An International Multicentre Retrospective Cohort Study Evaluating Robot-Assisted Total Mesorectal Excision in Experienced Dutch, French, and United Kingdom Centres—The EUREKA Collaborative
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
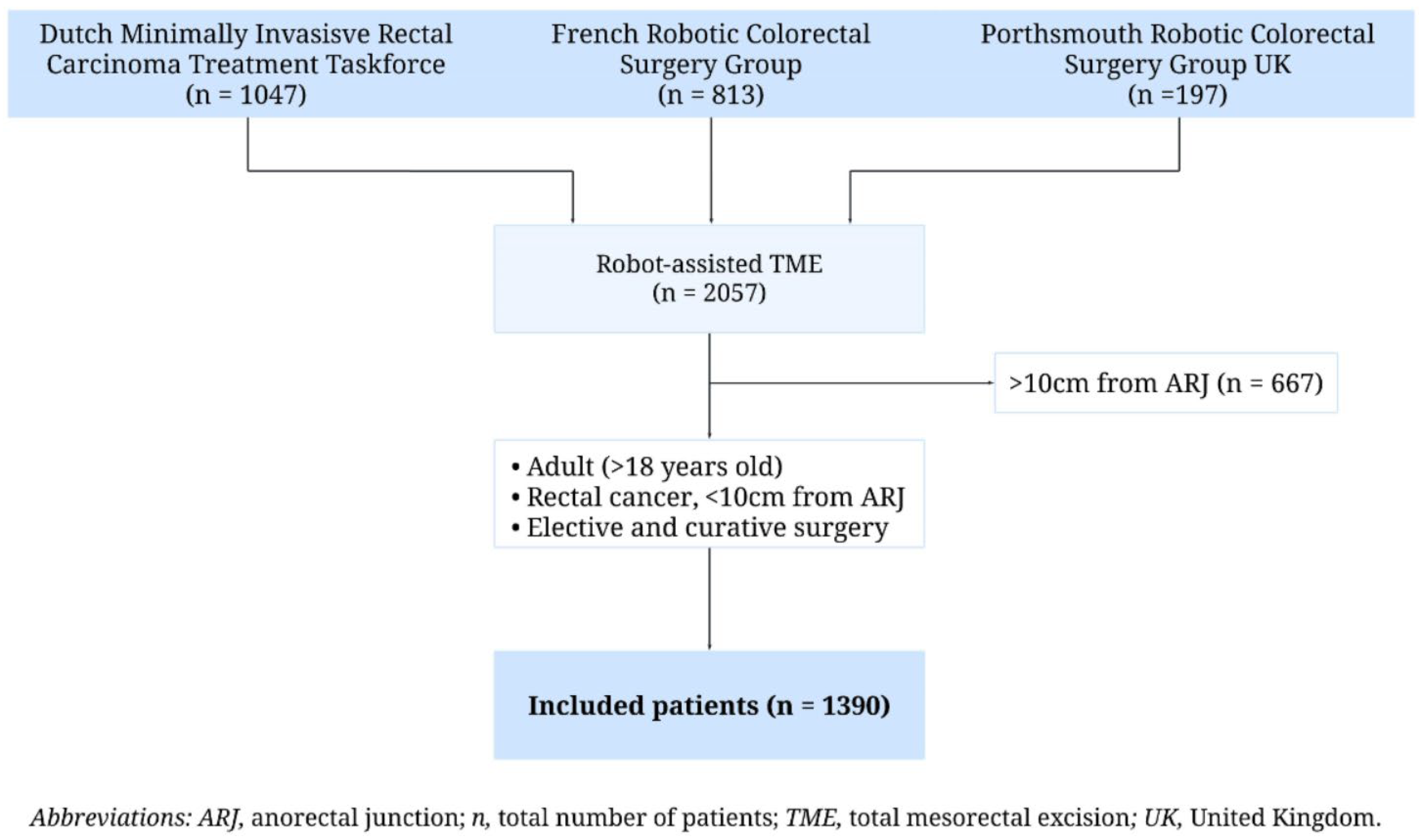
2.1. Study Design
2.2. Patients
2.3. Surgical Procedure
2.4. Outcome Parameters and Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Intraoperative Results
3.3. Postoperative Outcomes
3.4. Pathological Outcomes
3.5. Long-Term Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APR | Abdominoperineal resection |
| ARJ | Anorectal junction |
| ASA | American Society of Anesthesiology |
| BMI | Body mass index |
| CRM | Circumferential resection margin |
| CT | Computed tomography |
| ERAS | Enhanced recovery after surgery |
| EMVI | Extramural vascular invasion |
| ICG | Indocyanine green |
| ISREC | International Study Group of Rectal Cancer |
| IQR | Interquartile range |
| L-TME | Laparoscopic total mesorectal excision |
| MRI | Magnetic resonance imaging |
| MRF | Mesorectal fascia |
| nCRT | Neoadjuvant chemoradiotherapy |
| NAT | Neoadjuvant therapy |
| NRLAR | Non-restorative low anterior resection |
| PME | Partial mesorectal excision |
| RCT | Randomised controlled trial |
| RLAR | Restorative low anterior resection |
| R-TME | Robot-assisted total mesorectal excision |
| SCRT | Short-course radiotherapy |
| SD | Standard deviation |
| STROBE | Strengthening the reporting of observational studies in epidemiology guidelines for observational studies |
| TNT | Total neoadjuvant therapy |
| TME | Total mesorectal excision |
| TRG | Tumour regression grade |
Appendix A
References
- Kang, S.B.; Park, J.W.; Jeong, S.Y.; Nam, B.H.; Choi, H.S.; Kim, D.W.; Lim, S.B.; Lee, T.G.; Kim, D.Y.; Kim, J.S.; et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010, 11, 637–645. [Google Scholar] [CrossRef]
