Comparing the Prognostic Value of Quantitative Response Assessment Tools and LIRADS Treatment Response Algorithm in Patients with Hepatocellular Carcinoma Following Interstitial High-Dose-Rate Brachytherapy and Conventional Transarterial Chemoembolization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort, Design, and Endpoints
2.2. Imaging and Clinical Data Retrieval
2.3. Response Assessment Measurements
2.4. CT-Guided High-Dose-Rate Brachytherapy (iBT)
2.5. Conventional Transarterial Chemoembolization (cTACE)
2.6. Completed Treatment Cycles
2.7. Survival and Tumor Progression Measures
2.8. Statistics
3. Results
3.1. Patient and Disease Characteristics
3.2. Tumor Response
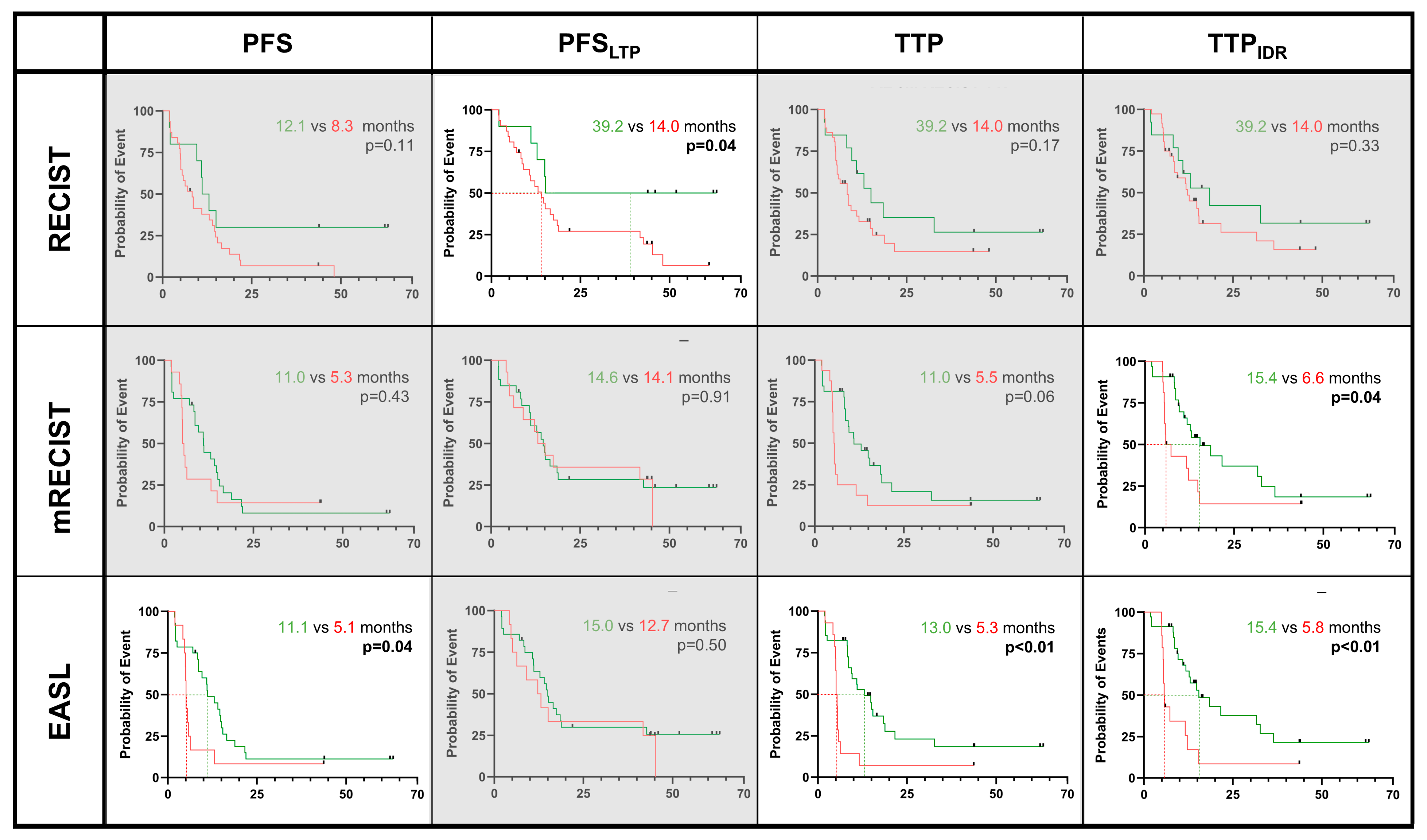
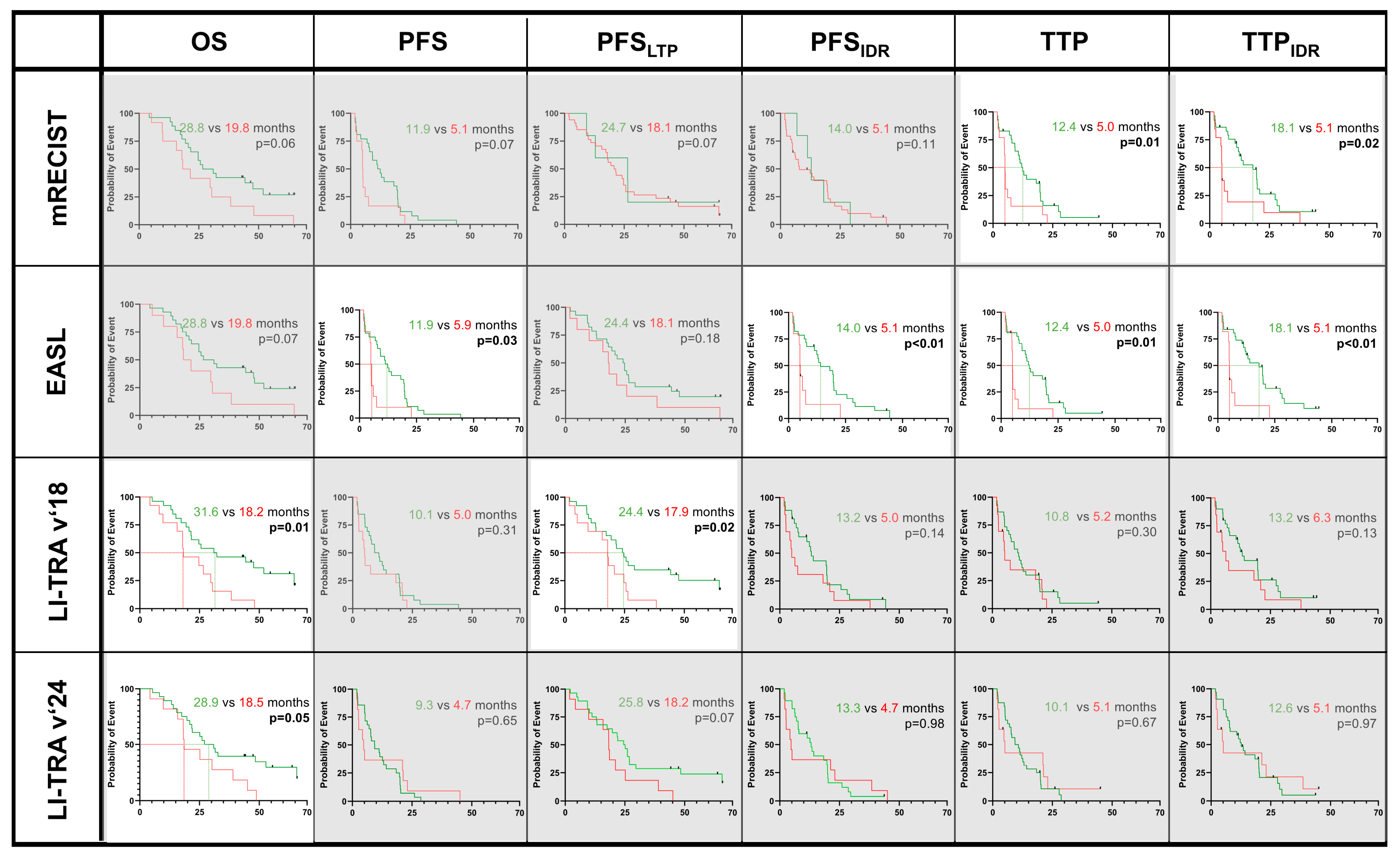
3.3. Predicting OS, PFS, and TTP Regarding Response Criteria
3.4. Confounders of OS, PFS, and TTP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALBI score | Albumin–bilirubin score |
| iBT | Interstitial (high-dose-rate) brachytherapy |
| CT | Computer tomography |
| EASL | European Association for the Study of the Liver |
| ESMO | European Society of Medical Oncology |
| HCC | Hepatocellular carcinoma |
| IDR | Intrahepatic distant recurrence |
| IQR | Interquartile range |
| MRI | Magnetic resonance imaging |
| MTB | Multidisciplinary Tumor Board |
| MWA | Microwave ablation |
| LI-RADS | Liver Imaging and Reporting and Data System |
| LR-TRA | LIRADS Treatment Response Algorithm |
| LRT | Locoregional therapy |
| LTP | Local tumor progression |
| OS | Overall survival |
| PFS | Progression-free survival |
| (m)RECIST | (Modified) Response Evaluation Criteria in Solid Tumors |
| RFA | Radiofrequency ablation |
| SD | Standard deviation |
| cTACE | Conventional transarterial chemoembolization |
| TTP | Time to progression |
