Synergy of Body Composition, Exercise Oncology, and Pharmacokinetics: A Narrative Review of Personalizing Paclitaxel Treatment for Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Paclitaxel in the Treatment of Breast Cancer
2.1. Toxicities
2.2. PTX Dose Intensity
2.3. Challenges Regarding Body Surface Area Dosing of PTX
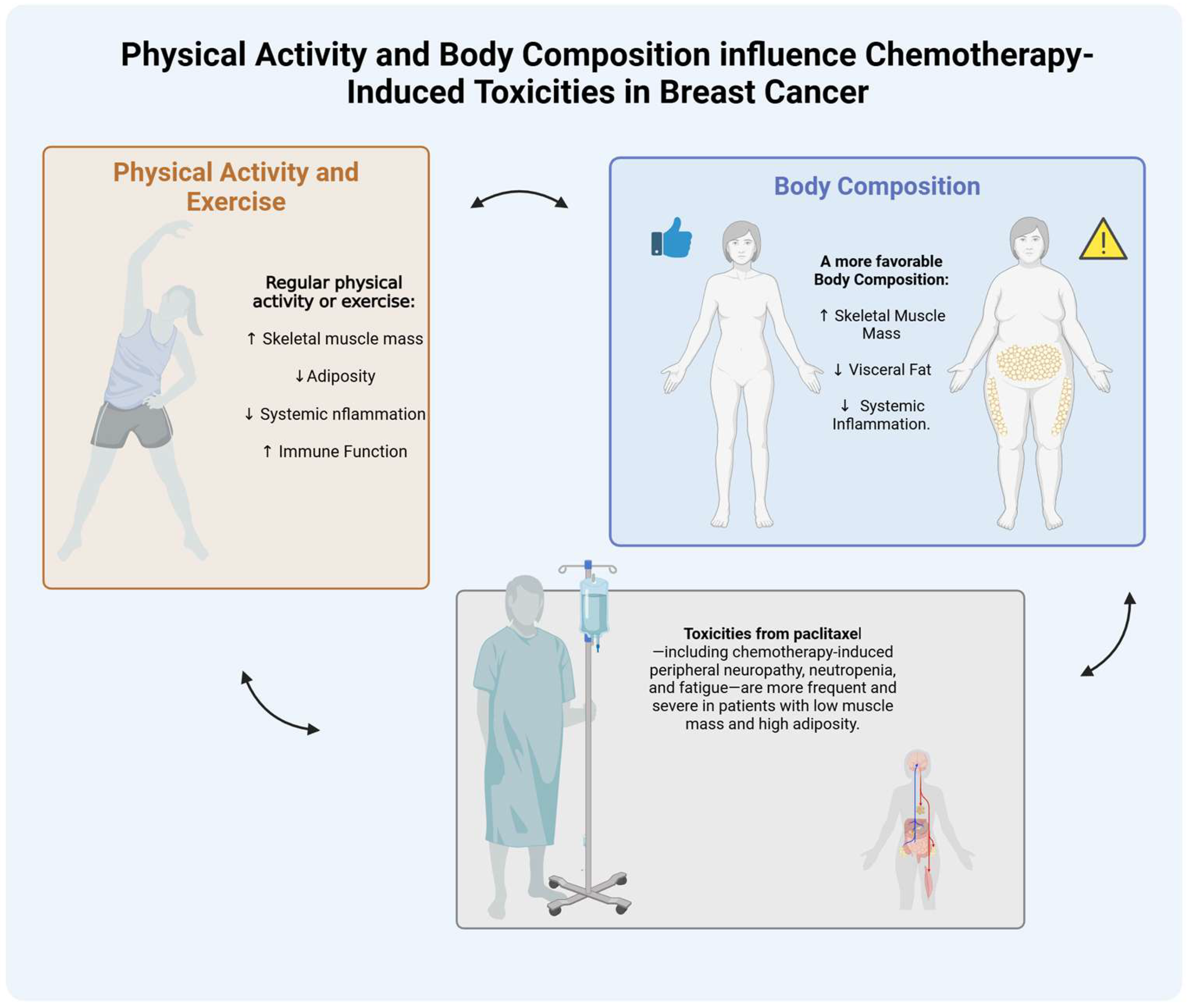
3. Role of Body Composition and Physical Activity in Breast Cancer Treatment
3.1. Body Composition and Chemotherapy Dosing
3.2. Body Composition, Paclitaxel Pharmacokinetics and Toxicities
4. Role of Physical Activity and Exercise in Breast Cancer Treatment
5. Exercise-Mediated Improvements in Body Composition and Their Impact on Paclitaxel Tolerance
6. Pharmacokinetics in Breast Cancer Treatment
7. Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [PubMed]
- World Health Organization Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 September 2024).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [PubMed]
- Pondé, N.F.; Zardavas, D.; Piccart, M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 2019, 16, 27–44. [Google Scholar]
- Gradishar, W. Taxanes for the treatment of metastatic breast cancer. Breast Cancer Basic. Clin. Res. 2012, 6, 159–171. [Google Scholar]
- Al-Mahayri, Z.N.; AlAhmad, M.M.; Ali, B.R. Current opinion on the pharmacogenomics of paclitaxel-induced toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 785–801. [Google Scholar] [PubMed]
- Klein, I.; Lehmann, H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar]
- Price, K.S.; Castells, M.C. Taxol reactions. In Allergy and Asthma Proceedings; OceanSide Publications: Providence, RI, USA, 2002. [Google Scholar]
- Weiss, R.B.; Donehower, R.; Wiernik, P.; Ohnuma, T.; Gralla, R.; Trump, D.; Baker, J., Jr.; Van Echo, D.; Von Hoff, D.; Leyland-Jones, B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar]
- Rowinsky, E.K.; Donehower, R.C. Paclitaxel (taxol). N. Eng. J. Med. 1995, 332, 1004–1014. [Google Scholar]
- Mielke, S.; Sparreboom, A.; Mross, K. Peripheral neuropathy: A persisting challenge in paclitaxel-based regimes. Eur. J. Cancer 2006, 42, 24–30. [Google Scholar] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Apfel, S.C.; Dutcher, J.P.; Rosenberg, R.; Kaplan, J.; Berger, A.; Einzig, A.; Wiernik, P.; Schaumburg, H. Taxol produces a predominantly sensory neuropathy. Neurology 1989, 39, 368. [Google Scholar]
- Gutiérrez-Gutiérrez, G.; Sereno, M.; Miralles, A.; Casado-Sáenz, E.; Gutiérrez-Rivas, E. Chemotherapy-induced peripheral neuropathy: Clinical features, diagnosis, prevention and treatment strategies. Clin. Transl. Oncol. 2010, 12, 81–91. [Google Scholar]
- van den Berg, M.M.; Kok, D.E.; Posthuma, L.; Kamps, L.; Kelfkens, C.S.; Buist, N.; Geenen, M.; Haringhuizen, A.; Heijns, J.B.; van Lieshout, R.H. Body composition is associated with risk of toxicity-induced modifications of treatment in women with stage I–IIIB breast cancer receiving chemotherapy. Breast Cancer Res. Treat. 2019, 173, 475–481. [Google Scholar] [CrossRef]
- Jivani, A.; Shinde, R.K. A Comprehensive Review of Taxane Treatment in Breast Cancer: Clinical Perspectives and Toxicity Profiles. Cureus 2024, 16, e59266. [Google Scholar]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncol. 2021, 26, e1609–e1618. [Google Scholar]
- Denduluri, N.; Patt, D.A.; Wang, Y.; Bhor, M.; Li, X.; Favret, A.M.; Morrow, P.K.; Barron, R.L.; Asmar, L.; Saravanan, S.; et al. Dose Delays, Dose Reductions, and Relative Dose Intensity in Patients With Cancer Who Received Adjuvant or Neoadjuvant Chemotherapy in Community Oncology Practices. J. Natl. Compr. Cancer Netw. 2015, 13, 1383–1393. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Reiner, M.; Morrow, P.K.; Watson, H.; Crawford, J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit. Rev. Oncol. Hematol. 2015, 93, 203–210. [Google Scholar] [CrossRef]
- Denduluri, N.; Lyman, G.H.; Wang, Y.; Morrow, P.K.; Barron, R.; Patt, D.; Bhowmik, D.; Li, X.; Bhor, M.; Fox, P.; et al. Chemotherapy Dose Intensity and Overall Survival Among Patients with Advanced Breast or Ovarian Cancer. Clin. Breast Cancer 2018, 18, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Skacel, T.; Nekljudova, V.; Luck, H.J.; Schwenkglenks, M.; Brodowicz, T.; Zielinski, C.; von Minckwitz, G. Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer—A pooled analysis. BMC Cancer 2011, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Gurney, H. How to calculate the dose of chemotherapy. Br. J. Cancer 2002, 86, 1297–1302. [Google Scholar] [CrossRef]
