Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy
Simple Summary
Abstract
1. Introduction
2. Neutrophils in Cancer
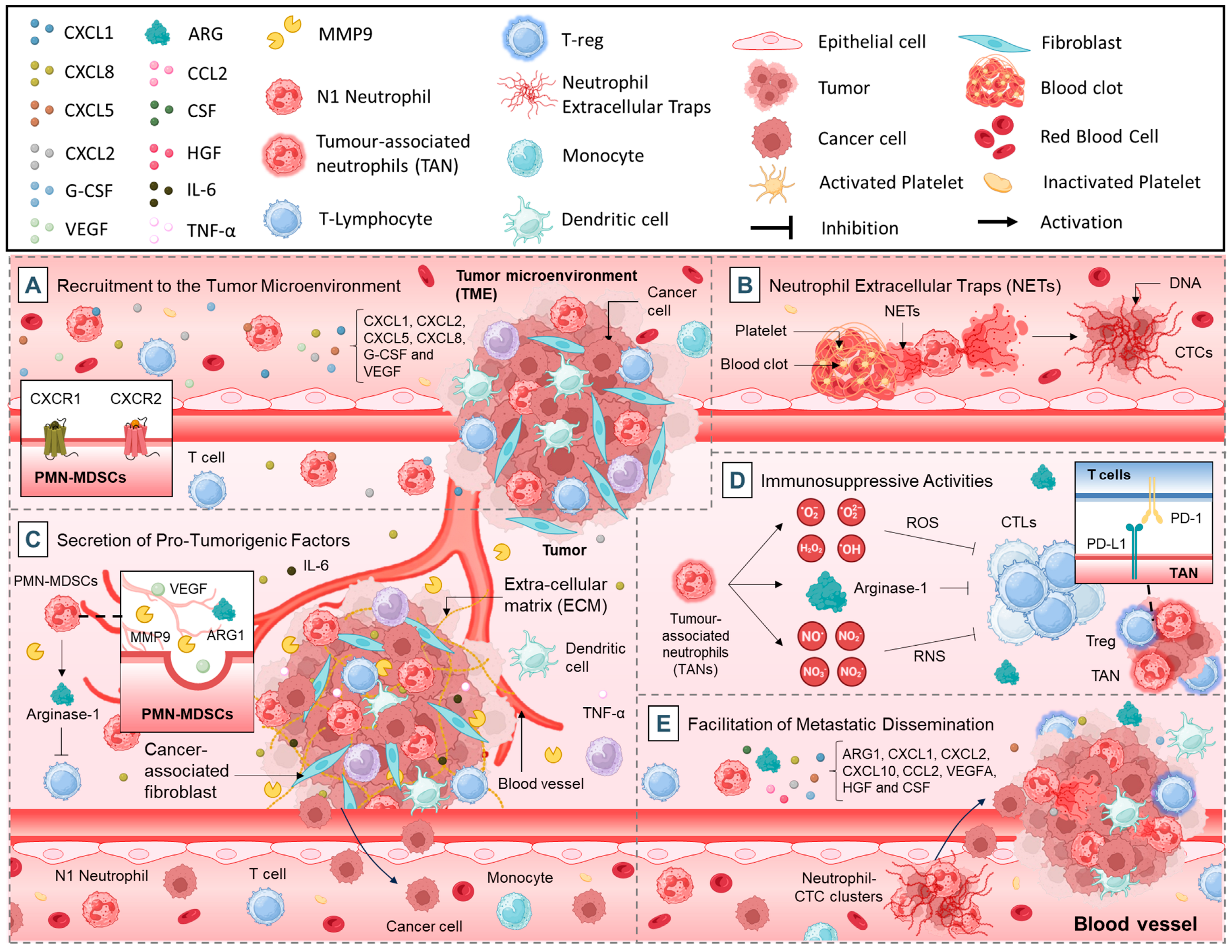
2.1. Pro-Tumorigenic Functions of Neutrophils
2.1.1. Recruitment to the TME
2.1.2. Neutrophil Extracellular Traps (NETs)
2.1.3. Secretion of Pro-Tumorigenic Factors
2.1.4. Immunosuppressive Activities
2.1.5. Facilitation of Metastatic Dissemination
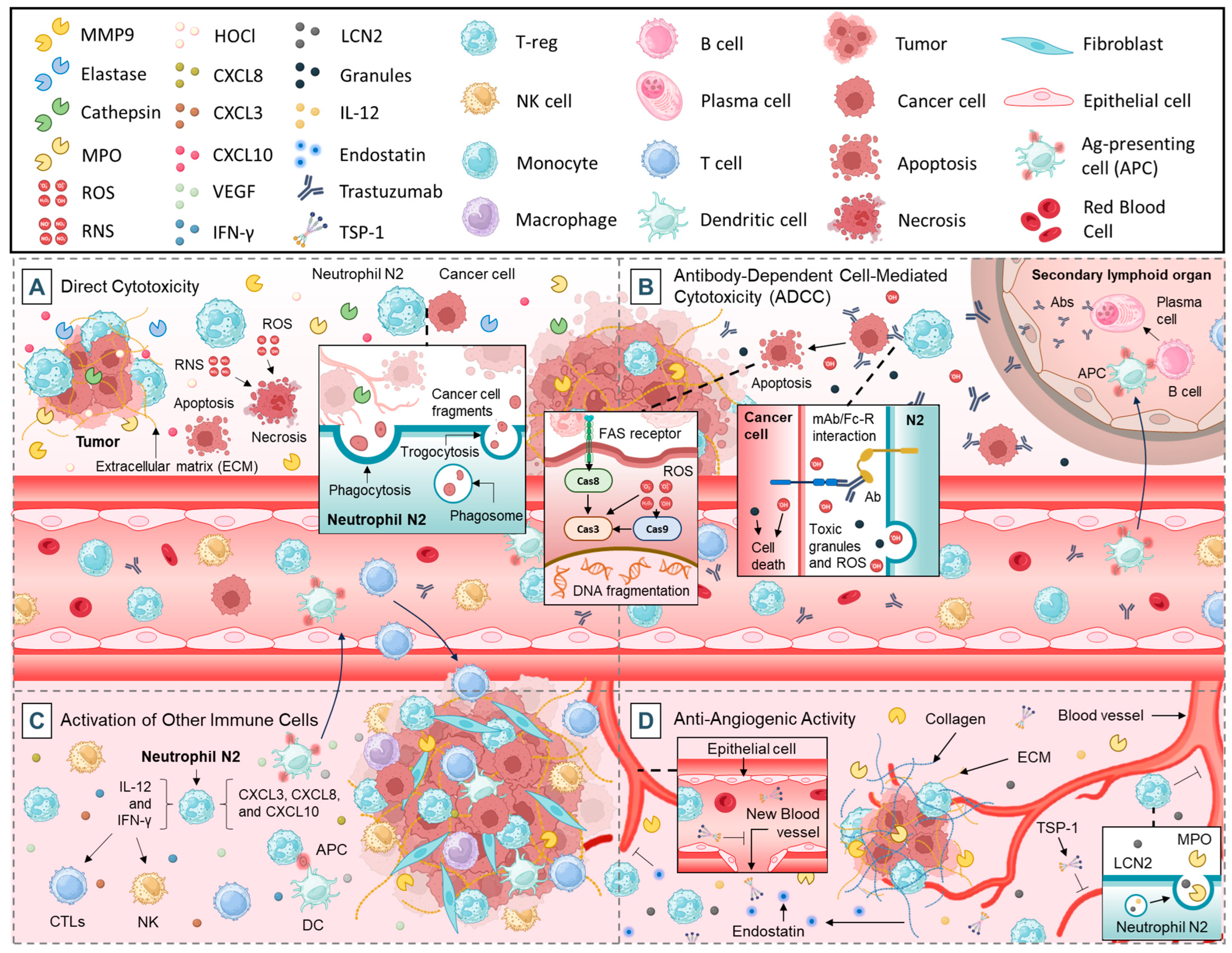
2.2. Anti-Tumorigenic Functions of Neutrophils
2.2.1. Direct Cytotoxicity
2.2.2. Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
2.2.3. Activation of Other Immune Cells
2.2.4. Anti-Angiogenic Activity
3. Therapeutic Strategies
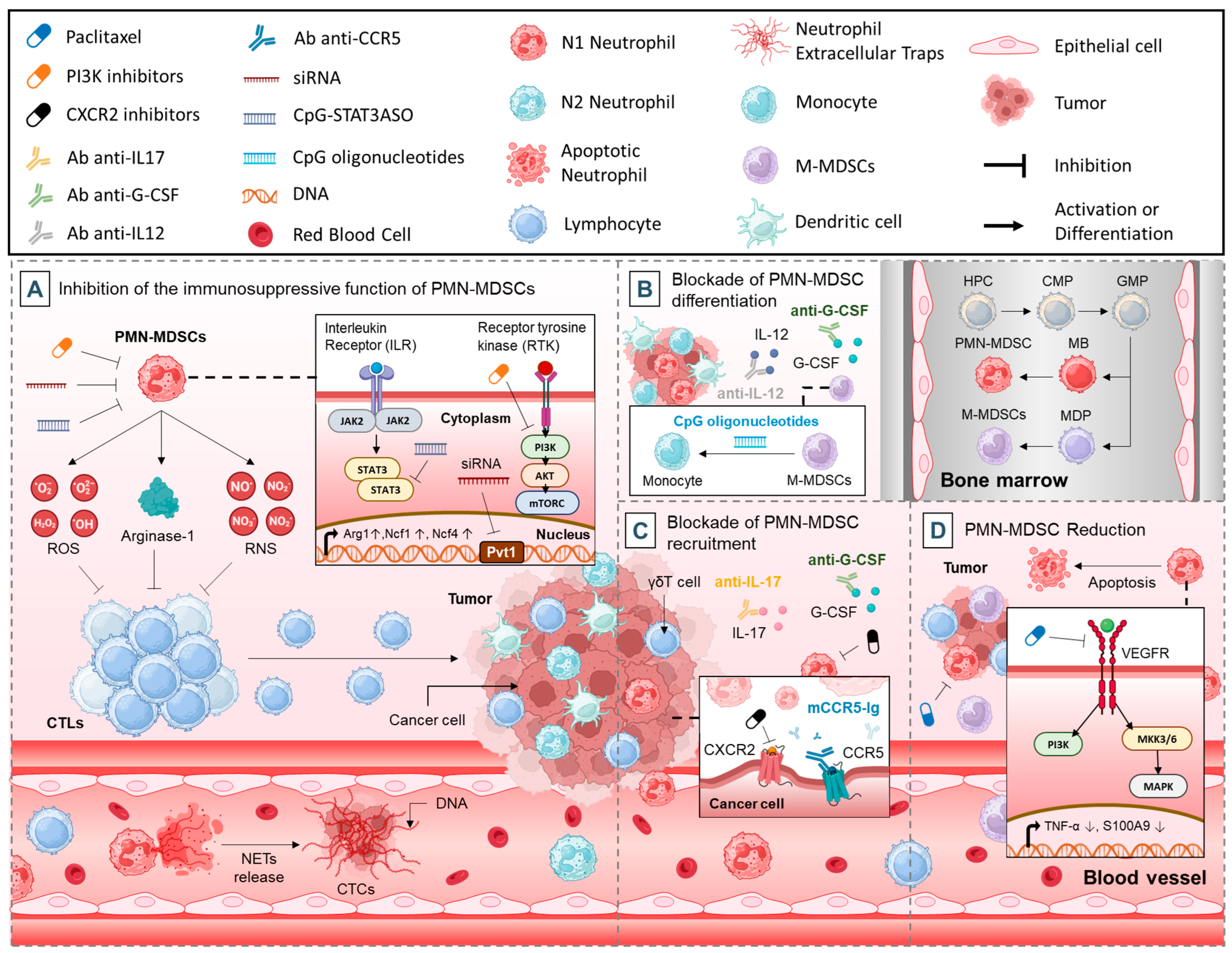
3.1. Inhibition of the Immunosuppressive Function of PMN-MDSCs
3.1.1. Targeting Immune Suppressors
3.1.2. Targeting NETs
3.1.3. Targeting Tyrosine Kinase Signaling
3.1.4. Targeting STAT3
3.1.5. Targeting Noncoding RNAs
3.2. Blockade of PMN-MDSCs Differentiation
3.3. Blockade of PMN-MDSCs Recruitment
3.4. PMN-MDSCs Reduction
4. Clinical Implications
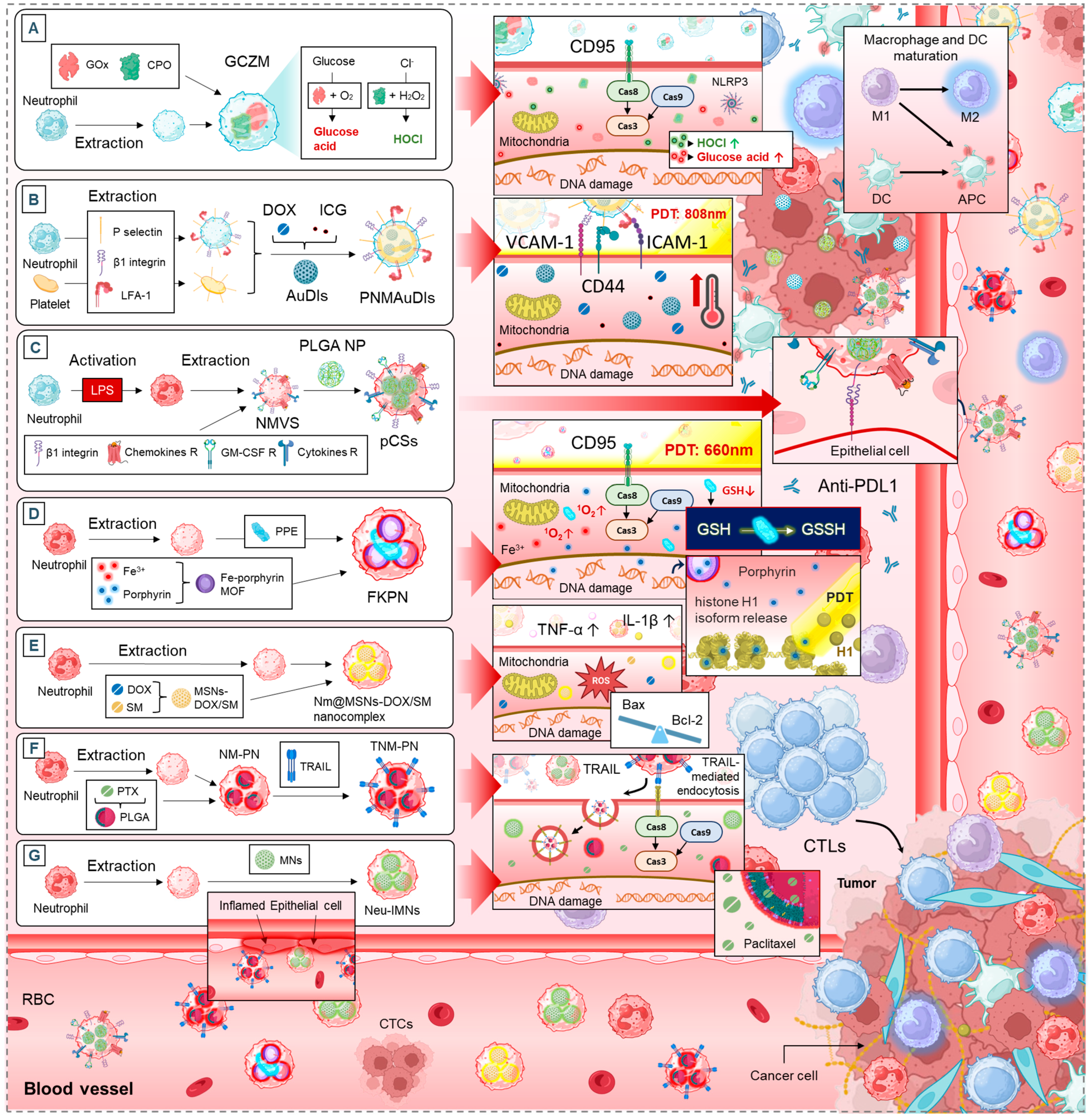
5. Neutrophil-Based Drug Delivery Systems
5.1. Neutrophil Membrane-Mimetics as Drug Delivery Vehicles
5.1.1. Apoptosis and Tumor Growth Suppression
5.1.2. TME Modulation and Immune Activation
5.1.3. Tumor Targeting and Drug Accumulation
5.1.4. Synergistic Effects with Standard Cancer Therapies
5.2. Neutrophils as Multifunctional Delivery Platforms for Multiple Therapies
5.2.1. Enhancing Tumor Targeting and Accumulation
5.2.2. Apoptosis Induction and Photothermal Therapy
5.2.3. Neutrophils to Reprogram the Tumor Microenvironment
5.2.4. Metastasis and Bone-Associated Cancers
6. Challenges, Research Gaps and Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5FU | 5-Fluorouracil |
| Acouscyte/O₂ | Nanoengineered Neutrophils as a Cellular Sonosensitizer |
| ADCC | Antibody-Dependent Cell-Mediated Cytotoxicity |
| ARG1 | Arginase-1 |
| ATRA | All-Trans Retinoic Acid |
| AuNR | Gold Nanorods |
| B16F10 | Murine Melanoma Cell Line |
| Bax | Pro-apoptotic Protein |
| Bcl-2 | Anti-apoptotic Protein |
| bEnd.3 cells | Mouse Brain Endothelial Cells |
| C6 | Murine Glioma Cell Line |
| Cal-27 | Human Oral Squamous Cell Carcinoma Cell Line |
| CCR5 | C-C Chemokine Receptor Type 5 |
| Ce6 | Chlorin e6 |
| COX2 | Cyclooxygenase-2 |
| CpG | Cytosine-phosphate-Guanine |
| CSF | Colony-Stimulating Factor |
| CTCs | Circulating Tumor Cells |
| CTLs | Cytotoxic T Lymphocytes |
| CTX-NPs@NEs | Cabazitaxel-Loaded Nanoparticles with Neutrophils |
| CXCL | Chemokine (C-X motif) Ligand |
| CXCR | C-X-C Chemokine Receptor |
| DC | Dendritic Cells |
| DNase | Deoxyribonuclease |
| DOX | Doxorubicin |
| ECM | Extracellular Matrix |