- Simillis, C.; Lal, N.; Thoukididou, S.N.; Kontovounisios, C.; Smith, J.J.; Hompes, R.; Adamina, M.; Tekkis, P.P. Open Versus Laparoscopic Versus Robotic Versus Transanal Mesorectal Excision for Rectal Cancer: A Systematic Review and Network Meta-analysis. Ann. Surg. 2019, 270, 59–68. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.; Sargent, D.J.; Boller, A.M.; George, V.; Abbas, M.; Peters, W.R., Jr.; Maun, D.; Chang, G.; Herline, A.; et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015, 314, 1346–1355. [Google Scholar] [CrossRef]
- Stevenson, A.R.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Davies, L.; Wilson, K.; Hague, W.; Simes, J. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015, 314, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Standard. Available online: https://www.davincisurgerycommunity.com/systems_i_a/standard (accessed on 16 October 2024).
- Park, J.S.; Lee, S.M.; Choi, G.S.; Park, S.Y.; Kim, H.J.; Song, S.H.; Min, B.S.; Kim, N.K.; Kim, S.H.; Lee, K.Y. Comparison of Laparoscopic Versus Robot-Assisted Surgery for Rectal Cancers: The COLRARRandomized Controlled Trial. Ann. Surg. 2023, 278, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Marshall, H.; Croft, J.; Copeland, J.; Jayne, D.; Brown, J. Exploring and adjusting for potential learning effects in ROLARR: A randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials 2018, 19, 339. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): Short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 991–1004. [Google Scholar] [CrossRef]
- Wang, X.; Cao, G.; Mao, W.; Lao, W.; He, C. Robot-assisted versus laparoscopic surgery for rectal cancer: A systematic review and meta-analysis. J. Cancer Res. Ther. 2020, 16, 979–989. [Google Scholar]
- Sun, Y.; Xu, H.; Li, Z.; Han, J.; Song, W.; Wang, J.; Xu, Z. Robotic versus laparoscopic low anterior resection for rectal cancer: A meta-analysis. World J. Surg. Oncol. 2016, 14, 61. [Google Scholar] [CrossRef]
- Xiong, B.; Ma, L.; Huang, W.; Zhao, Q.; Cheng, Y.; Liu, J. Robotic versus laparoscopic total mesorectal excision for rectal cancer: A meta-analysis of eight studies. J. Gastrointest. Surg. 2015, 19, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.A.; Duhoky, R.; Geitenbeek, R.T.J.; Moussion, A.; Bouazza, N.; Khan, J.; Cotte, E.; Dubois, A.; Rullier, E.; Hompes, R.; et al. Multicentre cohort study evaluating clinical, oncological and functional outcomes following robotic rectal cancer surgery—The EUREKA collaborative: Trial protocol. BJS Open 2024, 8, zrae019. [Google Scholar] [CrossRef] [PubMed]