- Hertz, D.L.; Joerger, M.; Bang, Y.-J.; Mathijssen, R.H.; Zhou, C.; Zhang, L.; Gandara, D.; Stahl, M.; Monk, B.J.; Jaehde, U.; et al. Paclitaxel therapeutic drug monitoring—International association of therapeutic drug monitoring and clinical toxicology recommendations. Eur. J. Cancer 2024, 202, 114024. [Google Scholar] [CrossRef]
- Hertz, D.L.; Chen, L.; Henry, N.L.; Griggs, J.J.; Hayes, D.F.; Derstine, B.A.; Su, G.L.; Wang, S.C.; Pai, M.P. Muscle mass affects paclitaxel systemic exposure and may inform personalized paclitaxel dosing. Br. J. Clin. Pharmacol. 2022, 88, 3222–3229. [Google Scholar] [CrossRef]
- Hertz, D.L.; Kidwell, K.M.; Vangipuram, K.; Li, F.; Pai, M.P.; Burness, M.; Griggs, J.J.; Schott, A.F.; Van Poznak, C.; Hayes, D.F. Paclitaxel plasma concentration after the first infusion predicts treatment-limiting peripheral neuropathy. Clin. Cancer Res. 2018, 24, 3602–3610. [Google Scholar] [CrossRef]
- Mielke, S.; Sparreboom, A.; Steinberg, S.M.; Gelderblom, H.; Unger, C.; Behringer, D.; Mross, K. Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin. Cancer Res. 2005, 11, 4843–4850. [Google Scholar] [CrossRef]
- Faisal, W.; Tang, H.-M.; Tiley, S.; Kukard, C. Not all body surface area formulas are the same, but does it matter? J. Glob. Oncol. 2016, 2, 436. [Google Scholar] [CrossRef] [PubMed]
- Redlarski, G.; Palkowski, A.; Krawczuk, M. Body surface area formulae: An alarming ambiguity. Sci. Rep. 2016, 6, 27966. [Google Scholar] [CrossRef]
- Purcell, S.A.; Kok, D.E.; Ketterl, T.; Garcia, M.B.; Joffe, L.; Brown, J.C.; Dieli-Conwright, C.M.; Williams, G.R. Pharmacokinetics of cancer therapeutics and energy balance: The role of diet intake, energy expenditure, and body composition. JNCI Monogr. 2023, 2023, 3–11. [Google Scholar] [CrossRef]
- Miller, A.A.; Rosner, G.L.; Egorin, M.J.; Hollis, D.; Lichtman, S.M.; Ratain, M.J. Prospective Evaluation of Body Surface Area as a Determinant of Paclitaxel Pharmacokinetics and Pharmacodynamics in Women with Solid Tumors: Cancer and Leukemia Group B Study 9763. Clin. Cancer Res. 2004, 10, 8325–8331. [Google Scholar] [CrossRef] [PubMed]
- Durkin, K.; Heetun, A.; Ewings, S.; Munday, R.; Wootton, S.A.; Turner, L.; Copson, E.R.; Cutress, R.I.; Group, C.-S. Body composition and chemotherapy toxicity in women with early breast cancer (CANDO-3): Protocol for an observational cohort study. BMJ Open 2022, 12, e054412. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.J.; Sawyer, M.B. A review of body composition and pharmacokinetics in oncology. Expert. Rev. Clin. Pharmacol. 2017, 10, 947–956. [Google Scholar] [CrossRef]
- Bruno, K.d.A.; Sobreira da Silva, M.J.; Chaves, G.V. Association of body composition with toxicity to first-line chemotherapy and three-year survival in women with ovarian adenocarcinoma. Acta Oncol. 2021, 60, 1611–1620. [Google Scholar] [CrossRef]
- Ryan, A.M.; Prado, C.M.; Sullivan, E.S.; Power, D.G.; Daly, L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019, 67–68, 110539. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.J.; Sawyer, M.B. Interactions of lean soft-tissue and chemotherapy toxicities in patients receiving anti-cancer treatments. Cancer Chemother. Pharmacol. 2018, 82, 1–29. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.; Korinek, J.; Allison, T.G.; Batsis, J.; Sert-Kuniyoshi, F.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Barret, M.; Antoun, S.; Dalban, C.; Malka, D.; Mansourbakht, T.; Zaanan, A.; Latko, E.; Taieb, J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr. Cancer 2014, 66, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Huillard, O.; Mir, O.; Peyromaure, M.; Tlemsani, C.; Giroux, J.; Boudou-Rouquette, P.; Ropert, S.; Delongchamps, N.B.; Zerbib, M.; Goldwasser, F.; et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br. J. Cancer 2013, 108, 1034–1041. [Google Scholar] [CrossRef]
- Tan, B.H.L.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J.; Catton, J.A. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur. J. Surg. Oncol. 2015, 41, 333–338. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar]
- Aleixo, G.; Shachar, S.; Nyrop, K.; Muss, H.; Malpica, L.; Williams, G. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar]
- Cousin, S.; Hollebecque, A.; Koscielny, S.; Mir, O.; Varga, A.; Baracos, V.; Soria, J.; Antoun, S. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Investig. New Drugs 2014, 32, 382–387. [Google Scholar]
- Jung, H.-W.; Kim, J.W.; Kim, J.-Y.; Kim, S.-W.; Yang, H.K.; Lee, J.W.; Lee, K.-W.; Kim, D.-W.; Kang, S.-B.; Kim, K.-i. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support. Care Cancer 2015, 23, 687–694. [Google Scholar]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil–based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar]