| EPI-SL | Sialic Acid-Modified Liposomal Epirubicin |
| FKPN | Neutrophil-Mimicking Nanodevice |
| GCZM | Artificial “Super Neutrophils” |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GSH | Glutathione |
| G422 | Murine Glioma Model |
| HCC | Hepatocellular Carcinoma |
| HC11 | Mouse Mammary Epithelial Cell Line |
| HeLa | Human Cervical Cancer Cell Line |
| HepG2 | Human Hepatocellular Carcinoma Cell Line |
| HGF | Hepatocyte Growth Factor |
| HL-7702 | Normal Human Liver Cell Line |
| HOCl | Hypochlorous Acid |
| hNVs | Hybrid Cellular Membrane Nanovesicles |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IC50 | 50% Inhibitory Concentration |
| ICB | Immune Checkpoint Blockade |
| ICG | Indocyanine Green |
| IFN-γ | Interferon-gamma |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-12 | Interleukin-12 |
| L02 | Human Normal Liver Cell Line |
| LCN2 | Lipocalin-2 |
| MCF-10A | Michigan Cancer Foundation-10A |
| MCF-7 | Human Breast Cancer Cell Line |
| MDA-MB-231 | Human Triple-Negative Breast Cancer Cell Line |
| MMPs | Matrix Metalloproteinases |
| MMSNs | Mesoporous Silica Nanoparticles |
| MPO | Myeloperoxidase |
| mTOR | Mechanistic Target of Rapamycin |
| ND-MMSNs | Neutrophil/Dox-loaded Mesoporous Silica Nanoparticles |
| NEs@STING-Mal-NP | Neotype Neutrophil Cytopharmaceutical with Liposomal STING Agonists |
| NETs | Neutrophil Extracellular Traps |
| Neu-IMNs | Neutrophil Membrane-Coated Immunomagnetic Nanoparticles |
| NK cells | Natural Killer Cells |
| NM-HB NPs | Neutrophil Membrane Hybrid Biomimetic Nanoparticles |
| NM-PN | Neutrophil Membrane-Coated PLGA Nanoparticles |
| Nm@MSNs-DOX/SM | Neutrophil Membrane-Coated Mesoporous Silica Nanoparticles Loaded with Doxorubicin and SM |
| NSNP | Nanoparticle–Neutrophil Composites |
| NSNP@Ne | Nanoparticle–Neutrophil Composites |
| PAD4 | Peptidylarginine Deiminase 4 |
| PAN | Photoactive Neutrophils |
| Pan02 | Mouse Pancreatic Adenocarcinoma Cell Line |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PDT | Photodynamic Therapy |
| PI3K | Phosphoinositide 3-Kinase |
| PLGA | Poly(lactic-co-glycolic acid) |
| PMN-MDSCs | Polymorphonuclear Myeloid-Derived Suppressor Cells |
| Ppa-loaded BSA NPs | Pyropheophorbide-a Loaded Albumin Nanoparticles |
| PTX-CL/NEs | Paclitaxel-Loaded Liposomal Neutrophils |
| pCSs | Pseudoneutrophil Cytokine Sponges |
| RA | RGD Apoptotic Peptide Conjugate |
| RA/Ce6 | RGD Apoptotic Peptide Conjugate Decorated Liposomal Photosensitizer Ce6 |
| RAGE | Receptor for Advanced Glycation Endproducts |
| RAW264.7 | Murine Macrophage Cell Line |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| S180 | Sarcoma 180 |
| SCNG | Supramolecular Core–Shell Nanogel System |
| siRNA | Small Interfering RNA |
| SIRPα | Signal Regulatory Protein Alpha |