- Burghgraef, T.A.; Sikkenk, D.J.; Crolla, R.M.P.H.; Fahim, M.; Melenhorst, J.; Moumni, M.E.; Schelling, G.V.; Smits, A.B.; Stassen, L.P.S.; Verheijen, P.M.; et al. Assessing the learning curve of robot-assisted total mesorectal excision: A multicenter study considering procedural safety, pathological safety, and efficiency. Int. J. Color. Dis. 2023, 38, 9. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Denost, Q.; Moreau, J.B.; Vendrely, V.; Celerier, B.; Rullier, A.; Assenat, V.; Rullier, E. Intersphincteric resection for low rectal cancer: The risk is functional rather than oncological. A 25-year experience from Bordeaux. Colorectal Dis. 2020, 22, 1603–1613. [Google Scholar] [CrossRef]
- Cotte, E.; Arquilliere, J.; Artru, P.; Bachet, J.B.; Benhaim, L.; Bibeau, F.; Christou, N.; Conroy, T.; Doyen, J.; Hoeffel, C.; et al. Rectal cancer—French intergroup clinical practice guidelines for diagnosis, treatment, and follow-up (TNCD, SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFP, RENAPE, SNFCP, AFEF, SFR, and GRECCAR). Dig. Liver Dis. 2024, 57, 669–679. [Google Scholar] [CrossRef]
- Ahmed, J.; Siddiqi, N.; Khan, L.; Kuzu, A.; Parvaiz, A. Standardized technique for single-docking robotic rectal surgery. Color. Dis. 2016, 18, O380–O384. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, N.K.; Min, B.S.; Lee, K.Y.; Sohn, S.K.; Cho, C.H. The Influence of the Number of Retrieved Lymph Nodes on Staging and Survival in Patients With Stage II and III Rectal Cancer Undergoing Tumor-Specific Mesorectal Excision. Ann. Surg. 2009, 249, 965–972. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for, R. Posit Software; PBC: Boston, MA, USA, 2024; Available online: http://www.posit.co/ (accessed on 10 October 2024).
