Prevalence and Genotype Distribution of High-Risk HPV Genotypes Among Women in Greece: A Retrospective Analysis of 3500 Women
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection for HR-HPV Screening
2.2. HR-HPV Screening with Roche Cobas 4800 HPV Test
2.3. NMPCR for the Identification of 12 HR-HPV Genotypes
2.4. Statistical Analysis
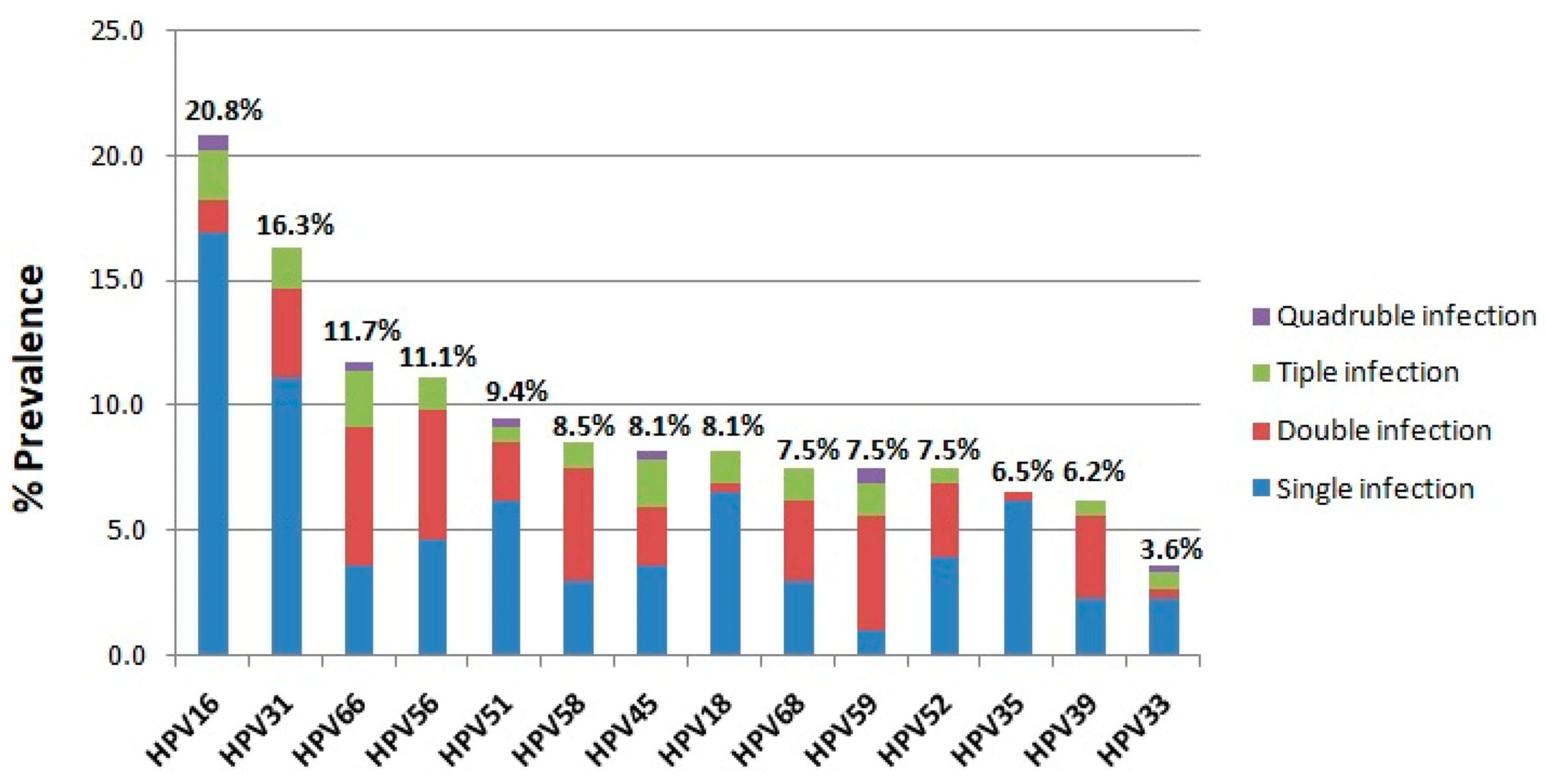
3. Results
3.1. The Prevalence of HR-HPV Infection in 3500 Women
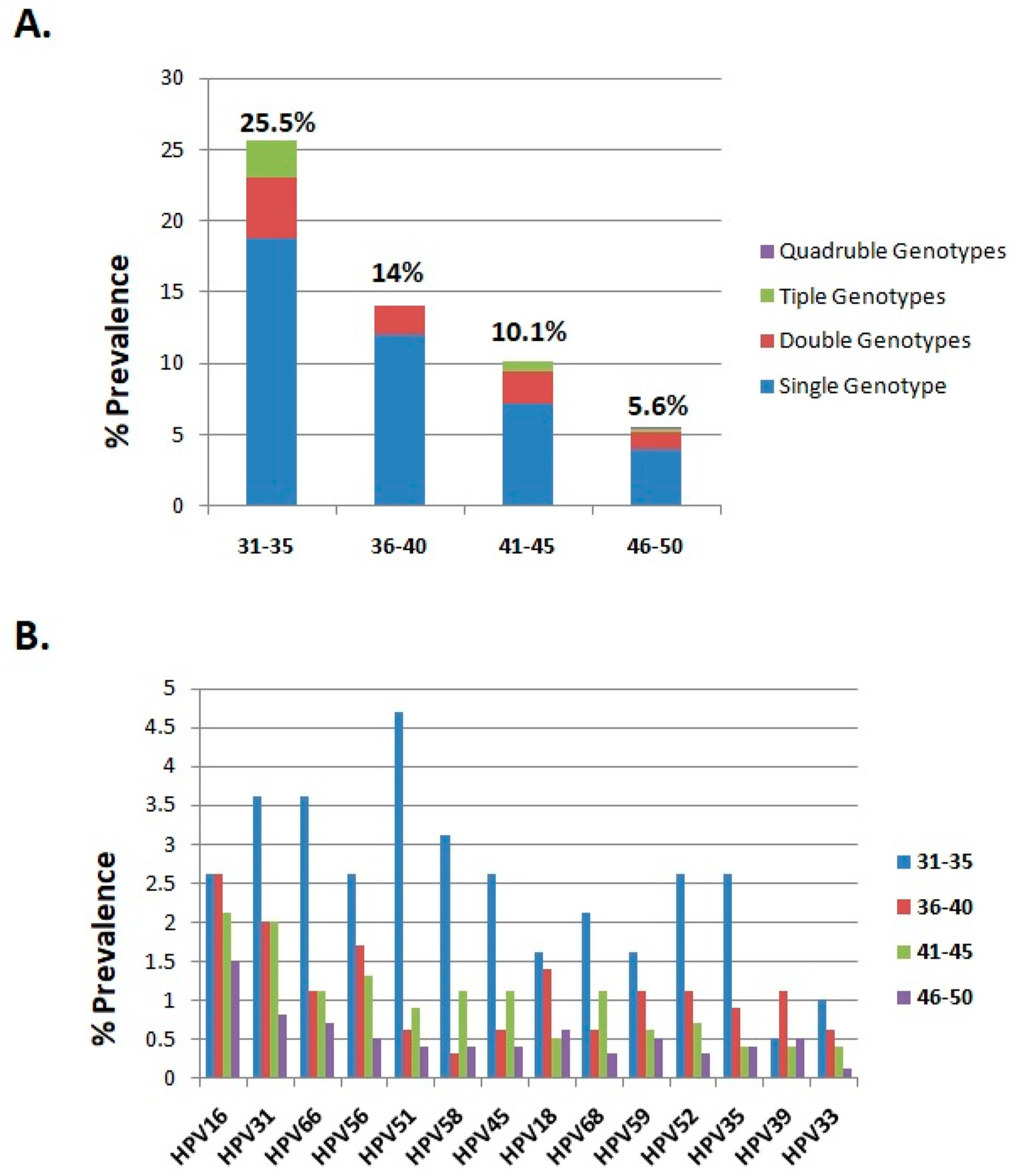
3.2. The Overall Prevalence of HR-HPV Infection in the Different Age Groups
3.3. The Prevalence of HR-HPV Genotypes Between 2021 and 2023
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; DeSalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Darmis, F.; Gortsilas, P.; Ruether, I.G.; Kyriakopoulou, Z.; Dimitriou, T.G.; Amoutzias, G.; Markoulatos, P. Nucleotide polymorphisms of the human papillomavirus 16 E1 gene. Arch. Virol. 2014, 159, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Tsakogiannis, D.; Ruether, I.G.; Kyriakopoulou, Z.; Pliaka, V.; Skordas, V.; Gartzonika, C.; Levidiotou-Stefanou, S.; Markoulatos, P. Molecular and phylogenetic analysis of the HPV 16 E4 gene in cervical lesions from women in Greece. Arch. Virol. 