| SKOV3 | Human Ovarian Cancer Cell Line |
| SNU-719 | Human Epstein–Barr Virus-Associated Gastric Carcinoma Cell Line |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| SU-DHL-2 | Human Diffuse Large B-Cell Lymphoma Cell Line |
| TANs | Tumor-Associated Neutrophils |
| TGF-β | Transforming Growth Factor-beta |
| TLR4 | Toll-Like Receptor 4 |
| TME | Tumor Microenvironment |
| TNF-α | Tumor Necrosis Factor-alpha |
| TNM-PN | Neutrophil Membrane-Coated Nanoparticles |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| Tregs | Regulatory T Cells |
| TSP-1 | Thrombospondin-1 |
| U87 | Human Glioblastoma Cell Line |
| UM-NEs | Urease Micromotor-Powered Neutrophils Nanodrug Delivery System |
| VEGF | Vascular Endothelial Growth Factor |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Ren, Y.; Liu, S.; Ba, Y.; Zuo, A.; Luo, P.; Cheng, Q.; Xu, H.; Han, X. Multi-stage mechanisms of tumor metastasis and therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 270. [Google Scholar] [CrossRef]
- Jackett, K.N.; Browne, A.T.; Aber, E.R.; Clements, M.; Kaplan, R.N. How the bone microenvironment shapes the pre-metastatic niche and metastasis. Nat. Cancer 2024, 5, 1800–1814. [Google Scholar] [CrossRef]
- Glaviano, A.; Lau, H.S.-H.; Carter, L.M.; Lee, E.H.C.; Lam, H.Y.; Okina, E.; Tan, D.J.J.; Tan, W.; Ang, H.L.; Carbone, D.; et al. Harnessing the tumor microenvironment: Targeted cancer therapies through modulation of epithelial-mesenchymal transition. J. Hematol. Oncol. 2025, 18, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Sun, M.; Lu, X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol. Med. 2020, 17, 32. [Google Scholar] [CrossRef]
- Malech, H.L.; Deleo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2014, 1124, 3–10. [Google Scholar] [CrossRef]
- Wahnou, H.; Ndayambaje, M.; Ouadghiri, Z.; Benayad, S.; Elattar, H.; Chgari, O.; Naya, A.; Zaid, Y.; Oudghiri, M. Artemisia herba-alba: Antioxidant capacity and efficacy in preventing chronic arthritis in vivo. Inflammopharmacology 2024, 32, 1855–1870. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Latif, M.; Elkoraichi, I.; El Faqer, O.; Wahnou, H.; Mtairag, E.M.; Oudghiri, M.; Rais, S. Phytochemical analysis and immunomodulatory activities in vitro and in vivo of Aframomum melegueta K Schum seed extracts. Inflammopharmacology 2024, 32, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- El Faqer, O.; Bendiar, S.; Rais, S.; Elkoraichi, I.; Dakir, M.; Elouaddari, A.; Amrani, A.E.; Oudghiri, M.; Mtairag, E.M. Phytochemical characterization and immunomodulatory effects of aqueous, ethanolic extracts and essential oil of Syzygium aromaticum L. on human neutrophils. Sci. Afr. 2022, 18, e01395. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Dutta, A.; Bhagat, S.; Paul, S.; Katz, J.P.; Sengupta, D.; Bhargava, D. Neutrophils in Cancer and Potential Therapeutic Strategies Using Neutrophil-Derived Exosomes. Vaccines 2023, 11, 1028. [Google Scholar] [CrossRef]