- Ali, R.; Baracos, V.E.; Sawyer, M.B.; Bianchi, L.; Roberts, S.; Assenat, E.; Mollevi, C.; Senesse, P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016, 5, 607–616. [Google Scholar]
- Anandavadivelan, P.; Brismar, T.B.; Nilsson, M.; Johar, A.M.; Martin, L. Sarcopenic obesity: A probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin. Nutr. 2016, 35, 724–730. [Google Scholar]
- Ott, C.D.; Twiss, J.J.; Waltman, N.L.; Gross, G.J.; Lindsey, A.M. Challenges of recruitment of breast cancer survivors to a randomized clinical trial for osteoporosis prevention. Cancer Nurs. 2006, 29, 21–31. [Google Scholar]
- MACVICAR, M.G.; Winningham, M.L.; NICKEL, J.L. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs. Res. 1989, 38, 348–353. [Google Scholar] [PubMed]
- Demark-Wahnefried, W.; Peterson, B.L.; Winer, E.P.; Marks, L.; Aziz, N.; Marcom, P.K.; Blackwell, K.; Rimer, B.K. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J. Clin. Oncol. 2001, 19, 2381–2389. [Google Scholar]
- Loprinzi, P.D.; Cardinal, B.J. Effects of physical activity on common side effects of breast cancer treatment. Breast Cancer 2012, 19, 4–10. [Google Scholar] [PubMed]
- Kazemi-Bajestani, S.M.R.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Brown, J.C.; Cespedes Feliciano, E.M.; Caan, B.J. The Evolution of Body Composition in Oncology—Epidemiology, Clinical Trials, and the Future of Patient Care: Facts and Numbers; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 1200–1208. [Google Scholar]
- Attanasio, S.; Forte, S.M.; Restante, G.; Gabelloni, M.; Guglielmi, G.; Neri, E. Artificial intelligence, radiomics and other horizons in body composition assessment. Quant. Imagining Med. Surg. 2020, 10, 1650–1660. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA A Cancer J. Clin. 2019, 69, 127–157. [Google Scholar]
- Desmedt, C.; Fornili, M.; Clatot, F.; Demicheli, R.; Bortoli, D.D.; Leo, A.D.; Viale, G.; Azambuja, E.d.; Crown, J.; Francis, P.A.; et al. Differential Benefit of Adjuvant Docetaxel-Based Chemotherapy in Patients With Early Breast Cancer According to Baseline Body Mass Index. J. Clin. Oncol. 2020, 38, 2883–2891. [Google Scholar] [PubMed]
- Hendrikx, J.; Haanen, J.; Voest, E.E.; Schellens, J.H.M.; Huitema, A.D.R.; Beijnen, J.H. Fixed Dosing of Monoclonal Antibodies in Oncology. Oncologist 2017, 22, 1212–1221. [Google Scholar]
- Prado, C.M.; Lima, I.S.; Baracos, V.E.; Bies, R.R.; McCargar, L.J.; Reiman, T.; Mackey, J.R.; Kuzma, M.; Damaraju, V.L.; Sawyer, M.B. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother. Pharmacol. 2011, 67, 93–101. [Google Scholar]
- Williams, G.R.; Al-Obaidi, M.; Rower, J.; Harmon, C.; Dai, C.; Acosta, E.; Giri, S.; Zamboni, W.; Lucas, A.T.; Shachar, S.S. Does Oxaliplatin Pharmacokinetics (PKs) Explain Associations Between Body Composition and Chemotherapy Toxicity Risk in Older Adults with Gastrointestinal (GI) Cancers? Wolters Kluwer Health: Philadelphia, PA, USA, 2021. [Google Scholar]
- Wong, A.; Seng, K.; Ong, E.; Wang, L.; Oscar, H.; Cordero, M.; Copones, R.; Fan, L.; Tan, S.; Goh, B. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res. Treat. 2014, 144, 143–152. [Google Scholar]
- Massicotte, M.H.; Borget, I.; Broutin, S.; Baracos, V.E.; Leboulleux, S.; Baudin, E.; Paci, A.; Deroussent, A.; Schlumberger, M.; Antoun, S. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: Results from a placebo-controlled study. J. Clin. Endocrinol. Metab. 2013, 98, 2401–2408. [Google Scholar] [CrossRef]
- Smorenburg, C.H.; ten Tije, A.J.; Verweij, J.; Bontenbal, M.; Mross, K.; van Zomeren, D.M.; Seynaeve, C.; Sparreboom, A. Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur. J. Cancer 2003, 39, 196–202. [Google Scholar] [CrossRef]
- Crombag, M.B.S.; de Vries Schultink, A.H.M.; Koolen, S.L.W.; Wijngaard, S.; Joerger, M.; Schellens, J.H.M.; Dorlo, T.P.C.; van Erp, N.P.; Mathijssen, R.H.J.; Beijnen, J.H.; et al. Impact of Older Age on the Exposure of Paclitaxel: A Population Pharmacokinetic Study. Pharm. Res. 2019, 36, 33. [Google Scholar]
- Barginear, M.; Dueck, A.C.; Allred, J.B.; Bunnell, C.; Cohen, H.J.; Freedman, R.A.; Hurria, A.; Kimmick, G.; Le-Rademacher, J.G.; Lichtman, S.; et al. Age and the Risk of Paclitaxel-Induced Neuropathy in Women with Early-Stage Breast Cancer (Alliance A151411): Results from 1,881 Patients from Cancer and Leukemia Group B (CALGB) 40101. Oncologist 2019, 24, 617–623. [Google Scholar]
- Sparreboom, A.; Wolff, A.C.; Mathijssen, R.H.; Chatelut, E.; Rowinsky, E.K.; Verweij, J.; Baker, S.D. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J. Clin. Oncol. 2007, 25, 4707–4713. [Google Scholar] [PubMed]