- Rouanet, P.; Guerrieri, M.; Lemercier, P.; Balik, E.; Cotte, E.; Spinelli, A.; Gómez-Ruiz, M.; Wolthuis, A.; Bertani, E.; Dubois, A.; et al. A Prospective European Trial Comparing Laparotomy, Laparoscopy, Robotic-Assisted, and Transanal Total Mesorectal Excision Procedures in High-Risk Patients with Rectal Cancer. Ann. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Baik, S.H.; Roh, Y.H.; Kang, J.; Hur, H.; Min, B.S.; Lee, K.Y.; Kim, N.K. Cost-effectiveness of robotic surgery for rectal cancer focusing on short-term outcomes: A propensity score-matching analysis. Medicine 2015, 94, e823. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Borstlap, W.A.A.; Westerduin, E.; Aukema, T.S.; Bemelman, W.A.; Tanis, P.J. Anastomotic Leakage and Chronic Presacral Sinus Formation After Low Anterior Resection: Results From a Large Cross-sectional Study. Ann. Surg. 2017, 266, 870–877. [Google Scholar] [CrossRef]
- Agger, E.A.; Jörgren, F.H.; Lydrup, M.A.; Buchwald, P.L. Risk of local recurrence of rectal cancer and circumferential resection margin: Population-based cohort study. Br. J. Surg. 2020, 107, 580–585. [Google Scholar] [CrossRef]
- Kusters, M.; Marijnen, C.A.; van de Velde, C.J.; Rutten, H.J.; Lahaye, M.J.; Kim, J.H.; Beets-Tan, R.G.; Beets, G.L. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 470–476. [Google Scholar] [CrossRef]
- Sammour, T.; Malakorn, S.; Bednarski, B.K.; Kaur, H.; Shin, U.S.; Messick, C.; You, Y.N.; Chang, G.J. Oncological Outcomes After Robotic Proctectomy for Rectal Cancer: Analysis of a Prospective Database. Ann. Surg. 2018, 267, 521–526. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; van der Pas, M.H.; de Lange-de Klerk, E.S.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef]
- Daabiss, M. American society of anaesthesiologists physical status classification. Indian J. Anaesth. 2011, 55, 111–115. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; van de Velde, C.J.; van der Worp, E.; Kapiteijn, E.; Quirke, P.; van Krieken, J.H. Macroscopic evaluation of rectal cancer resection specimen: Clinical significance of the pathologist in quality control. J. Clin. Oncol. 2002, 20, 1729–1734. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef] [PubMed]