- Yin, H.; Gao, S.; Chen, Q.; Liu, S.; Shoucair, S.; Ji, Y.; Lou, W.; Yu, J.; Wu, W.; Pu, N. Tumor-associated N1 and N2 neutrophils predict prognosis in patients with resected pancreatic ductal adenocarcinoma: A preliminary study. MedComm 2022, 3, e183. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, Y.; Ding, P.; Wu, T.; Ji, G. The role of CXCL family members in different diseases. Cell Death Discov. 2023, 9, 212. [Google Scholar] [CrossRef]
- Sabroe, I.; Jones, E.C.; Whyte, M.K.; Dower, S.K. Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology 2005, 115, 90–98. [Google Scholar] [CrossRef]
- Ohki, Y.; Heissig, B.; Sato, Y.; Akiyama, H.; Zhu, Z.; Hicklin, D.J.; Shimada, K.; Ogawa, H.; Daida, H.; Hattori, K.; et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. Faseb J. 2005, 19, 2005–2007. [Google Scholar] [CrossRef]
- SenGupta, S.; Hein, L.E.; Parent, C.A. The Recruitment of Neutrophils to the Tumor Microenvironment Is Regulated by Multiple Mediators. Front. Immunol. 2021, 12, 734188. [Google Scholar] [CrossRef]
- Chen, R.; Pan, S. Increased neutrophil infiltration as a body-wide effect in pancreatic cancer development. EBioMedicine 2022, 81, 104089. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, Y.; Shi, Y.; Shao, C. Neutrophils in the tumor microenvironment and their functional modulation by mesenchymal stromal cells. Cell. Immunol. 2022, 379, 104576. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, G.; Sun, Y.; Li, M.; Hu, Z.; Cao, P.; Li, X.; Song, Z.; Chen, J. Neutrophil infiltration associated genes on the prognosis and tumor immune microenvironment of lung adenocarcinoma. Front. Immunol. 2023, 14, 1304529. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, J.; Peng, Y.; Sun, J.; Cheng, P.; Huang, Q. Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers 2022, 14, 4755. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef]
- Kim, J.; Bae, J.S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef]
- Yan, M.; Gu, Y.; Sun, H.; Ge, Q. Neutrophil extracellular traps in tumor progression and immunotherapy. Front. Immunol. 2023, 14, 1135086. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Nasrollahzadeh, E.; Razi, S.; Keshavarz-Fathi, M.; Mazzone, M.; Rezaei, N. Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol. Immunother. CII 2020, 69, 1673–1697. [Google Scholar] [CrossRef]
- Hu, W.; Lee, S.M.L.; Bazhin, A.V.; Guba, M.; Werner, J.; Nieß, H. Neutrophil extracellular traps facilitate cancer metastasis: Cellular mechanisms and therapeutic strategies. J. Cancer Res. Clin. Oncol. 2023, 149, 2191–2210. [Google Scholar] [CrossRef]