- Mosli, R.H.; Mosli, H.H. Obesity and morbid obesity associated with higher odds of hypoalbuminemia in adults without liver disease or renal failure. Diabetes Metab. Syndr. Obes. 2017, 10, 467–472. [Google Scholar]
- Kjaergaard, A.D.; Teumer, A.; Witte, D.R.; Stanzick, K.J.; Winkler, T.W.; Burgess, S.; Ellervik, C. Obesity and Kidney Function: A Two-Sample Mendelian Randomization Study. Clin. Chem. 2022, 68, 461–472. [Google Scholar] [PubMed]
- Zamboni, W.C.; Charlab, R.; Burckart, G.J.; Stewart, C.F. Effect of Obesity on the Pharmacokinetics and Pharmacodynamics of Anticancer Agents. J. Clin. Pharmacol. 2023, 63 (Suppl. 2), S85–S102. [Google Scholar]
- Chmielewski, N.N.; Limoli, C.L. Sex Differences in Taxane Toxicities. Cancers 2022, 14, 3325. [Google Scholar] [CrossRef]
- Mizrahi, D.; Lai, J.K.L.; Wareing, H.; Ren, Y.; Li, T.; Swain, C.T.; Smith, D.P.; Adams, D.; Martiniuk, A.; David, M. Effect of exercise interventions on hospital length of stay and admissions during cancer treatment: A systematic review and meta-analysis. Br. J. Sports Med. 2024, 58, 97–109. [Google Scholar]
- Watson, G.; Coyne, Z.; Houlihan, E.; Leonard, G. Exercise oncology: An emerging discipline in the cancer care continuum. Postgrad. Med. 2022, 134, 26–36. [Google Scholar]
- Cannioto, R.A.; Dighe, S.; Mahoney, M.C.; Moysich, K.B.; Sen, A.; Hulme, K.; McCann, S.E.; Ambrosone, C.B. Habitual recreational physical activity is associated with significantly improved survival in cancer patients: Evidence from the Roswell Park Data Bank and BioRepository. Cancer Causes Control. 2019, 30, 1–12. [Google Scholar] [PubMed]
- Kim, J.; Choi, W.J.; Jeong, S.H. The effects of physical activity on breast cancer survivors after diagnosis. J. Cancer Prev. 2013, 18, 193. [Google Scholar]
- Cannioto, R.A.; Hutson, A.; Dighe, S.; McCann, W.; McCann, S.E.; Zirpoli, G.R.; Barlow, W.; Kelly, K.M.; DeNysschen, C.A.; Hershman, D.L. Physical activity before, during, and after chemotherapy for high-risk breast cancer: Relationships with survival. JNCI J. Natl. Cancer Inst. 2021, 113, 54–63. [Google Scholar]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [PubMed]
- Wirtz, P.; Baumann, F.T. Physical activity, exercise and breast cancer-what is the evidence for rehabilitation, aftercare, and survival a review. Breast Care 2018, 13, 92–100. [Google Scholar]
- Spence, R.R.; Heesch, K.C.; Brown, W.J. Exercise and cancer rehabilitation: A systematic review. Cancer Treat. Rev. 2010, 36, 185–194. [Google Scholar] [PubMed]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar]
- Gauchez, L.; Boyle, S.L.L.; Eekman, S.S.; Harnie, S.; Decoster, L.; Van Ginderdeuren, F.; De Nys, L.; Adriaenssens, N. Recommended Physiotherapy Modalities for Oncology Patients with Palliative Needs and Its Influence on Patient-Reported Outcome Measures: A Systematic Review. Cancers 2024, 16, 3371. [Google Scholar] [CrossRef]
- Adriaenssens, N.; Strimpakos, N.; Rotem, N.; Sheill, G.; Cannone, M.; Gigli, L.; Tiesnese, L.; Descloux, A.; MacKenzie, A.; Pérez Navarro, M.; et al. The Role of Physiotherapy in Cancer Care in the Europe Region: A Position Paper of the Cancer Working Group of Europe Region World Physiotherapy. J. Cancer Rehabil. 2023, 6, 70–79. [Google Scholar]
- Mock, V.; Burke, M.B.; Sheehan, P.; Creaton, E.M.; Winningham, M.L.; McKenney-Tedder, S.; Schwager, L.P.; Liebman, M. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncol. Nurs. Forum 1994, 21, 899–907. [Google Scholar]
- Winningham, M.L.; MacVicar, M.G.; Bondoc, M.; Anderson, J.I.; Minton, J.P. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol. Nurs. Forum 1989, 16, 683–689. [Google Scholar] [PubMed]
- Winningham, M.L.; MacVicar, M.G.; Burke, C.A. Exercise for Cancer Patients: Guidelines and Precautions. Phys. Sport. 1986, 14, 125–134. [Google Scholar]
- Brown, J.K.; Byers, T.; Doyle, C.; Coumeya, K.S.; Demark-Wahnefried, W.; Kushi, L.H.; McTieman, A.; Rock, C.L.; Aziz, N.; Bloch, A.S.; et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2003, 53, 268–291. [Google Scholar] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.H.; Lane, K.; et al. Effects of Aerobic and Resistance Exercise in Breast Cancer Patients Receiving Adjuvant Chemotherapy: A Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar]
- Sweegers, M.G.; van Doormaal, M.C.M.; Conijn, D.; Stuiver, M.M. KNGF Guideline on Oncology. Available online: https://www.kennisplatformfysiotherapie.nl/app/uploads/sites/2/2024/10/kngf-guideline-on-oncology.pdf (accessed on 2 April 2025).