| R-TME (n = 1390) | |
|---|---|
| Age (mean (SD)) | 67 (11) |
| Sex | |
| Male | 907 (65.3) |
| Female | 483 (34.7) |
| BMI (mean (SD)) | 26.3 (4.4) |
| Categorical BMI | |
| Underweight (BMI < 18.5) | 21 (1.5) |
| Normal weight (BMI 18.5–24.9) | 557 (40.2) |
| Overweight (BMI 25.0–29.9) | 563 (40.6) |
| Obese (BMI > 30.0) | 245 (17.7) |
| Missing | 4 |
| Comorbidities Diabetes | |
| No | 1230 (88.9) |
| Yes | 154 (11.1) |
| Missing | 6 |
| Cardiovascular | |
| No | 912 (69.4) |
| Yes | 403 (30.6) |
| Missing | 75 |
| ASA physical status score | |
| 1 | 229 (16.5) |
| 2 | 871 (62.7) |
| 3 | 283 (20.4) |
| 4 | 7 (0.5) |
| Previous abdominal surgery | |
| No | 1001 (72.6) |
| Yes | 378 (27.4) |
| Missing | 11 |
| Clinical T stage | |
| cT1 | 43 (3.1) |
| cT2 | 375 (27.5) |
| cT3 | 830 (60.8) |
| cT4 | 118 (8.6) |
| Missing | 24 |
| Clinical N stage | |
| cN0 | 532 (46.2) |
| cN1 | 420 (36.5) |
| cN2 | 199 (17.3) |
| Missing | 239 |
| Clinical M stage | |
| cM0 | 1231 (92.6) |
| cM1 | 98 (7.4) |
| Missing | 61 |
| MRF involvement on MRI | |
| MRF− | 735 (55.9) |
| MRF+ | 580 (44.1) |
| Missing | 75 |
| EMVI involvement on MRI | |
| EMVI− | 413 (45.2) |
| EMVI+ | 501 (54.8) |
| Missing | 476 |
| Tumour location in lumen determined on MRI | |
| Anterior | 187 (14.6) |
| Circumferential | 546 (42.6) |
| Lateral | 122 (9.5) |
| Posterior | 139 (10.8) |
| Semi-circumferential | 13 (1) |
| Other | 276 (21.5) |
| Missing | 107 |
| Tumour distance from ARJ (median [IQR]) | 5.00 [2.00, 7.00] |
| Neoadjuvant therapy | |
| Total (%) received NAT | 830 (59.7) |
| Only chemotherapy | 41 (4.9) |
| Short-course radiotherapy | 225 (27.1) |
| Chemoradiotherapy | 283 (34.1) |
| Total neoadjuvant therapy | 281 (33.8) |
| ycT-stage | |
| ycT0 | 34 (7.2) |
| ycT1 | 16 (3.4) |
| ycT2 | 124 (24.5) |
| ycT3 | 256 (54.7) |
| ycT4 | 38 (8.1) |
| Missing | 362 |
| ycN-stage | |
| ycN0 | 306 (65.4) |
| ycN1 | 138 (29.5) |
| ycN2 | 24 (5.1) |
| Missing | 362 |
| MRF involvement on preoperative MRI after neoadjuvant therapy | |
| yMRF− | 172 (54.1) |
| yMRF+ | 146 (44.9) |