2012, 157, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef]
- Bernard, H.U.; Calleja-Macias, I.E.; Dunn, S.T. Genome variation of human papillomavirus types: Phylogenetic and medical implications. Int. J. Cancer 2006, 118, 1071–1076. [Google Scholar] [CrossRef]
- Humans IWGotEoCRt. Humans IWGotEoCRt. Human papillomaviruses. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2007; Volume 90, pp. 1–636. [Google Scholar]
- Li, Y.; Xu, C. Human Papillomavirus-Related Cancers. Adv. Exp. Med. Biol. 2017, 1018, 23–34. [Google Scholar] [CrossRef]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Shimada, M.; Yamashita, A.; Saito, M.; Ichino, M.; Kinjo, T.; Mizuki, N.; Klinman, D.M.; Okuda, K. The human papillomavirus E6 protein targets apoptosis-inducing factor (AIF) for degradation. Sci. Rep. 2020, 10, 14195. [Google Scholar] [CrossRef]
- Aarthy, M.; Kumar, D.; Giri, R.; Singh, S.K. E7 oncoprotein of human papillomavirus: Structural dynamics and inhibitor screening study. Gene 2018, 658, 159–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, K.; Ren, J.; Zhao, Y.; Cheng, P. Roles of human papillomavirus in cancers: Oncogenic mechanisms and clinical use. Signal Transduct. Target. Ther. 2025, 10, 44. [Google Scholar] [CrossRef]
- Bletsa, G.; Zagouri, F.; Amoutzias, G.D.; Nikolaidis, M.; Zografos, E.; Markoulatos, P.; Tsakogiannis, D. Genetic variability of the HPV16 early genes and LCR. Present and future perspectives. Expert Rev. Mol. Med. 2021, 23, e19. [Google Scholar] [CrossRef] [PubMed]
- Zygouras, I.; Leventakou, D.; Pouliakis, A.; Panagiotou, S.; Tsakogiannis, D.; Konstantopoulos, G.; Logotheti, E.; Samaras, M.; Kyriakopoulou, Z.; Beloukas, A.; et al. Human Papillomavirus 16 DNA Methylation Patterns and Investigation of Integration Status in Head and Neck Cancer Cases. Int. J. Mol. Sci. 2023, 24, 14593. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bruni, L.A.G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Greece. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/GRC.pdf (accessed on 26 February 2025).