- Strain, T.; Strain, T.; Flaxman, S.; Flaxman, S.; Guthold, R.; Guthold, R.; Semenova, E.; Semenova, E.; Cowan, M.; Cowan, M.; et al. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: A pooled analysis of 507 population-based surveys with 5·7 million participants. Lancet Glob. Health 2024, 12, e1232–e1243. [Google Scholar]
- Hanson, E.D.; Sakkal, S.; Evans, W.S.; Violet, J.A.; Battaglini, C.L.; McConell, G.K.; Hayes, A. Altered stress hormone response following acute exercise during prostate cancer treatment. Scand. J. Med. Sci. Sports 2018, 28, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Schauer, T.; Mazzoni, A.S.; Henriksson, A.; Demmelmaier, I.; Berntsen, S.; Raastad, T.; Nordin, K.; Pedersen, B.K.; Christensen, J.F. Exercise intensity and markers of inflammation during and after (neo-) adjuvant cancer treatment. Endocr. Relat. Cancer 2021, 28, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Lillelund, C.; Midtgaard, J.; Andersen, C.; Pedersen, B.K.; Christensen, J.F.; Hojman, P. Exercise regulates breast cancer cell viability: Systemic training adaptations versus acute exercise responses. Breast Cancer Res. Treat. 2016, 159, 469–479. [Google Scholar] [CrossRef]
- Tanay, M.A.L.; Armes, J.; Moss-Morris, R.; Rafferty, A.M.; Robert, G. A systematic review of behavioural and exercise interventions for the prevention and management of chemotherapy-induced peripheral neuropathy symptoms. J. Cancer Surviv. 2023, 17, 254–277. [Google Scholar] [CrossRef] [PubMed]
- Streckmann, F.; Zopf, E.M.; Lehmann, H.C.; May, K.; Rizza, J.; Zimmer, P.; Gollhofer, A.; Bloch, W.; Baumann, F.T. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med. 2014, 44, 1289–1304. [Google Scholar]
- McLaughlin, M.; Jacobs, I. Exercise Is Medicine, But Does It Interfere With Medicine? Exerc. Sport Sci. Rev. 2017, 45, 127–135. [Google Scholar] [CrossRef]
- Persky, A.M.; Eddington, N.D.; Derendorf, H. A review of the effects of chronic exercise and physical fitness level on resting pharmacokinetics. Int. J. Clin. Pharmacol. Ther. 2003, 41, 504–516. [Google Scholar] [CrossRef]
- Sasso, J.P.; Eves, N.D.; Christensen, J.F.; Koelwyn, G.J.; Scott, J.; Jones, L.W. A framework for prescription in exercise-oncology research. J. Cachexia Sarcopenia Muscle 2015, 6, 115–124. [Google Scholar]
- Jacobsen, P.B.; Hann, D.M.; Azzarello, L.M.; Horton, J.; Balducci, L.; Lyman, G.H. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. J. Pain. Symptom Manag. 1999, 18, 233–242. [Google Scholar] [CrossRef]
- Finne, E.; Glausch, M.; Exner, A.-K.; Sauzet, O.; Stölzel, F.; Seidel, N. Behavior change techniques for increasing physical activity in cancer survivors: A systematic review and meta-analysis of randomized controlled trials. Cancer Manag. Res. 2018, 10, 5125–5143. [Google Scholar] [CrossRef]
- Hailey, V.; Rojas-Garcia, A.; Kassianos, A.P. A systematic review of behaviour change techniques used in interventions to increase physical activity among breast cancer survivors. Breast Cancer 2022, 29, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Stacey, F.G.; James, E.L.; Chapman, K.; Courneya, K.S.; Lubans, D.R. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J. Cancer Surviv. 2015, 9, 305–338. [Google Scholar]
- Pudkasam, S.; Polman, R.; Pitcher, M.; Fisher, M.; Chinlumprasert, N.; Stojanovska, L.; Apostolopoulos, V. Physical activity and breast cancer survivors: Importance of adherence, motivational interviewing and psychological health. Maturitas 2018, 116, 66–72. [Google Scholar]
- Seven, M.; Reid, A.; Abban, S.; Madziar, C.; Faro, J.M. Motivational interviewing interventions aiming to improve health behaviors among cancer survivors: A systematic scoping review. J. Cancer Surviv. 2023, 17, 795–804. [Google Scholar] [PubMed]
- Huy, C.; Schmidt, M.E.; Vrieling, A.; Chang-Claude, J.; Steindorf, K. Physical activity in a German breast cancer patient cohort: One-year trends and characteristics associated with change in activity level. Eur. J. Cancer 2012, 48, 297–304. [Google Scholar]