- Demkow, U. Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis. Cancers 2021, 13, 4495. [Google Scholar] [CrossRef]
- Wienkamp, A.K.; Erpenbeck, L.; Rossaint, J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022, 13, 953129. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Chamardani, T.M.; Amiritavassoli, S. Inhibition of NETosis for treatment purposes: Friend or foe? Mol. Cell. Biochem. 2022, 477, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Barillari, G. The Impact of Matrix Metalloproteinase-9 on the Sequential Steps of the Metastatic Process. Int. J. Mol. Sci. 2020, 21, 4526. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 120–122. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-α with Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. A 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Martí, I.L.A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. CMLS 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Wang, L.; Kuang, Z.; Zhang, D.; Gao, Y.; Ying, M.; Wang, T. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccines Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. Contextual Regulation of Inflammation: A Duet by Transforming Growth Factor-β and Interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef]
- Ujike, A.; Takeda, K.; Nakamura, A.; Ebihara, S.; Akiyama, K.; Takai, T. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(-/-) mice. Nat. Immunol. 2002, 3, 542–548. [Google Scholar] [CrossRef]
- Hu, A.; Sun, L.; Lin, H.; Liao, Y.; Yang, H.; Mao, Y. Harnessing innate immune pathways for therapeutic advancement in cancer. Signal Transduct. Target. Ther. 2024, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Xie, J.; Zhao, Z.Z.; Li, P.; Liu, Q.; Guo, Y.; Meng, Y.; Wan, X.J.; Bian, J.J.; Deng, X.M.; et al. PD-L1 maintains neutrophil extracellular traps release by inhibiting neutrophil autophagy in endotoxin-induced lung injury. Front. Immunol. 2022, 13, 949217. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, R.-R.; Miao, N.-J.; Tang, J.-J.; Zhang, Y.-W.; Lu, X.-R.; Yan, P.-Y.; Wang, J.; Jia, X.-M. PD-L1 negatively regulates antifungal immunity by inhibiting neutrophil release from bone marrow. Nat. Commun. 2022, 13, 6857. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, M.; Tan, Z.; Zheng, H.; Liu, X. Neutrophil: A New Player in Metastatic Cancers. Front. Immunol. 2020, 11, 565165. [Google Scholar] [CrossRef]
- Huang, X.; Nepovimova, E.; Adam, V.; Sivak, L.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K. Neutrophils in Cancer immunotherapy: Friends or foes? Mol. Cancer 2024, 23, 107. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef]