- Tsakogiannis, D.; Nikolaidis, M.; Zagouri, F.; Zografos, E.; Kottaridi, C.; Kyriakopoulou, Z.; Tzioga, L.; Markoulatos, P.; Amoutzias, G.D.; Bletsa, G. Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas. Viruses 2022, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfstrom, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Lew, J.B.; Simms, K.T.; Smith, M.A.; Hall, M.; Kang, Y.J.; Xu, X.M.; Caruana, M.; Velentzis, L.S.; Bessell, T.; Saville, M.; et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: Effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health 2017, 2, e96–e107. [Google Scholar] [CrossRef] [PubMed]
- Ostrbenk Valencak, A.; Kroon, K.R.; Fabjan, D.; Mlakar, J.; Seme, K.; Berkhof, J.; Poljak, M. Clinically validated HPV assays offer comparable long-term safety in primary cervical cancer screening: A 9-year follow-up of a population-based screening cohort. Int. J. Cancer 2025, 156, 788–801. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Gartzonika, C.; Levidiotou-Stefanou, S.; Markoulatos, P. Molecular approaches for HPV genotyping and HPV-DNA physical status. Expert Rev. Mol. Med. 2017, 19, e1. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gao, K.; Gu, S.; You, L.; Qian, S.; Tang, M.; Wang, J.; Chen, K.; Jin, M. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer 2021, 127, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ahrlund-Richter, A.; Nasman, A.; Dalianis, T. Human papilloma virus (HPV) prevalence upon HPV vaccination in Swedish youth: A review based on our findings 2008-2018, and perspectives on cancer prevention. Arch. Gynecol. Obstet. 2021, 303, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Oyervides-Munoz, M.A.; Perez-Maya, A.A.; Sanchez-Dominguez, C.N.; Berlanga-Garza, A.; Antonio-Macedo, M.; Valdez-Chapa, L.D.; Cerda-Flores, R.M.; Trevino, V.; Barrera-Saldana, H.A.; Garza-Rodriguez, M.L. Multiple HPV Infections and Viral Load Association in Persistent Cervical Lesions in Mexican Women. Viruses 2020, 12, 380. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, N.; Li, B.; Shang, E.; Wang, J.; Zhang, M.; Yang, X. Prevalence and genotype distribution of human papillomavirus infections in Beijing, China between 2016 and 2020. Virol. J. 2023, 20, 11. [Google Scholar] [CrossRef]
- Sotlar, K.; Diemer, D.; Dethleffs, A.; Hack, Y.; Stubner, A.; Vollmer, N.; Menton, S.; Menton, M.; Dietz, K.; Wallwiener, D.; et al. Detection and typing of human papillomavirus by e6 nested multiplex PCR. J. Clin. Microbiol. 2004, 42, 3176–3184. [Google Scholar] [CrossRef]
- Malagon, T.; Franco, E.L.; Tejada, R.; Vaccarella, S. Epidemiology of HPV-associated cancers past, present and future: Towards prevention and elimination. Nat. Rev. Clin. Oncol. 2024, 21, 522–538. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Arroyo Muhr, L.S.; Gini, A.; Yilmaz, E.; Hassan, S.S.; Lagheden, C.; Hultin, E.; Garcia Serrano, A.; Ure, A.E.; Andersson, H.; Merino, R.; et al. Concomitant human papillomavirus (HPV) vaccination and screening for elimination of HPV and cervical cancer. Nat. Commun. 2024, 15, 3679. [Google Scholar] [CrossRef]
- Kutz, J.M.; Rausche, P.; Gheit, T.; Puradiredja, D.I.; Fusco, D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: A systematic review. BMC Public Health 2023, 23, 974. [Google Scholar] [CrossRef]
- Wendland, E.M.; Villa, L.L.; Unger, E.R.; Domingues, C.M.; Benzaken, A.S. Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: The POP-Brazil Study. Sci. Rep. 2020, 10, 4920. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Romero, L.D.C.; Organista-Nava, J.; Gomez-Gomez, Y.; Ortiz-Ortiz, J.; Hernandez-Sotelo, D.; Del Moral-Hernandez, O.; Mendoza-Catalan, M.A.; Antano-Arias, R.; Leyva-Vazquez, M.A.; Sales-Linares, N.; et al. Prevalence and Distribution of Human Papillomavirus Genotypes (1997–2019) and Their Association with Cervical Cancer and Precursor Lesions in Women from Southern Mexico. Cancer Control 2022, 29, 10732748221103331. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Reiter, P.L.; McRee, A.L. Nativity status and genital HPV infection among adults in the U.S. Hum. Vaccin. Immunother. 2019, 15, 1897–1903. [Google Scholar] [CrossRef]
- Hu, J.P.; Wang, J.L.; Li, Y.; Feng, Y.; Tian, C.Q.; Zhang, G.H.; Chen, X.Q.; Liu, H.X.; Yang, J.S.; Fang, Z.W.; et al. Prevalence and genotype distribution of human papillomavirus infection among 66000 women from 2014 to 2023 in the plateau region of Southwest China. Virol. J. 2024, 21, 176. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 26 February 2025).
