Manji, A.; Allotey, J.; Heazell, A.E.P.; Jorgensen, L.; Magee, L.A.; Mol, B.W.; Snell, K.I.E.; Riley, R.D.; Sandall, J.; et al. Can Risk Prediction Models Help Us Individualise Stillbirth Prevention? A Systematic Review and Critical Appraisal of Published Risk Models. BJOG Int. J. Obstet. Gynaecol. 2020, 128, 214–224. [Google Scholar] [CrossRef]
- Xu, Y.; Goodacre, R. On Splitting Training and Validation Set: A Comparative Study of Cross-Validation, Bootstrap and Systematic Sampling for Estimating the Generalization Performance of Supervised Learning. J. Anal. Test. 2018, 2, 249–262. [Google Scholar] [CrossRef]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; Van Diepen, M. External Validation of Prognostic Models: What, Why, How, When and Where? Clin. Kidney J. 2020, 14, 49–58. [Google Scholar] [CrossRef]
- El-Sherbini, A.H.; Krittanawong, C.; Hassan Virk, H.U.; Wang, Z.; Glicksberg, B.S. Machine-Learning-Based Prediction Modelling in Primary Care: State-of-the-Art Review. AI 2023, 4, 437–460. [Google Scholar] [CrossRef]

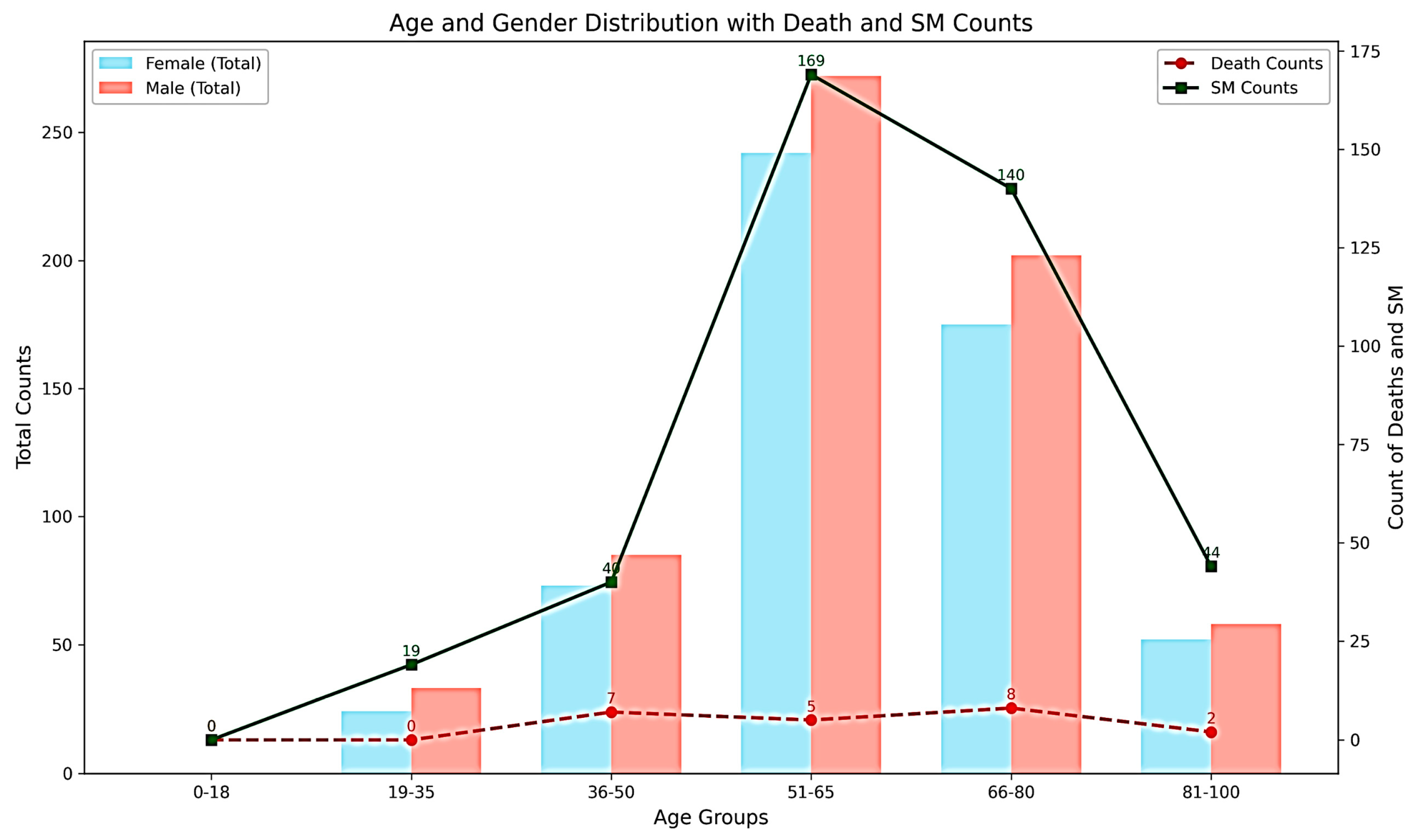



| Cancer Patients [Counts (%total pts in MIMIC-IV)] | 42,296 (unique) |
| Total | |
| Chemotherapy [Total Counts—in MIMIC-IV] | 3797 |
| Surgery [Total Counts—in MIMIC-IV] | 59,228 |
| Immunotherapy [Total Counts as treated in MIMIC-IV] | 3296 |
| Secondary Malignancy [Total Counts diagnosed in MIMIC-IV] | 35,143 (includes total pts with RT and non-RT) |
| Radiotherapy [Total Counts—in MIMIC-IV] | 3851 (with duplicates) |
| Mean Time Since Radiation Exposure (years) | 2.4 (Max: 11.7 y) |
| Radiotherapy [TOTAL RT Cohort-Counts] | 1443 (without duplicates) |
| Radiotherapy [TOTAL RT Cohort-Counts used for analysis] | 1216 |
| Radiation-Induced Secondary Malignancy [RISM-Counts] | 608 |
| Age [Mean (sd)] | 62.32 y ± 13.6 |
| Male [Counts (%)] | 632 (51.97%) |
| Female [Counts (%)] | 584 (48.02%) |
| Death Events [ RISM cohort] | |
| Male [Counts (%)] | 10 (1.5%) |
| Female [Counts (%)] | 12 (2%) |
| Secondary Malignancy [(RISM cohort)] | |
| Male [Counts (%)] | 286 (47.03%) |
| Female [Counts (%)] | 322 (52.96%) |
| Subgroups [TOTAL RT (RISM cohort)] | |
| High-LET [Counts available in MIMIC-IV] | 13 (11) |
| Low-LET [Counts available in MIMIC-IV] | 1122 (502) |
| Surgery [Counts available in MIMIC-IV] | 27 (20) |