| Missing | 512 |
| Tumour response | |
| No response | 60 (15.7) |
| Partial response | 261 (68.1) |
| Complete response | 62 (16.2) |
| Missing | 447 |
| R-TME (n = 1390) | |
|---|---|
| Procedure type | |
| RLAR | 842 (60.6) |
| NRLAR | 199 (14.3) |
| APR | 349 (25.1) |
| Operative time (minutes) (median [IQR]) | 223 [171.00, 273.50] |
| Intraoperative complications | |
| No | 1148 (94.5) |
| Yes | 67 (5.5) |
| Missing | 175 |
| Type of intraoperative complication | |
| Bleed | 3 (4.5) |
| Perforation | 20 (29.9) |
| Other | 44 (65.7) |
| Conversion | |
| No | 1338 (96.3) |
| Yes | 52 (3.7) |
| Fluorescence used for evaluation of perfusion * | |
| No | 446 (54.3) |
| Yes | 376 (45.7) |
| Missing | 20 |
| Stoma * | |
| No | 350 (41.6) |
| Yes | 492 (58.4) |
| Stoma type | |
| Ileostomy | 490 (99.6) |
| Colostomy | 2 (0.4) |
| R-TME (n = 1390) | |
|---|---|
| Length of stay (days) (median [IQR]) | 7.00 [5.00, 11.00] |
| Postoperative complications within 31 days | |
| No | 750 (71.3) |
| Yes | 302 (28.7) |
| Missing | 338 |
| Ileus * | |
| No | 1037 (89.5) |
| Yes | 122 (10.5) |
| Missing | 231 |
| Wound infection * | |
| No | 1105 (94.3) |
| Yes | 67 (5.7) |
| Missing | 218 |
| Bleed * | |
| No | 1138 (97.9) |
| Yes | 25 (2.1) |
| Missing | 227 |
| Other complication * | |
| No | 1020 (91.4) |
| Yes | 96 (8.6) |
| Missing | 274 |
| Readmission within 30 days | |
| No | 1197 (86.4) |
| Yes | 189 (13.6) |
| Missing | 4 |
| Reintervention within 31 days | |
| No | 1244 (89.6) |
| Yes | 144 (10.4) |
| Missing | 2 |
| Type of reintervention within 31 days | |
| Radiologic | 14 (10.5) |
| Laparoscopic surgery | 42 (31.6) |
| Open surgery | 49 (36.8) |
| Transanal surgery | 13 (9.8) |
| Endoscopic intervention | 2 (1.5) |
| Other | 13 (9.8) |
| Missing | 11 |
| Clavien–Dindo classification * | |
| None | 769 (55.4) |
| I | 131 (9.4) |
| II | 262 (18.9) |
| IIIa | 38 (2.7) |
| IIIb | 146 (10.5) |
| IV | 6 (0.4) |
| IVa | 17 (1.2) |
| IVb | 5 (0.4) |
| V | 14 (1.0) |
| Missing | 2 |
| Anastomotic leakage ** | |
| No | 667 (85.3) |
| Yes | 115 (14.7) |
| Missing | 60 |
| Time | |