- Lucas, A.R.; Levine, B.J.; Avis, N.E. Posttreatment trajectories of physical activity in breast cancer survivors. Cancer 2017, 123, 2773–2780. [Google Scholar] [PubMed]
- Godinho-Mota, J.C.M.; Mota, J.F.; Goncalves, L.V.; Soares, L.R.; Schincaglia, R.M.; Prado, C.M.; Martins, K.A.; Freitas-Junior, R. Chemotherapy negatively impacts body composition, physical function and metabolic profile in patients with breast cancer. Clin. Nutr. 2021, 40, 3421–3428. [Google Scholar]
- Jung, G.H.; Kim, J.H.; Chung, M.S. Changes in weight, body composition, and physical activity among patients with breast cancer under adjuvant chemotherapy. Eur. J. Oncol. Nurs. 2020, 44, 101680. [Google Scholar]
- Visovsky, C. Muscle strength, body composition, and physical activity in women receiving chemotherapy for breast cancer. Integr. Cancer Ther. 2006, 5, 183–191. [Google Scholar]
- Li, X.; Wang, J.; Zhang, J.; Zhang, N.; Wu, C.; Geng, Z.; Zhou, J.; Dong, L. The Effect of Exercise on Weight and Body Composition of Breast Cancer Patients Undergoing Chemotherapy: A Systematic Review. Cancer Nurs. 2023, 47, 207–220. [Google Scholar]
- Aires, I.; Duarte, J.A.; Vitorino, R.; Moreira-Goncalves, D.; Oliveira, P.; Ferreira, R. Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy. Int. J. Mol. Sci. 2024, 25, 7533. [Google Scholar] [CrossRef] [PubMed]
- Kudiarasu, C.; Lopez, P.; Galvao, D.A.; Newton, R.U.; Taaffe, D.R.; Mansell, L.; Fleay, B.; Saunders, C.; Fox-Harding, C.; Singh, F. What are the most effective exercise, physical activity and dietary interventions to improve body composition in women diagnosed with or at high-risk of breast cancer? A systematic review and network meta-analysis. Cancer 2023, 129, 3697–3712. [Google Scholar] [PubMed]
- Wopat, H.; Harrod, T.; Brem, R.F.; Kaltman, R.; Anderson, K.; Robien, K. Body composition and chemotherapy toxicity among women treated for breast cancer: A systematic review. J. Cancer Surviv. 2024, 18, 1356–1369. [Google Scholar] [CrossRef]
- Poltronieri, T.S.; Persico, R.S.; Falcetta, F.S.; Viana, L.V. Changes in Body Adiposity in Women Undergoing Breast Cancer Treatment: A Scoping Review. Nutr. Cancer 2022, 74, 3431–3445. [Google Scholar] [CrossRef] [PubMed]
- Barnes, O.; Wilson, R.L.; Gonzalo-Encabo, P.; Kang, D.W.; Christopher, C.N.; Bentley, T.; Dieli-Conwright, C.M. The Effect of Exercise and Nutritional Interventions on Body Composition in Patients with Advanced or Metastatic Cancer: A Systematic Review. Nutrients 2022, 14, 2110. [Google Scholar] [CrossRef]
- Bland, K.A.; Kouw, I.W.K.; van Loon, L.J.C.; Zopf, E.M.; Fairman, C.M. Exercise-Based Interventions to Counteract Skeletal Muscle Mass Loss in People with Cancer: Can We Overcome the Odds? Sports Med. 2022, 52, 1009–1027. [Google Scholar]
- Gerland, L.; Baumann, F.T.; Niels, T. Resistance Exercise for Breast Cancer Patients? Evidence from the Last Decade. Breast Care 2021, 16, 657–663. [Google Scholar] [CrossRef]
- Altundag, K. Correlation between exercise and skeletal muscle index in early breast cancer patients: Is it worth mentioning? J. BUON 2020, 25, 1268. [Google Scholar]
- An, K.Y.; Morielli, A.R.; Kang, D.W.; Friedenreich, C.M.; McKenzie, D.C.; Gelmon, K.; Mackey, J.R.; Reid, R.D.; Courneya, K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J. Cancer 2020, 146, 150–160. [Google Scholar] [CrossRef]
- Rosenberg, J.; Hyde, P.N.; Yancy, W.S., Jr.; Ford, K.M.; Champ, C.E. Quantity of Resistance Exercise for Breast Cancer Patients: Does the Dose Match the Objective? J. Strength. Cond. Res. 2021, 35, 1467–1476. [Google Scholar]
- Mijwel, S.; Jervaeus, A.; Bolam, K.A.; Norrbom, J.; Bergh, J.; Rundqvist, H.; Wengstrom, Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J. Cancer Surviv. 2019, 13, 244–256. [Google Scholar] [PubMed]
- Winters-Stone, K.M.; Torgrimson-Ojerio, B.; Dieckmann, N.F.; Stoyles, S.; Mitri, Z.; Luoh, S.W. A randomized-controlled trial comparing supervised aerobic training to resistance training followed by unsupervised exercise on physical functioning in older breast cancer survivors. J. Geriatr. Oncol. 2022, 13, 152–160. [Google Scholar]