| WHO | World Health Organization |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Schnapauff, D.; Tegel, B.R.; Powerski, M.J.; Colletini, F.; Hamm, B.; Gebauer, B. Interstitial Brachytherapy in Combination with Previous Transarterial Embolization in Patients with Unresectable Hepatocellular Carcinoma. Anticancer. Res. 2019, 39, 1329–1336. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv238–iv255. [Google Scholar] [CrossRef]
- Collettini, F.; Schnapauff, D.; Poellinger, A.; Denecke, T.; Schott, E.; Berg, T.; Wust, P.; Hamm, B.; Gebauer, B. Hepatocellular carcinoma: Computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5–7 cm) and very large (>7 cm) tumours. Eur. Radiol. 2012, 22, 1101–1109. [Google Scholar] [CrossRef]
- Makary, M.S.; Ramsell, S.; Miller, E.; Beal, E.W.; Dowell, J.D. Hepatocellular carcinoma locoregional therapies: Outcomes and future horizons. World J. Gastroenterol. 2021, 27, 7462–7479. [Google Scholar] [CrossRef]
- Yaghmai, V.; Besa, C.; Kim, E.; Gatlin, J.L.; Siddiqui, N.A.; Taouli, B. Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. AJR Am. J. Roentgenol. 2013, 201, 80–96. [Google Scholar] [CrossRef]
- Chaudhry, M.; McGinty, K.A.; Mervak, B.; Lerebours, R.; Li, C.; Shropshire, E.; Ronald, J.; Commander, L.; Hertel, J.; Luo, S.; et al. The LI-RADS Version 2018 MRI Treatment Response Algorithm: Evaluation of Ablated Hepatocellular Carcinoma. Radiology 2020, 294, 320–326. [Google Scholar] [CrossRef]
- Harmath, C.; Fung, A.; Aslam, A.; Kamath, A.; Lall, C.; Surabhi, V.; Borhani, A.A.; Mendiratta-Lala, M.; Do, R. LI-RADS radiation-based treatment response algorithm for HCC: What to know and how to use it. Abdom. Radiol. 2024. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Lyu, R.; Jia, K.; Xu, P.-J. LI-RADS version 2018 treatment response algorithm on extracellular contrast-enhanced MRI in patients treated with transarterial chemoembolization for hepatocellular carcinoma: Diagnostic performance and the added value of ancillary features. Abdom. Radiol. 2024, 49, 3045–3055. [Google Scholar] [CrossRef] [PubMed]