- Agorastos, T.; Chatzistamatiou, K.; Zafrakas, M.; Siamanta, V.; Katsamagkas, T.; Constantinidis, T.C.; Lampropoulos, A.F.; group, L.s. Epidemiology of HPV infection and current status of cervical cancer prevention in Greece: Final results of the LYSISTRATA cross-sectional study. Eur. J. Cancer Prev. 2014, 23, 425–431. [Google Scholar] [CrossRef]
- Agorastos, T.; Lambropoulos, A.F.; Sotiriadis, A.; Mikos, T.; Togaridou, E.; Emmanouilides, C.J. Prevalence and distribution of high-risk human papillomavirus in Greece. Eur. J. Cancer Prev. 2009, 18, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Argyri, E.; Papaspyridakos, S.; Tsimplaki, E.; Michala, L.; Myriokefalitaki, E.; Papassideri, I.; Daskalopoulou, D.; Tsiaoussi, I.; Magiakos, G.; Panotopoulou, E. A cross sectional study of HPV type prevalence according to age and cytology. BMC Infect. Dis. 2013, 13, 53. [Google Scholar] [CrossRef]
- Tsiodras, S.; Hatzakis, A.; Spathis, A.; Margari, N.; Meristoudis, C.; Chranioti, A.; Kyrgiou, M.; Panayiotides, J.; Kassanos, D.; Petrikkos, G.; et al. Molecular epidemiology of HPV infection using a clinical array methodology in 2952 women in Greece. Clin. Microbiol. Infect. 2011, 17, 1185–1188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panotopoulou, E.; Tserkezoglou, A.; Kouvousi, M.; Tsiaousi, I.; Chatzieleftheriou, G.; Daskalopoulou, D.; Magiakos, G. Prevalence of human papillomavirus types 6, 11, 16, 18, 31, and 33 in a cohort of Greek women. J. Med. Virol. 2007, 79, 1898–1905. [Google Scholar] [CrossRef]
- Salazar, K.L.; Zhou, H.S.; Xu, J.; Peterson, L.E.; Schwartz, M.R.; Mody, D.R.; Ge, Y. Multiple Human Papilloma Virus Infections and Their Impact on the Development of High-Risk Cervical Lesions. Acta Cytol. 2015, 59, 391–398. [Google Scholar] [CrossRef]
- Bruno, M.T.; Scalia, G.; Cassaro, N.; Boemi, S. Multiple HPV 16 infection with two strains: A possible marker of neoplastic progression. BMC Cancer 2020, 20, 444. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, D.; Zhang, M.; Kang, P.; Cui, M.; Zhu, L.; Luo, L. Characteristic of persistent human papillomavirus infection in women worldwide: A meta-analysis. PeerJ 2023, 11, e16247. [Google Scholar] [CrossRef]
- Na, J.; Li, Y.; Wang, J.; Wang, X.; Lu, J.; Han, S. The correlation between multiple HPV infections and the occurrence, development, and prognosis of cervical cancer. Front. Microbiol. 2023, 14, 1220522. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mei, B.; Ouyang, Y.; Li, C. Prevalence and genotype distribution of human papillomavirus infection among women in Jingzhou, China: A population-based study of 51,720 women. Virol. J. 2023, 20, 297. [Google Scholar] [CrossRef] [PubMed]
- Pisani, T.; Cenci, M. Prevalence of Multiple High Risk Human Papilloma Virus (HR-HPV) Infections in Cervical Cancer Screening in Lazio Region, Italy. Cancer Diagn. Progn. 2024, 4, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xue, J.; Sun, Y.; Zhu, L.; Zhao, M.; Cui, M.; Zhang, M.; Jia, J.; Luo, L. Patterns of single and multiple HPV infections in female: A systematic review and meta-analysis. Heliyon 2024, 10, e35736. [Google Scholar] [CrossRef]