| Brachytherapy [Counts available in MIMIC-IV] | 101 (14) |
| Radiotherapy Regimens [TOTAL RT (RISM cohort)] | |
| Short course [Counts available in MIMIC-IV] | 366 (257) |
| Long course [Counts available in MIMIC-IV] | 409 (242) |
| RT Treatment Type [TOTAL RT (RISM cohort)] | |
| Neoadjuvant [Counts available in MIMIC-IV] | 72 (27) |
| Adjuvant [Counts available in MIMIC-IV] | 144 (40) |
| Definitive [Counts available in MIMIC-IV] | 144 (30) |
| Dose of Radiation [TOTAL RT (RISM cohort)] | |
| 0–2 Gy [Counts available in MIMIC-IV] | 3 (1) |
| 2–10 Gy [Counts available in MIMIC-IV] | 10 (3) |
| 10–20 Gy [Counts available in MIMIC-IV] | 19 (3) |
| 20–30 Gy [Counts available in MIMIC-IV] | 16 (1) |
| 30–40 Gy [Counts available in MIMIC-IV] | 11 (3) |
| 40–50 Gy [Counts available in MIMIC-IV] | 17 (8) |
| 50–60 Gy [Counts available in MIMIC-IV] | 16 (5) |
| 60–70 Gy [Counts available in MIMIC-IV] | 4 (2) |
| >200 Gy [Counts available in MIMIC-IV] | 3 (1) |
| Tumor Staging [TOTAL RT (RISM cohort)] | |
| TUMOUR (T) [Counts available in MIMIC-IV] | 1267 (365) |
| NODE (N) [Counts available in MIMIC-IV] | 967 (264) |
| METASTASIS (M) [Counts available in MIMIC-IV] | 758 (201) |
| Primary Condition as per ICD Codes Treated with RT [RISM Cohort- in MIMIC-IV] | |
| Other/Non-Cancer or Unclassified [Counts (%)] | 523 (43.01%) |
| Lung Cancer [Counts (%)] | 67 (5.5%) |
| Brain/CNS Cancer [Counts (%)] | 61 (5.02%) |
| Lymphoma [Counts (%)] | 52 (4.28%) |
| Myeloma [Counts (%)] | 52 (4.28%) |
| Leukemia Total (ALL + AML + CLL + CML) [Counts (%)] | 48 (20 + 19 + 6 + 1) (3.95%) |
| Bone Cancer [Counts (%)] | 46 (3.78%) |
| Gynecological Cancer [Counts (%)] | 30 (2.47%) |
| Other Specified Cancers (incl Pancreatic, Liver, Oral, esophageal, Colorectal, Prostate, bladder, etc.) [Counts (%)] | 108 (<3%) |
| RISM Counts as per ICD Codes Post-Radiotherapy [RISM Cohort- in MIMIC-IV] | 608 |
| Other Cancer Types (Unspecified) [Counts (%)] | 273 (44.90%) |
| Bone Cancer [Counts (%)] | 134 (22.04%) |
| Brain Cancer [Counts (%)] | 93 (15.29%) |
| Lung Cancer [Counts (%)] | 77 (12.66%) |
| Liver Cancer [Counts (%)] | 24 (3.95%) |
| Other Cancers (Rectal, Bladder, Kidney, etc.) [Counts (%)] | 7 (1.15%) |
| Primary Hematological Conditions as per ICD Codes treated with RT [RISM Cohort- in MIMIC-IV] | |
| Myeloma/Plasma Cell Disorder [Counts] | 52 |