- Feng, Y.; Feng, X.; Wan, R.; Luo, Z.; Qu, L.; Wang, Q. Impact of exercise on cancer: Mechanistic perspectives and new insights. Front. Immunol. 2024, 15, 1474770. [Google Scholar]
- Caru, M.; Curnier, D. The Potential Role of Exercise on the Bioavailability of Cancer Treatments. Acad. J. Pediatr. Neonatol. 2019, 7, 555775. [Google Scholar]
- Chen, L.; Chen, C.S.; Sun, Y.; Henry, N.L.; Stringer, K.A.; Hertz, D.L. Feasibility of pharmacometabolomics to identify potential predictors of paclitaxel pharmacokinetic variability. Cancer Chemother. Pharmacol. 2021, 88, 475–483. [Google Scholar]
- Bagheri, R.; Shakibaee, A.; Camera, D.M.; Sobhani, V.; Ghobadi, H.; Nazar, E.; Fakhari, H.; Dutheil, F. Effects of 8 weeks of resistance training in combination with a high protein diet on body composition, muscular performance, and markers of liver and kidney function in untrained older ex-military men. Front. Nutr. 2023, 10, 1205310. [Google Scholar]
- Bagheri, R.; Kargarfard, M.; Sadeghi, R.; Scott, D.; Camera, D.M. Effects of 16 weeks of two different high-protein diets with either resistance or concurrent training on body composition, muscular strength and performance, and markers of liver and kidney function in resistance-trained males. J. Int. Soc. Sports Nutr. 2023, 20, 2236053. [Google Scholar]
- De Nys, L.; Barzegar-Fallah, A.; Lanckmans, K.; Steurbaut, S.; Beckwee, D.; de Haar-Holleman, A.; Provyn, S.; Gasthuys, E.; Vande Casteele, S.; De Sutter, P.J.; et al. Dose-Limiting Toxicities of Paclitaxel in Breast Cancer Patients: Studying Interactions Between Pharmacokinetics, Physical Activity, and Body Composition-A Protocol for an Observational Cohort Study. Cancers 2024, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Brown, J.C.; Irwin, M.L.; Robien, K.; Scott, J.M.; Berger, N.A.; Caan, B.; Cercek, A.; Crane, T.E.; Evans, S.R.; et al. Exercise and Nutrition to Improve Cancer Treatment-Related Outcomes (ENICTO). J. Natl. Cancer Inst. 2025, 117, 9–19. [Google Scholar]
- Khazaeinia, T.; Ramsey, A.A.; Tam, Y.K. The effects of exercise on the pharmacokinetics of drugs. J. Pharm. Pharm. Sci. 2000, 3, 292–302. [Google Scholar]
- Guo, Z.; Gao, J.; Liu, L.; Liu, X. Quantitatively Predicting Effects of Exercise on Pharmacokinetics of Drugs Using a Physiologically Based Pharmacokinetic Model. Drug Metab. Dispos. 2024, 52, 1271–1287. [Google Scholar] [PubMed]
- Lenz, T.L.; Lenz, N.J.; Faulkner, M.A. Potential interactions between exercise and drug therapy. Sports Med. 2004, 34, 293–306. [Google Scholar]
- Ylitalo, P. Effect of exercise on pharmacokinetics. Ann. Med. 1991, 23, 289–294. [Google Scholar] [PubMed]
- Dunvald, A.D.; Jarvinen, E.; Mortensen, C.; Stage, T.B. Clinical and Molecular Perspectives on Inflammation-Mediated Regulation of Drug Metabolism and Transport. Clin. Pharmacol. Ther. 2022, 112, 277–290. [Google Scholar]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical pharmacokinetics of paclitaxel monotherapy: An updated literature review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar]
- Kipouros, M.; Vamvakari, K.; Kalafati, I.P.; Evangelou, I.; Kasti, A.N.; Kosti, R.I.; Androutsos, O. The Level of Adherence to the ESPEN Guidelines for Energy and Protein Intake Prospectively Influences Weight Loss and Nutritional Status in Patients with Cancer. Nutrients 2023, 15, 4232. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar]
- Baldessari, C.; Guaitoli, G.; Valoriani, F.; Bonacini, R.; Marcheselli, R.; Reverberi, L.; Pecchi, A.; Menozzi, R.; Torricelli, P.; Bertolini, F.; et al. Impact of body composition, nutritional and inflammatory status on outcome of non-small cell lung cancer patients treated with immunotherapy. Clin. Nutr. ESPEN 2021, 43, 64–75. [Google Scholar]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Williams, G.R.; Nyrop, K.A.; Popuri, K.; Choi, S.K.; Muss, H.B. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane–Based Chemotherapy for Early-Stage Breast Cancer. Clin. Cancer Res. 2017, 23, 3537–3543. [Google Scholar]