- Kielar, A.; Fowler, K.J.; Lewis, S.; Yaghmai, V.; Miller, F.H.; Yarmohammadi, H.; Kim, C.; Chernyak, V.; Yokoo, T.; Meyer, J.; et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom. Radiol. 2018, 43, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Schmidt, R.; Hamm, C.A.; Schobert, I.T.; He, Y.; Böning, G.; Jonczyk, M.; Hamm, B.; Gebauer, B.; Savic, L.J. Comparison of intrahepatic progression patterns of hepatocellular carcinoma and colorectal liver metastases following CT-guided high dose-rate brachytherapy. Ther. Adv. Med. Oncol. 2021, 13, 17588359211042304. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Wust, P.; Stohlmann, A.; Beck, A.; Cho, C.H.; Pech, M.; Felix, R. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: Phase I–II results of a novel technique. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1496–1505. [Google Scholar] [CrossRef]
- Mohnike, K.; Wieners, G.; Schwartz, F.; Seidensticker, M.; Pech, M.; Ruehl, R.; Ricke, J. Computed tomography-guided high-dose-rate brachytherapy in hepatocellular carcinoma: Safety, efficacy, and effect on survival. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 172–179. [Google Scholar] [CrossRef]
- Bretschneider, T.; Ricke, J.; Gebauer, B.; Streitparth, F. Image-guided high-dose-rate brachytherapy of malignancies in various inner organs—Technique, indications, and perspectives. J. Contemp. Brachyther. 2016, 8, 251–261. [Google Scholar] [CrossRef]
- Savic, L.J.; Chen, E.; Nezami, N.; Murali, N.; Hamm, C.A.; Wang, C.; Lin, M.; Schlachter, T.; Hong, K.; Georgiades, C.; et al. Conventional vs. Drug-Eluting Beads Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma-A Propensity Score Weighted Comparison of Efficacy and Safety. Cancers 2022, 14, 5847. [Google Scholar] [CrossRef]