| Lymphoma—(Other/Hodgkins) [Counts] | 49/3 |
| Leukemia (Other/AML/ALL/CML/CLL) [Counts] | 7/19/15/6/1 |
| Neutropenia [Counts] | 8 |
| Myeloid Neoplasm/MDS/MPN [Counts] | 6 |
| Anemia [Counts] | 3 |
| Thrombocytopenia [Counts] | 1 |
| Pancytopenia [Counts] | 1 |
| Inflammatory Markers [RISM Cohort—in MIMIC-IV] | |
| Absolute Neutrophil Count [Mean (sd)] (cells/µL) | 71.3 ± 12.2 |
| Absolute Lymphocyte Count [Mean (sd)] (cells/µL) | 17.6 ± 10.7 |
| Platelet Count [Mean(sd)] (×103/µL) | 229.8 ± 104.86 |
| Albumin(g/dL)-[Mean(sd)] | 3.55 ± 0.65 |
| Globulin(g/dL)-[Mean(sd)] | 2.7 ± 0.74 |
| Hemoglobin [Mean (sd)] (g/dL) | 10.8 ± 1.9 |
| Hematocrit [Mean (sd)] (%) | 32.9 ± 5.4 |
| Creatinine [Mean (sd)] (mg/dL) | 1 ± 0.8 |
| Sodium [Mean (sd)] (mEq/L) | 138 ± 3.28 |
| Potassium [Mean (sd)] (mEq/L) | 4.15 ± 0.35 |
| Chloride [Mean (sd)] (mEq/L) | 101.74 ± 3.96 |
| Blood Urea Nitrogen (BUN) [Mean (sd)] (mg/dL) | 19.83 ± 11.96 |
| Glucose [Mean (sd)] (mg/dL) | 120.8 ± 33.22 |
| Bicarbonate [Mean(sd)] (mEq/L) | 25.9 ± 2.99 |
| Anion Gap [Mean (sd)] (mEq/L) | 13.7 ± 2.08 |
| BMI | 79.7 ± 2313 |
| Vital Signs [RISM Cohort- in MIMIC-IV] | |
| heart-rate [Mean(sd)] (bpm) | 88.28 ± 14.5 |
| mbp [Mean (sd)] (mmHg) | 79.33 ± 9.68 |
| resp_rate [Mean (sd)] (%). | 19.3 ± 3.4 |
| temperature [Mean (sd)] | 36.8 ± 0.42 |
| sPO2 [Mean (sd)] (%) | 96.7 ± 1.87 |
| Severity Score [RISM Cohort- in MIMIC-IV] | |
| Oasis [Mean (sd)] | 30.44 ± 7.55 |
| gcs_score [Mean (sd)] | 1.9 ± 2.37 |
| sapsii [Mean (sd)] | 38.1 ± 13 |
| sepsis3 [Mean (sd)] | 0.25 ± 0.436 |
| sirs [Mean (sd)] | 2.57 ± 0.83 |
| apsii [Mean (sd)] | 43.7 ± 17.9 |
| Coagulation Parameters [RISM Cohort- in MIMIC-IV] | |
| inr [Mean (sd)] | 1.28 ± 0.42 |
| pt [Mean (sd)] | 14.1 ± 4.29 |
| ptt [Mean (sd)] | 34.4 ± 11.68 |
| Charlson Comorbidity Index [Mean(sd)] [RISM Cohort- in MIMIC-IV] | 4.38 ± 2.88 |
| Standard Inflammation Ratios (Literature reported) [calculated in RISM cohort of MIMIC-IV DATABASE] | |
| NLR [median (IQR)] | 6.14(10.6) |
| PLR (median (IQR)] | 274 (378.5) |
| ALI [median (IQR)] | 16.44 (29.11) |
| SII [median (IQR)] | 1333.6 (2490.9) |
| CAR (median (IQR)) | 4.89 (24.98) |
| GNRI [median (IQR)] | −25.24 ± 198.2 |
| mGNRI [median (IQR)] | −1197.15 ± 8378.7 |
| NRI [median (IQR)] | −1285.2 ± 8330.5 |
| AGR [median (IQR)] | 1.36 ± 89.3 |