- Shi, H.; Yu, S.; Li, W.; Li, X.; Wu, X.; Qu, X.; Ma, Y.; Zheng, C.; Che, X. Neutrophils in the tumor microenvironment: Role in tumor progression and potential targeted therapeutic strategy. J. Cancer Metastasis Treat. 2024, 10, 13. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Radisky, E.S. Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis. J. Biol. Chem. 2024, 300, 107347. [Google Scholar] [CrossRef]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e3946. [Google Scholar] [CrossRef]
- Ustyanovska Avtenyuk, N.; Visser, N.; Bremer, E.; Wiersma, V.R. The Neutrophil: The Underdog That Packs a Punch in the Fight against Cancer. Int. J. Mol. Sci. 2020, 21, 7820. [Google Scholar] [CrossRef]
- Westman, J.; Grinstein, S.; Marques, P.E. Phagocytosis of Necrotic Debris at Sites of Injury and Inflammation. Front. Immunol. 2019, 10, 3030. [Google Scholar] [CrossRef]
- Miyake, K.; Karasuyama, H. The Role of Trogocytosis in the Modulation of Immune Cell Functions. Cells 2021, 10, 1255. [Google Scholar] [CrossRef]
- Rajgopal, S.; Nakano, K.; Cook, L.M. Beyond the horizon: Neutrophils leading the way in the evolution of immunotherapy. Cancer Med. 2023, 12, 21885–21904. [Google Scholar] [CrossRef]
- Behrens, L.M.; van Egmond, M.; van den Berg, T.K. Neutrophils as immune effector cells in antibody therapy in cancer. Immunol. Rev. 2023, 314, 280–301. [Google Scholar] [CrossRef]
- Treffers, L.W.; van Houdt, M.; Bruggeman, C.W.; Heineke, M.H.; Zhao, X.W.; van der Heijden, J.; Nagelkerke, S.Q.; Verkuijlen, P.; Geissler, J.; Lissenberg-Thunnissen, S.; et al. FcγRIIIb Restricts Antibody-Dependent Destruction of Cancer Cells by Human Neutrophils. Front. Immunol. 2018, 9, 3124. [Google Scholar] [CrossRef]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef]
- Mandó, P.; Rivero, S.G.; Rizzo, M.M.; Pinkasz, M.; Levy, E.M. Targeting ADCC: A different approach to HER2 breast cancer in the immunotherapy era. Breast 2021, 60, 15–25. [Google Scholar] [CrossRef]
- Weiner, G.J. Rituximab: Mechanism of Action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Irvine, E.B.; Nikolov, A.; Khan, M.Z.; Peters, J.M.; Lu, R.; Sixsmith, J.; Wallace, A.; van Woudenbergh, E.; Shin, S.; Karpinski, W.; et al. Fc-engineered antibodies promote neutrophil-dependent control of Mycobacterium tuberculosis. Nat. Microbiol. 2024, 9, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Gogesch, P.; Dudek, S.; van Zandbergen, G.; Waibler, Z.; Anzaghe, M. The Role of Fc Receptors on the Effectiveness of Therapeutic Monoclonal Antibodies. Int. J. Mol. Sci. 2021, 22, 8947. [Google Scholar] [CrossRef]
- Mata-Molanes, J.J.; Rebollo-Liceaga, J.; Martínez-Navarro, E.M.; Manzano, R.G.; Brugarolas, A.; Juan, M.; Sureda, M. Relevance of Fc Gamma Receptor Polymorphisms in Cancer Therapy with Monoclonal Antibodies. Front. Oncol. 2022, 12, 926289. [Google Scholar] [CrossRef]