- Lencioni, R.; Petruzzi, P.; Crocetti, L. Chemoembolization of hepatocellular carcinoma. Semin. Intervent Radiol. 2013, 30, 3–11. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ahmed, M.; Adam, A.; Arai, Y.; Arellano, R.; de Baère, T.; Bale, R.; Bellera, C.; Binkert, C.A.; Brace, C.L.; et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology 2021, 301, 533–540. [Google Scholar] [CrossRef]
- Tirkes, T.; Hollar, M.A.; Tann, M.; Kohli, M.D.; Akisik, F.; Sandrasegaran, K. Response criteria in oncologic imaging: Review of traditional and new criteria. Radiographics 2013, 33, 1323–1341. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Zhao, Y.; Duran, R.; Bai, W.; Sahu, S.; Wang, W.; Kabus, S.; Lin, M.; Han, G.; Geschwind, J.-F. Which Criteria Applied in Multi-Phasic CT Can Predict Early Tumor Response in Patients with Hepatocellular Carcinoma Treated Using Conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc. Intervent Radiol. 2018, 41, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Lin, M.; Pellerin, O.; Bhagat, N.; Rao, P.P.; Loffroy, R.; Ardon, R.; Mory, B.; Reyes, D.K.; Geschwind, J.-F. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: Feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J. Vasc. Interv. Radiol. 2012, 23, 1629–1637. [Google Scholar]
- Shropshire, E.L.; Chaudhry, M.; Miller, C.M.; Allen, B.C.; Bozdogan, E.; Cardona, D.M.; King, L.Y.; Janas, G.L.; Do, R.K.; Kim, C.Y.; et al. LI-RADS Treatment Response Algorithm: Performance and Diagnostic Accuracy. Radiology 2019, 292, 226–234. [Google Scholar] [CrossRef]
- Moussa, A.M.; Ziv, E. Radiogenomics in Interventional Oncology. Curr. Oncol. Rep. 2021, 23, 9. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Hong, H.; Kamath, S.; Schlegel, A.; Fujiki, M.; Hashimoto, K.; Aucejo, F. Tumor Mutational Burden from Circulating Tumor DNA Predicts Recurrence of Hepatocellular Carcinoma After Resection: An Emerging Biomarker for Surveillance. Ann. Surg. 2024, 280, 504–513. [Google Scholar] [CrossRef]