- Berza, N.; Zodzika, J.; Kivite-Urtane, A.; Baltzer, N.; Curkste, A.; Pole, I.; Nygard, M.; Parna, K.; Stankunas, M.; Tisler, A.; et al. Understanding the high-risk human papillomavirus prevalence and associated factors in the European country with a high incidence of cervical cancer. Eur. J. Public Health 2024, 34, 826–832. [Google Scholar] [CrossRef]
- Clarke, M.A.; Risley, C.; Stewart, M.W.; Geisinger, K.R.; Hiser, L.M.; Morgan, J.C.; Owens, K.J.; Ayyalasomayajula, K.; Rives, R.M.; Jannela, A.; et al. Age-specific prevalence of human papillomavirus and abnormal cytology at baseline in a diverse statewide prospective cohort of individuals undergoing cervical cancer screening in Mississippi. Cancer Med. 2021, 10, 8641–8650. [Google Scholar] [CrossRef]
- Reynders, C.; Lerho, T.; Goebel, E.A.; Crum, C.P.; Vandenput, S.; Beaudart, C.; Herfs, M. Prevalence and genotype distribution of human papillomavirus in cervical adenocarcinoma (usual type and variants): A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e29190. [Google Scholar] [CrossRef]
- Lin, X.; Chen, L.; Zheng, Y.; Yan, F.; Li, J.; Zhang, J.; Yang, H. Age-specific prevalence and genotype distribution of human papillomavirus in women from Northwest China. Cancer Med. 2022, 11, 4366–4373. [Google Scholar] [CrossRef]
- Huber, J.; Mueller, A.; Sailer, M.; Regidor, P.A. Human papillomavirus persistence or clearance after infection in reproductive age. What is the status? Review of the literature and new data of a vaginal gel containing silicate dioxide, citric acid, and selenite. Women’s Health 2021, 17, 17455065211020702. [Google Scholar] [CrossRef] [PubMed]


| 31–35 | 36–40 | 41–45 | 46–50 | Total | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Samples | 192 (5.5) | 351 (10) | 951 (27.2) | 2006 (57.3) | 3500 (100) |
| HPV infection | 49 (25.5) | 49 (14) | 96 (10.1) | 113 (5.6) | 307 (8.8) |
| Single genotypes | 36 (18.8) | 42 (12) | 68 (7.2) | 81 (4) | 227 (6.5) |
| Double genotypes | 8 (4.2) | 7 (2) | 22 (2.3) | 24 (1.2) | 61 (1.7) |
| Triple genotypes | 5 (2.6) | 0 | 6 (0.6) | 6 (0.3) | 17 (0.5) |
| Quadruple genotypes | 0 (0) | 0 | 0 | 2 (0.1) | 2 (0.1) |
| HPV genotypes | |||||
| HPV16 | 5 (2.6) | 9 (2.6) | 20 (2.1) | 30 (1.5) | 64 (1.8) |
| HPV31 | 7 (3.6) | 7 (2) | 19 (2) | 17 (0.8) | 50 (1.4) |
| HPV66 | 7 (3.6) | 4 (1.1) | 10 (1.1) | 15 (0.7) | 36 (1) |
| HPV56 | 5 (2.6) | 6 (1.7) | 12 (1.3) | 11 (0.5) | 34 (1) |
| HPV51 | 9 (4.7) | 3 (0.9) | 9 (0.9) | 8 (0.4) | 29 (0.8) |
| HPV58 | 6 (3.1) | 1 (0.3) | 10 (1.1) | 9 (0.4) | 26 (0.7) |
| HPV45 | 5 (2.6) | 2 (0.6) | 10 (1.1) | 8 (0.4) | 25 (0.7) |
| HPV18 | 3 (1.6) | 5 (1.4) | 5 (0.5) | 12 (0.6) | 25 (0.7) |
| HPV68 | 4 (2.1) | 2 (0.6) | 10 (1.1) | 7 (0.3) | 23 (0.7) |
| HPV59 | 3 (1.6) | 4 (1.1) | 6 (0.6) | 10 (0.5) | 23 (0.7) |
| HPV52 | 5 (2.6) | 4 (1.1) | 7 (0.7) | 7 (0.3) | 23 (0.7) |
| HPV35 | 5 (2.6) | 3 (0.9) | 4 (0.4) | 8 (0.4) | 20 (0.6) |