| Early (≤30 days) | 118 (79.2) |
| Late (>30 days) | 31 (20.8) |
| Missing | 6 |
| Grade | |
| Grade A, subclinical | 9 (8) |
| Grade B, clinical, requiring radiologic or transanal drainage | 40 (35.4) |
| Grade C, clinical, requiring re-laparotomy | 64 (56.6) |
| Missing | 42 |
| Treatment | |
| Medicine, no reintervention | 15 (13.3) |
| Radiological drainage of abscess | 12 (10.6) |
| Transanal drainage of abscess | 9 (7.9) |
| Endosponge | 4 (3.5) |
| Relaparotomy with deviating stoma | 23 (20.4) |
| Relaparotomy with reversal of anastomosis and endostomy | 28 (24.8) |
| Other | 22 (19.5) |
| Missing | 42 |
| R-TME (n = 1390) | |
|---|---|
| pT-staging | |
| pT0 | 104 (7.5) |
| pT1 | 123 (8.9) |
| pT2 | 468 (33.8) |
| pT3 | 632 (45.7) |
| pT4 | 29 (2.1) |
| pTx | 27 (2.0) |
| Missing | 7 |
| pN-staging | |
| pN0 | 882 (63.6) |
| pN1 | 390 (28.1) |
| pN2 | 96 (6.9) |
| pNx | 18 (1.3) |
| Missing | 4 |
| Quality of TME | |
| Complete | 1105 (81.9) |
| Nearly complete | 178 (13.1) |
| Incomplete | 71 (5.2) |
| Missing | 36 |
| CRM− | 1180 (94.5) |
| CRM+ | 69 (5.5) |
| Missing | 141 |
| Resection | |
| R0 | 1296 (94.7) |
| R1 | 69 (5.0) |
| R2 | 3 (0.2) |
| Missing | 36 |
| Perforation | |
| No | 1070 (96.7) |
| Yes | 36 (3.3) |
| Missing | 284 |
| Pathological nodes removed (mean (SD)) | 17.3 (8.94) |
| Positive nodes (median [IQR]) | 0 [0.00, 1.00] |
| R-TME (n = 1390) | |
|---|---|
| Adjuvant chemotherapy | |
| No | 1067 (82.3) |
| Yes | 230 (17.7) |
| Missing | 93 |
| 3-year local recurrence rate | |
| No | 1317 (97.2) |
| Yes | 38 (2.9) |
| Missing | 35 |
| Location | |
| Anterior Above | 0 |
| Anterior Below | 4 (12.5) |
| Inferior | 2 (6.3) |
| Central anastomotic | 8 (25) |
| Central non-anastomotic | 10 (31.3) |
| Posterior | 5 (14.6) |
| Lateral at right | 2 (6.3) |
| Lateral at left | 1 (3.1) |
| Peritoneal reflection | 0 |
| Missing | 6 |
| 3-year systemic recurrence rate | |
| No | 1155 (85.4) |
| Yes | 198 (14.6) |
| Missing | 37 |
| Location | |
| Lung | 109 (56.8) |
| Liver | 66 (34.4) |
| Peritoneal | 4 (2.1) |
| Bone | 1 (0.5) |
| Brain | 1 (0.5) |
| Other | 11 (5.7) |