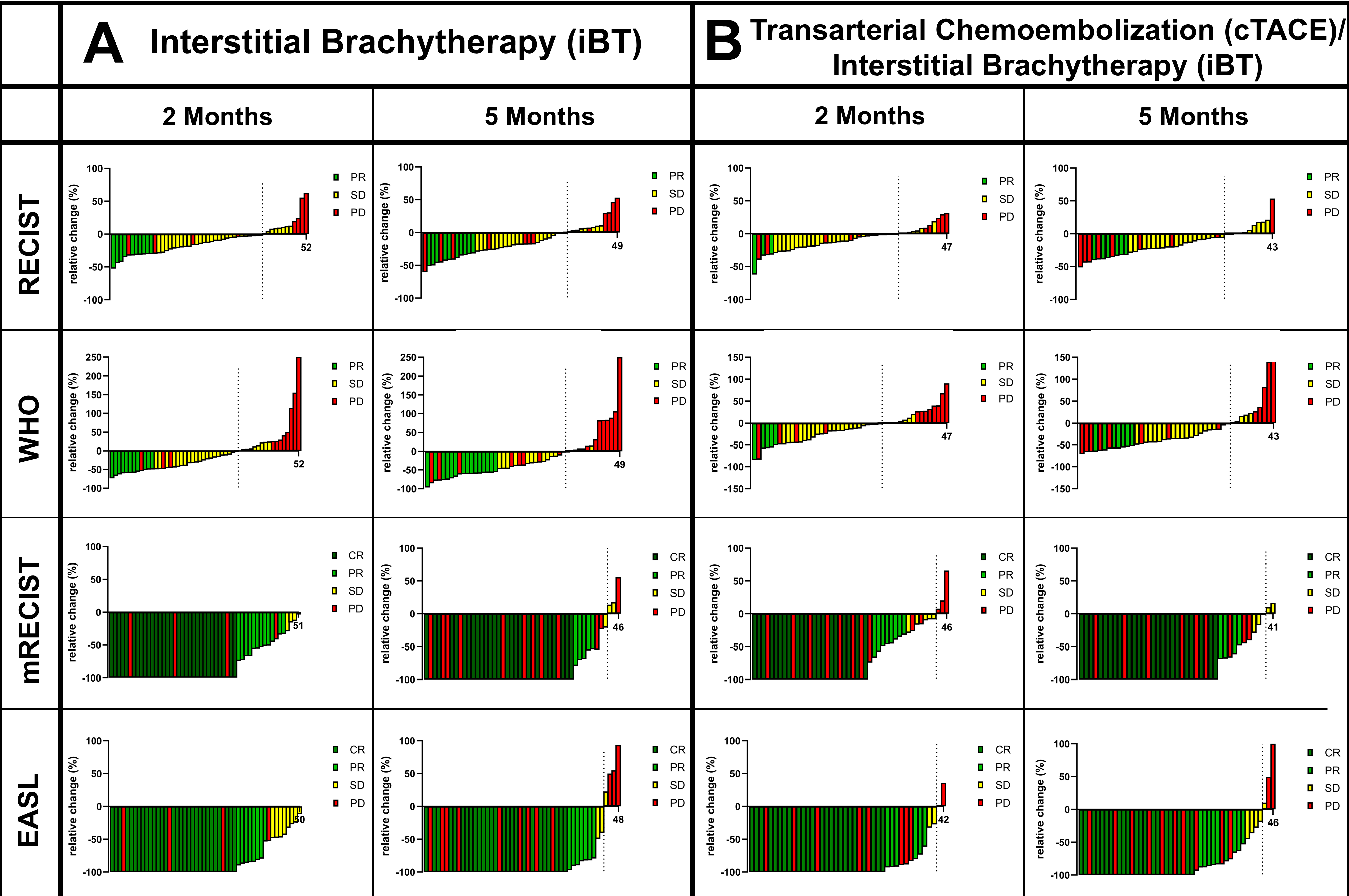

| Demographics | iBT Patients | cTACE/iBT Patients | p-Value |
|---|---|---|---|
| Patient characteristics | |||
| Number of patients | 48 | 47 | - |
| Age, mean ± SD | 70.6 ± 9.3 years | 70.3 ± 9.5 years | 0.84 |
| Male:Female, n | 38:10 | 36:11 | 0.81 |
| Tumor characteristics | |||
| Lesions, n | 89 | 70 | - |
| Sum of lesion diameter, mean ± SD | 26.5 ± 10.6 mm | 44.3 ± 20.4 mm | <0.01 |
| Unifocal:Multifocal, n | 41:19 | 38:13 | 0.53 |
| Completed treatment cycles, n | 60 | 51 | - |
| HCC diagnosis, n (biopsy vs. imaging) | 43:17 | 24:27 | - |
| HCC grading, n (%) | |||
| GX | 28 (65.1%) | 6 (25.0%) | - |
| G1 | 1 (2.3%) | 2 (8.3%) | - |
| G2 | 7 (16.3%) | 13 (54.2%) | - |
| G3 | 7 (16.3%) | 3 (12.5%) | - |
| Disease characteristics | |||
| Cirrhosis, n (%) | 41 (85.4%) | 43 (91.5%) | 0.52 |
| Etiology of cirrhosis, n (%) | |||
| Viral hepatitis (Hepatitis B/C) | 12 (29.3%) | 11 (23.2%) | 0.98 |
| Alcoholic steatohepatitis | 11 (26.8%) | 18 (41.9%) | 0.17 |
| Metabolic-dysfunction associated steatohepatitis | 13 (31.7%) | 14 (32.6%) | 0.99 |
| Others * | 5 (12.2%) | 0 (0.0%) | 0.02 |
| Child Pugh class, n (%) | |||
| A | 34 (82.9%) | 41 (97.7%) | 0.09 |
| B | 7 (17.1%) | 2 (2.3%) | 0.09 |
| Barcelona Clinic Liver Cancer stage, n (%) | |||
| A | 41 (85.4%) | 22 (46.8%) | <0.01 |
| B | 6 (12.5%) | 17 (36.2%) | 0.01 |
| C | 1 (2.1%) | 8 (17.0%) | 0.02 |
| Laboratory values of liver function, mean ± SD or median [IQR] | |||
| Alpha-fetoprotein (AFP) (U/mL) | 8.0 [5.4, 46.2] | 13.1 [5.2, 69.8] | 0.46 |
| Albumine (g/L) | 39.7 [35.3, 42.9] | 39.0 [37.3, 40.5] | 0.96 |
| Bilirubin (mg/dL) | 0.6 [0.4, 1.1] | 0.7 [0.5, 1.1] | 0.23 |