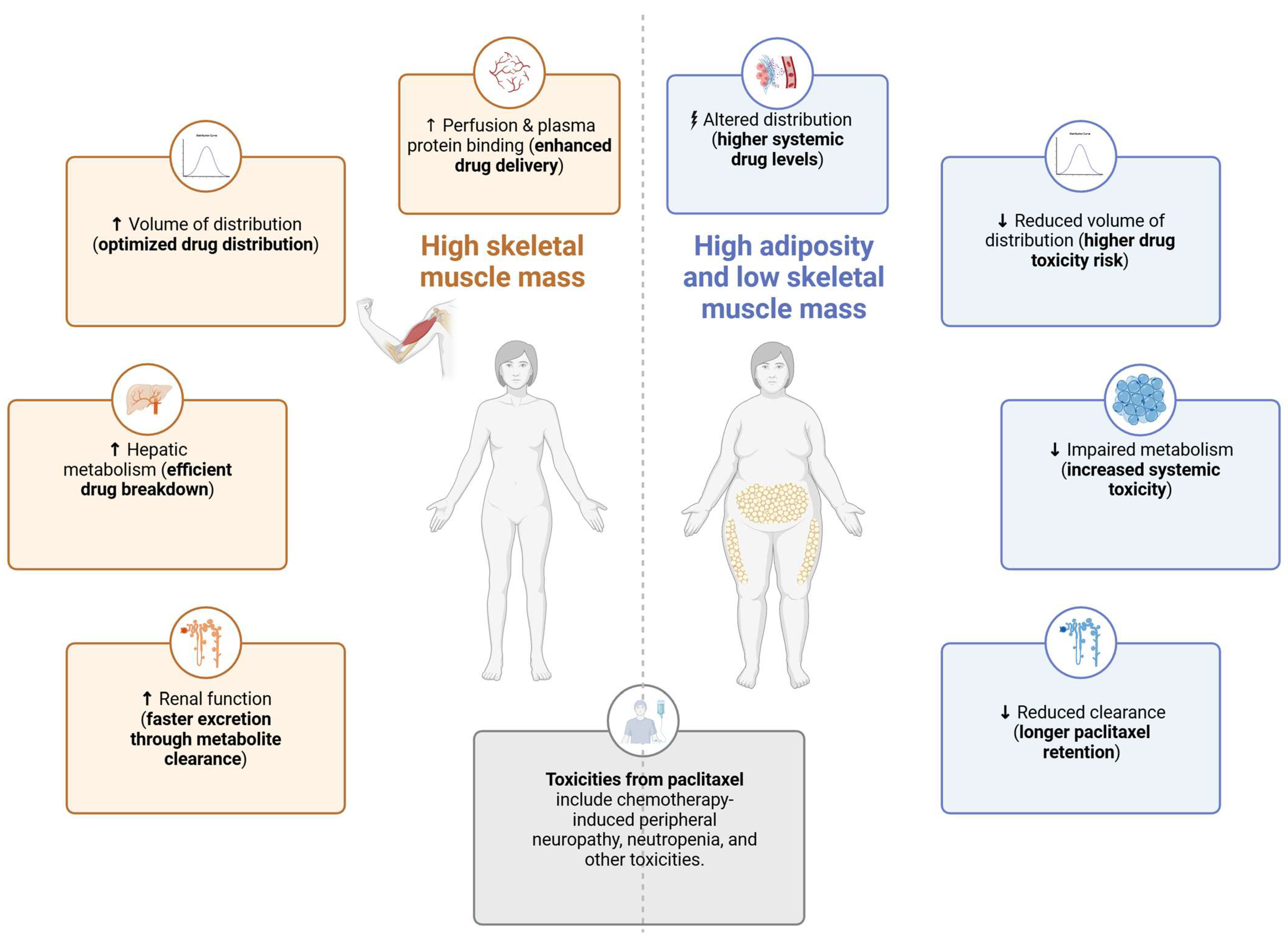
| Pharmacokinetic Process | Exercise Effect | Proposed Mechanism | Relationship to Body Composition | Clinical Implications |
|---|---|---|---|---|
| Absorption | Minimal direct effect (for IV drugs); potential changes in initial plasma levels. | Increased cardiovascular output and blood flow may influence early drug distribution rather than absorption. | High adiposity alters plasma protein binding, potentially increasing free PTX concentrations. Low muscle mass (sarcopenia) may lead to higher peak plasma drug levels. | Exercise may help optimize early PTX distribution by enhancing vascular function. Patients with sarcopenia or high adiposity may experience altered plasma drug levels, requiring personalized monitoring. |
| Distribution | Greater volume of distribution (Vd) with higher lean mass. | Higher skeletal muscle mass enhances tissue perfusion and drug dispersion. Reduced adipose tissue prevents excessive retention in circulation. | Sarcopenia reduces Vd, leading to higher PTX plasma concentrations and increased toxicity risk. High adiposity alters PTX storage and plasma exposure. | Patients with low muscle mass may require lower PTX doses to prevent toxicity. Exercise that increases lean mass may improve PTX distribution and reduce side effects. |
| Metabolism | Enhanced CYP3A4 and CYP2C8 activity. | Exercise reduces systemic inflammation, indirectly improving hepatic metabolism via CYP enzymes. | Higher skeletal muscle mass is associated with increased hepatic enzyme activity, while adiposity-related inflammation suppresses metabolism. | Exercise may help optimize PTX metabolism by reducing inflammation. Obese patients may exhibit a slower metabolism and higher toxicity risk. |
| Excretion | Improved hepatic elimination and renal function. | Exercise reduces glomerular filtration transiently, but long-term exercise improves renal function. | Obesity and sarcopenia may alter hepatic elimination, affecting PTX elimination and toxicity risk. | Exercise may help regulate overall metabolic function, potentially lowering systemic toxicity in high-risk patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adriaenssens, N.; Wuyts, S.C.M.; Steurbaut, S.; De Sutter, P.-J.; Vermeulen, A.; de Haar-Holleman, A.; Beckwée, D.; Provyn, S.; Vande Casteele, S.; Zhou, J.; et al. Synergy of Body Composition, Exercise Oncology, and Pharmacokinetics: A Narrative Review of Personalizing Paclitaxel Treatment for Breast Cancer. Cancers 2025, 17, 1271. https://doi.org/10.3390/cancers17081271
Adriaenssens N, Wuyts SCM, Steurbaut S, De Sutter P-J, Vermeulen A, de Haar-Holleman A, Beckwée D, Provyn S, Vande Casteele S, Zhou J, et al. Synergy of Body Composition, Exercise Oncology, and Pharmacokinetics: A Narrative Review of Personalizing Paclitaxel Treatment for Breast Cancer. Cancers. 2025; 17(8):1271. https://doi.org/10.3390/cancers17081271
Chicago/Turabian StyleAdriaenssens, Nele, Stephanie C. M. Wuyts, Stephane Steurbaut, Pieter-Jan De Sutter, An Vermeulen, Amy de Haar-Holleman, David Beckwée, Steven Provyn, Sofie Vande Casteele, Jinyu Zhou, and et al. 2025. "Synergy of Body Composition, Exercise Oncology, and Pharmacokinetics: A Narrative Review of Personalizing Paclitaxel Treatment for Breast Cancer" Cancers 17, no. 8: 1271. https://doi.org/10.3390/cancers17081271
APA StyleAdriaenssens, N., Wuyts, S. C. M., Steurbaut, S., De Sutter, P.-J., Vermeulen, A., de Haar-Holleman, A., Beckwée, D., Provyn, S., Vande Casteele, S., Zhou, J., Lanckmans, K., Van Bocxlaer, J., & De Nys, L. (2025). Synergy of Body Composition, Exercise Oncology, and Pharmacokinetics: A Narrative Review of Personalizing Paclitaxel Treatment for Breast Cancer. Cancers, 17(8), 1271. https://doi.org/10.3390/cancers17081271