| Missing | 6 |
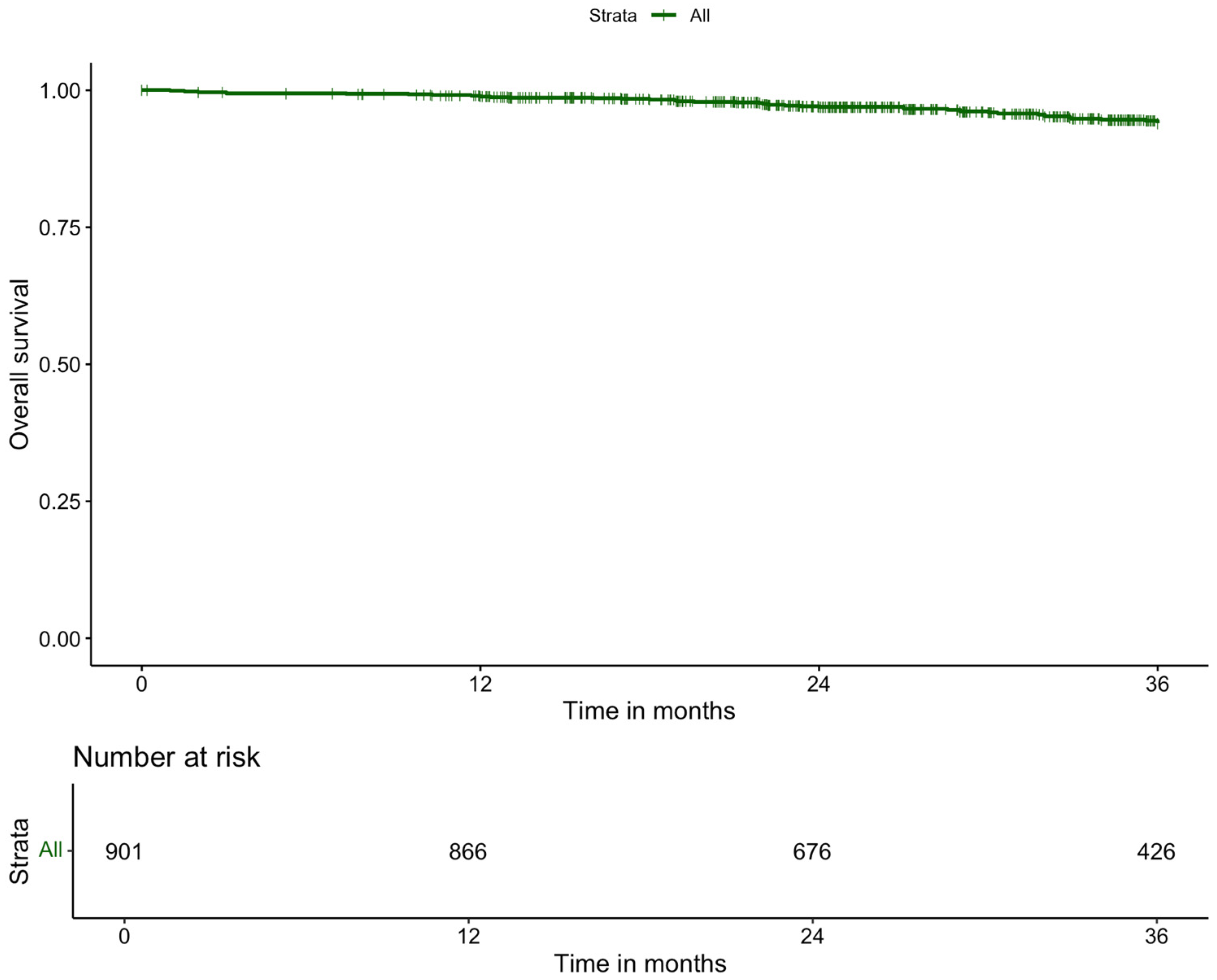
| 3-year overall survival | |
| No | 136 (9.9) |
| Yes | 1238 (90.1) |
| Missing | 16 |
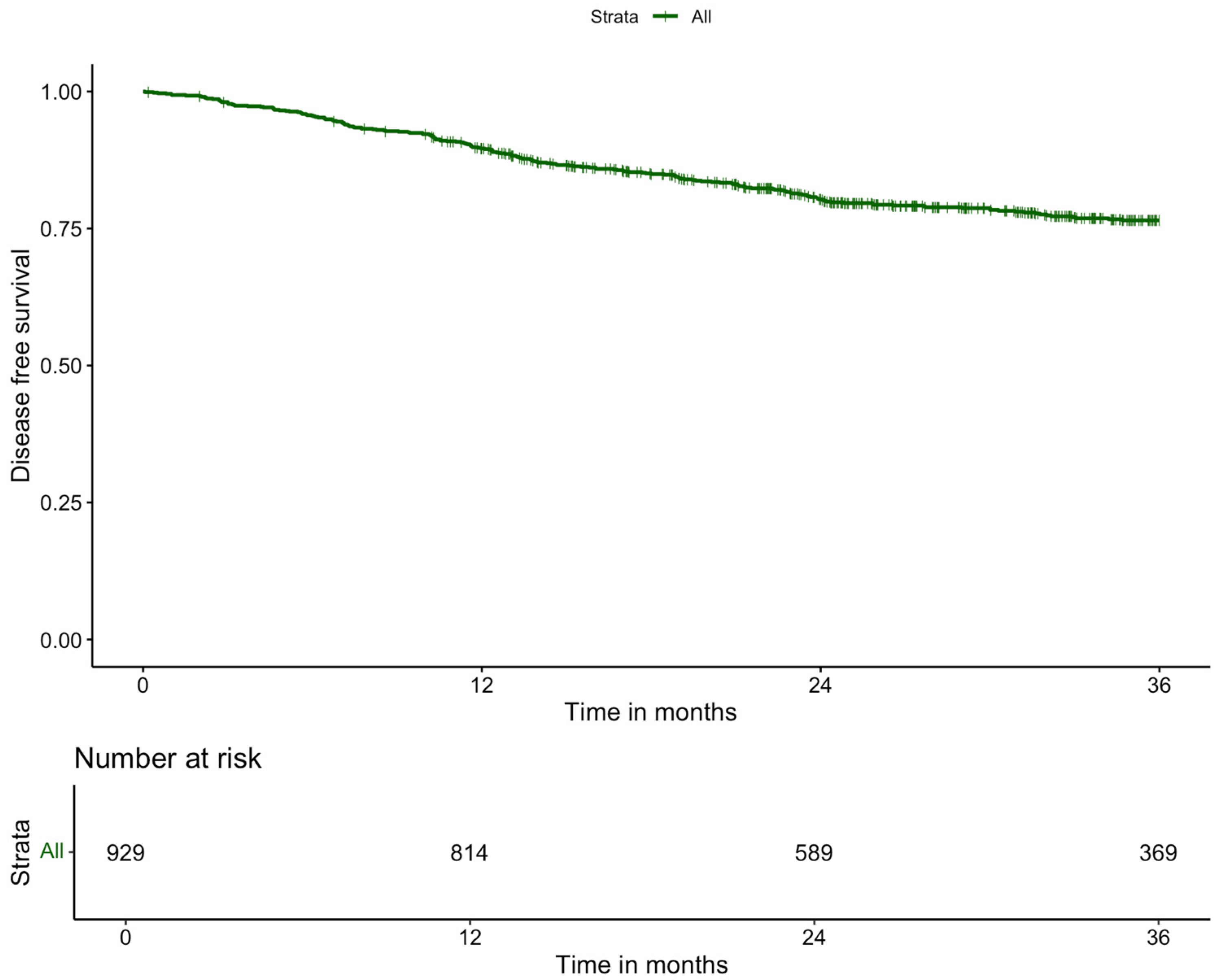
| 3-year disease free survival | |
| No | 157 (11.4) |
| Yes | 1215 (88.6) |
| Missing | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geitenbeek, R.T.J.; Genders, C.M.S.; Taoum, C.; Duhoky, R.; Burghgraef, T.A.; Fleming, C.A.; Cotte, E.; Dubois, A.; Rullier, E.; Denost, Q.; et al. An International Multicentre Retrospective Cohort Study Evaluating Robot-Assisted Total Mesorectal Excision in Experienced Dutch, French, and United Kingdom Centres—The EUREKA Collaborative. Cancers 2025, 17, 1268. https://doi.org/10.3390/cancers17081268
Geitenbeek RTJ, Genders CMS, Taoum C, Duhoky R, Burghgraef TA, Fleming CA, Cotte E, Dubois A, Rullier E, Denost Q, et al. An International Multicentre Retrospective Cohort Study Evaluating Robot-Assisted Total Mesorectal Excision in Experienced Dutch, French, and United Kingdom Centres—The EUREKA Collaborative. Cancers. 2025; 17(8):1268. https://doi.org/10.3390/cancers17081268
Chicago/Turabian StyleGeitenbeek, Ritch T. J., Charlotte M. S. Genders, Christophe Taoum, Rauand Duhoky, Thijs A. Burghgraef, Christina A. Fleming, Eddy Cotte, Anne Dubois, Eric Rullier, Quentin Denost, and et al. 2025. "An International Multicentre Retrospective Cohort Study Evaluating Robot-Assisted Total Mesorectal Excision in Experienced Dutch, French, and United Kingdom Centres—The EUREKA Collaborative" Cancers 17, no. 8: 1268. https://doi.org/10.3390/cancers17081268
APA StyleGeitenbeek, R. T. J., Genders, C. M. S., Taoum, C., Duhoky, R., Burghgraef, T. A., Fleming, C. A., Cotte, E., Dubois, A., Rullier, E., Denost, Q., Khan, J. S., Hompes, R., Rouanet, P., & Consten, E. C. J., on behalf of the EUREKA Study Group. (2025). An International Multicentre Retrospective Cohort Study Evaluating Robot-Assisted Total Mesorectal Excision in Experienced Dutch, French, and United Kingdom Centres—The EUREKA Collaborative. Cancers, 17(8), 1268. https://doi.org/10.3390/cancers17081268