| ALBI dcore | −2.56 ± 0.37 | −2.56 ± 0.60 | 0.96 |
| AST/ALT tatio | 1.3 [1.0, 1.5] | 1.2 [1.1, 1.5] | 0.67 |
| γ-GT (U/L) | 119 [56, 218] | 152 [105, 240] | 0.24 |
| AP (U/L) | 3.4 [0.9, 5.6] | 4.4 [2.4, 6.7] | 0.73 |
| iBT | cTACE/iBT | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criterion | OS | PFS | PFS | PFS | TTP | TTP | TTP | OS | PFS | PFS | PFS | TTP | TTP | TTP | |
| LTP | IDR | LTP | IDR | LTP | IDR | LTP | IDR | ||||||||
| 2 Months | RECIST | 0.52 | 0.96 | 0.80 | 0.79 | 0.83 | 0.78 | 0.77 | 0.83 | 0.86 | 0.72 | 0.64 | 0.56 | 0.53 | 0.54 |
| WHO | 0.24 | 0.18 | 0.26 | 0.08 | 0.63 | 0.61 | 0.28 | 0.21 | 0.13 | 0.31 | 0.09 | 0.93 | 0.99 | 0.67 | |
| mRECIST | 0.52 | 0.15 | 0.33 | 0.98 | 0.65 | 0.20 | 0.32 | 0.99 | <0.01 | 0.9 | <0.01 | <0.01 | 0.77 | <0.01 | |
| EASL | 0.91 | 0.80 | 0.82 | 0.59 | 0.19 | 0.24 | 0.08 | 0.81 | <0.01 | 0.86 | <0.01 | <0.01 | 0.72 | <0.01 | |
| LI-TRA v2018 | 0.87 | 0.67 | 0.48 | 0.50 | 0.93 | 0.34 | 0.08 | 0.13 | 0.42 | 0.24 | 0.88 | 0.60 | 0.31 | 0.95 | |
| LI-TRA v2024 | 0.61 | 0.98 | 0.32 | 0.73 | 0.98 | 0.22 | 0.93 | 0.06 | 0.81 | 0.01 | 0.29 | 0.27 | 0.21 | 0.18 | |
| 5 Months | RECIST | 0.12 | 0.11 | 0.04 | 0.13 | 0.17 | 0.26 | 0.33 | 0.17 | 0.83 | 0.65 | 0.91 | 0.80 | 0.44 | 0.62 |
| WHO | 0.86 | 0.43 | 0.14 | 0.91 | 0.75 | 0.17 | 0.72 | 0.55 | 0.87 | 0.75 | 0.71 | 0.61 | 0.64 | 0.92 | |
| mRECIST | 0.90 | 0.43 | 0.91 | 0.50 | 0.06 | 0.61 | 0.04 | 0.06 | 0.07 | 0.07 | 0.11 | 0.01 | 0.62 | 0.02 | |
| EASL | 0.82 | 0.04 | 0.50 | 0.08 | <0.01 | 0.62 | <0.01 | 0.07 | 0.03 | 0.18 | <0.01 | 0.01 | 0.93 | <0.01 | |
| LI-TRA v2018 | 0.40 | 0.72 | 0.92 | 0.68 | 0.57 | 0.26 | 0.57 | 0.01 | 0.31 | 0.02 | 0.14 | 0.3 | 0.18 | 0.13 | |