| PNI [median (IQR)] | 36 ± 9.9 |
| LCR [median (IQR)] | 320.6 ± 19.18 |
| Coefficient | Feature | Hazard Ratio |
|---|---|---|
| 1.065059427 | risk_index (SIRI-RT) | 2.901011 |
| −0.280998265 | CT1 (Chemotherapy) | 0.75503 |
| 0.318255732 | Charlson comorbidity index | 1.374728 |
| −0.578412143 | Creatinine | 0.560788 |
| 0.061338537 | sirs | 1.063259 |
| −0.030315099 | Hemoglobin | 0.97014 |
| −0.061052613 | Calcium | 0.940774 |
| −0.140983725 | Sepsis31 | 0.868503 |
| 0.044216404 | AGR | 1.045209 |
| −0.292879789 | Potassium | 0.746112 |
| Variable | Coefficient (β) | HR (exp(β)) | 95% CI | p-Value | Interpretation |
|---|---|---|---|---|---|
| Risk index (SIRI-RT) | 1.1089 | 3.03 | 2.79–3.30 | <2 × 10−16 *** | A unit increase in risk_index triples the hazard (~203% higher risk). Strongest risk factor. |
| Chemotherapy | 0.6761 | 1.97 | 1.63–2.37 | 7.39 × 10−13 *** | Presence of Chemotherapy nearly doubles the hazard (~97% higher risk). |
| Charlson comorbidity index | 0.3848 | 1.47 | 1.41–1.53 | <2 × 10−16 *** | For each unit increase in the comorbidity score, the hazard increases by 47%. |
| Creatinine | −0.7246 | 0.48 | 0.40–0.58 | 3.86 × 10−14 *** | Higher creatinine is protective, reducing hazard by 52%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, S.; Chandna, S.; Chinnadurai, V.; Vidyarthi, P. A Novel Serum Inflammation Risk-Index (SIRI-RT)-Driven Nomogram for Predicting Secondary Malignancy Outcomes Post-Radiotherapy. Cancers 2025, 17, 1290. https://doi.org/10.3390/cancers17081290
Gandhi S, Chandna S, Chinnadurai V, Vidyarthi P. A Novel Serum Inflammation Risk-Index (SIRI-RT)-Driven Nomogram for Predicting Secondary Malignancy Outcomes Post-Radiotherapy. Cancers. 2025; 17(8):1290. https://doi.org/10.3390/cancers17081290
Chicago/Turabian StyleGandhi, Sonia, Sudhir Chandna, Vijayakumar Chinnadurai, and Pankaj Vidyarthi. 2025. "A Novel Serum Inflammation Risk-Index (SIRI-RT)-Driven Nomogram for Predicting Secondary Malignancy Outcomes Post-Radiotherapy" Cancers 17, no. 8: 1290. https://doi.org/10.3390/cancers17081290
APA StyleGandhi, S., Chandna, S., Chinnadurai, V., & Vidyarthi, P. (2025). A Novel Serum Inflammation Risk-Index (SIRI-RT)-Driven Nomogram for Predicting Secondary Malignancy Outcomes Post-Radiotherapy. Cancers, 17(8), 1290. https://doi.org/10.3390/cancers17081290