| HPV39 | 1 (0.5) | 4 (1.1) | 4 (0.4) | 10 (0.5) | 19 (0.5) |
| HPV33 | 2 (1) | 2 (0.6) | 4 (0.4) | 3 (0.1) | 11 (0.3) |
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Samples | 1139 | 1108 | 1253 |
| HPV infection | 93 (8.2) | 104 (9.4) | 110 (8.8) |
| Single genotype | 69 (6.1) | 76 (6.9) | 82 (6.5) |
| Double genotypes | 18 (1.6) | 22 (2) | 21 (1.7) |
| Triple genotypes | 6 (0.5) | 5 (0.5) | 6 (0.5) |
| Quadruple genotypes | 0 (0) | 1 (0.1) | 1 (0.1) |
| HPV genotypes | |||
| HPV16 | 17 (1.5) | 23 (2.1) | 24 (1.9) |
| HPV31 | 15 (1.3) | 20 (1.8) | 15 (1.2) |
| HPV66 | 13 (1.1) | 11 (1) | 12 (1.0) |
| HPV56 | 9 (0.8) | 8 (0.7) | 17 (1.4) |
| HPV51 | 10 (0.9) | 10 (0.9) | 9 (0.7) |
| HPV58 | 13 (1.1) | 9 (0.8) | 4 (0.1) |
| HPV45 | 6 (0.5) | 10 (0.9) | 9 (0.7) |
| HPV18 | 5 (0.4) | 9 (0.8) | 11 (0.9) |
| HPV68 | 7 (0.6) | 10 (0.9) | 6 (0.5) |
| HPV59 | 7 (0.6) | 8 (0.7) | 8 (0.6) |
| HPV52 | 7 (0.6) | 9 (0.8) | 7 (0.6) |
| HPV35 | 3 (0.3) | 7 (0.6) | 10 (0.8) |
| HPV39 | 7 (0.6) | 3 (0.3) | 9 (0.7) |
| HPV33 | 4 (0.4) | 2 (0.2) | 5 (0.4) |
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| HPV-positive samples (n) | 93 | 104 | 110 |
| HPV genotypes | n (%) | n (%) | n (%) |
| HPV16 | 17 (18.3) | 23 (22.1) | 24 (21.8) |
| HPV31 | 15 (16.1) | 20 (19.2) | 15 (13.6) |
| HPV66 | 13 (14) | 11 (10.6) | 12 (10.9) |
| HPV56 | 9 (9.7) | 8 (7.7) | 17 (15.5) |
| HPV51 | 10 (10.8) | 10 (9.6) | 9 (8.2) |
| HPV58 | 13 (14) | 9 (8.7) | 4 (3.6) |
| HPV45 | 6 (6.5) | 10 (9.6) | 9 (8.2) |
| HPV18 | 5 (5.4) | 9 (8.7) | 11 (10) |
| HPV68 | 7 (7.5) | 10 (9.6) | 6 (8.2) |
| HPV59 | 7 (7.5) | 8 (7.7) | 8 (7.3) |
| HPV52 | 7 (7.5) | 9 (8.7) | 7 (6.4) |
| HPV35 | 3 (3.2) | 7 (6.7) | 10 (9.1) |
| HPV39 | 7 (7.5) | 3 (2.9) | 9 (8.2) |
| HPV33 | 4 (4.3) | 2 (1.9) | 5 (4.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakogiannis, D.; Zografos, E.; Tzioga, L.; Zografos, C.G.; Zagouri, F.; Bletsa, G. Prevalence and Genotype Distribution of High-Risk HPV Genotypes Among Women in Greece: A Retrospective Analysis of 3500 Women. Cancers 2025, 17, 1267. https://doi.org/10.3390/cancers17081267
Tsakogiannis D, Zografos E, Tzioga L, Zografos CG, Zagouri F, Bletsa G. Prevalence and Genotype Distribution of High-Risk HPV Genotypes Among Women in Greece: A Retrospective Analysis of 3500 Women. Cancers. 2025; 17(8):1267. https://doi.org/10.3390/cancers17081267
Chicago/Turabian StyleTsakogiannis, Dimitris, Eleni Zografos, Lamprini Tzioga, Constantinos G. Zografos, Flora Zagouri, and Garyfalia Bletsa. 2025. "Prevalence and Genotype Distribution of High-Risk HPV Genotypes Among Women in Greece: A Retrospective Analysis of 3500 Women" Cancers 17, no. 8: 1267. https://doi.org/10.3390/cancers17081267
APA StyleTsakogiannis, D., Zografos, E., Tzioga, L., Zografos, C. G., Zagouri, F., & Bletsa, G. (2025). Prevalence and Genotype Distribution of High-Risk HPV Genotypes Among Women in Greece: A Retrospective Analysis of 3500 Women. Cancers, 17(8), 1267. https://doi.org/10.3390/cancers17081267