| LI-TRA v2024 | 0.65 | 0.91 | 0.98 | 0.76 | 0.77 | 0.31 | 0.91 | 0.05 | 0.65 | 0.07 | 0.98 | 0.67 | 0.64 | 0.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, R.; Rueger, C.; Xu, H.; He, Y.; Yilmaz, E.Y.; Heidemann, L.; Sulejmani, O.; Liu, Y.; Noack, L.; Hesse, F.; et al. Comparing the Prognostic Value of Quantitative Response Assessment Tools and LIRADS Treatment Response Algorithm in Patients with Hepatocellular Carcinoma Following Interstitial High-Dose-Rate Brachytherapy and Conventional Transarterial Chemoembolization. Cancers 2025, 17, 1275. https://doi.org/10.3390/cancers17081275
Schmidt R, Rueger C, Xu H, He Y, Yilmaz EY, Heidemann L, Sulejmani O, Liu Y, Noack L, Hesse F, et al. Comparing the Prognostic Value of Quantitative Response Assessment Tools and LIRADS Treatment Response Algorithm in Patients with Hepatocellular Carcinoma Following Interstitial High-Dose-Rate Brachytherapy and Conventional Transarterial Chemoembolization. Cancers. 2025; 17(8):1275. https://doi.org/10.3390/cancers17081275
Chicago/Turabian StyleSchmidt, Robin, Christopher Rueger, Han Xu, Yubei He, Emine Yaren Yilmaz, Luisa Heidemann, Ornela Sulejmani, Yu Liu, Lasse Noack, Friederike Hesse, and et al. 2025. "Comparing the Prognostic Value of Quantitative Response Assessment Tools and LIRADS Treatment Response Algorithm in Patients with Hepatocellular Carcinoma Following Interstitial High-Dose-Rate Brachytherapy and Conventional Transarterial Chemoembolization" Cancers 17, no. 8: 1275. https://doi.org/10.3390/cancers17081275
APA StyleSchmidt, R., Rueger, C., Xu, H., He, Y., Yilmaz, E. Y., Heidemann, L., Sulejmani, O., Liu, Y., Noack, L., Hesse, F., Ruppel, R., Abosabie, S. A., Hamm, C. A., Penzkofer, T., Gebauer, B., & Savic, L. J. (2025). Comparing the Prognostic Value of Quantitative Response Assessment Tools and LIRADS Treatment Response Algorithm in Patients with Hepatocellular Carcinoma Following Interstitial High-Dose-Rate Brachytherapy and Conventional Transarterial Chemoembolization. Cancers, 17(8), 1275. https://doi.org/10.3390/cancers17081275