- Gaggero, S.; Witt, K.; Carlsten, M.; Mitra, S. Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy. Front. Immunol. 2020, 11, 621225. [Google Scholar] [CrossRef]
- Liu, J.; Cao, S.; Kim, S.; Chung, E.Y.; Homma, Y.; Guan, X.; Jimenez, V.; Ma, X. Interleukin-12: An update on its immunological activities, signaling and regulation of gene expression. Curr. Immunol. Rev. 2005, 1, 119–137. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- van Gisbergen, K.P.; Sanchez-Hernandez, M.; Geijtenbeek, T.B.; van Kooyk, Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005, 201, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes. Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. CMLS 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Lewis, C.E.; Murdoch, C. Neutrophils: Key mediators of tumour angiogenesis. Int. J. Exp. Pathol. 2009, 90, 222–231. [Google Scholar] [CrossRef]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell. Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar]
- Economopoulou, M.; Bdeir, K.; Cines, D.B.; Fogt, F.; Bdeir, Y.; Lubkowski, J.; Hammes, H.P.; Chavakis, T. Inhibition of Pathologic Retinal Neovascularization by –Defensins. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1438. [Google Scholar]
- Williams, M.R.; Azcutia, V.; Newton, G.; Alcaide, P.; Luscinskas, F.W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011, 32, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Guardado, S.; Ojeda-Juárez, D.; Kaul, M.; Nordgren, T.M. Comprehensive review of lipocalin 2-mediated effects in lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L726–L733. [Google Scholar] [CrossRef]
- Okuno, K.; Naito, Y.; Yasumura, S.; Sawada, H.; Asakura, M.; Masuyama, T.; Ishihara, M. Haploinsufficiency of Transferrin Receptor 1 Impairs Angiogenesis with Reduced Mitochondrial Complex I in Mice with Limb Ischemia. Sci. Rep. 2019, 9, 13658. [Google Scholar] [CrossRef]
- Ozel, I.; Duerig, I.; Domnich, M.; Lang, S.; Pylaeva, E.; Jablonska, J. The Good, the Bad, and the Ugly: Neutrophils, Angiogenesis, and Cancer. Cancers 2022, 14, 536. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Neutrophils and polymorphonuclear myeloid-derived suppressor cells: An emerging battleground in cancer therapy. Oncogenesis 2022, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, X.; Wang, T.; Xia, X.; Cao, F.; Tian, J.; Xu, P.; Ma, J.; Xu, H.; Wang, S. Long noncoding RNA Pvt1 regulates the immunosuppression activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Mol. Cancer 2019, 18, 61. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Nefedova, Y.; Yoder, D.; Gabrilovich, D.I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 2004, 172, 989–999. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar] [CrossRef]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e865. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, X.; Zhang, L.; Lu, X.; Chaudhary, S.; Teng, R.; Frederickson, C.; Champion, M.M.; Zhao, R.; Cheng, L.; et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA 2018, 115, 10094–10099. [Google Scholar] [CrossRef]
- Nagaraj, S.; Schrum, A.G.; Cho, H.I.; Celis, E.; Gabrilovich, D.I. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010, 184, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Olsson, A.K. Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res. 2016, 76, 4311–4315. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Swanson, K.D.; Csizmadia, E.; Solanki, A.; Landon-Brace, N.; Gehring, M.P.; Helenius, K.; Olson, B.M.; Pyzer, A.R.; Wang, L.C.; et al. Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity. Cancer Discov. 2017, 7, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef]
- Davis, R.J.; Moore, E.C.; Clavijo, P.E.; Friedman, J.; Cash, H.; Chen, Z.; Silvin, C.; Van Waes, C.; Allen, C. Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kδ/γ. Cancer Res. 2017, 77, 2607–2619. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Moreira, D.; Adamus, T.; Zhao, X.; Su, Y.L.; Zhang, Z.; White, S.V.; Swiderski, P.; Lu, X.; DePinho, R.A.; Pal, S.K.; et al. STAT3 Inhibition Combined with CpG Immunostimulation Activates Antitumor Immunity to Eradicate Genetically Distinct Castration-Resistant Prostate Cancers. Clin. Cancer Res. 2018, 24, 5948–5962. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, W.; Xu, J.; Li, J.; Hong, Z.; Yin, Z.; Wang, X. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget 2016, 7, 8866–8878. [Google Scholar] [CrossRef]
- Mao, Y.; Sarhan, D.; Steven, A.; Seliger, B.; Kiessling, R.; Lundqvist, A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 2014, 20, 4096–4106. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Kohanbash, G.; Fellows-Mayle, W.; Hamilton, R.L.; Komohara, Y.; Decker, S.A.; Ohlfest, J.R.; Okada, H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011, 71, 2664–2674. [Google Scholar] [CrossRef]
- Zelenay, S.; van der Veen, A.G.; Böttcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef]
- Mao, Y.; Poschke, I.; Wennerberg, E.; Pico de Coaña, Y.; Egyhazi Brage, S.; Schultz, I.; Hansson, J.; Masucci, G.; Lundqvist, A.; Kiessling, R. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013, 73, 3877–3887. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Qin, H.; Lerman, B.; Sakamaki, I.; Wei, G.; Cha, S.C.; Rao, S.S.; Qian, J.; Hailemichael, Y.; Nurieva, R.; Dwyer, K.C.; et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat. Med. 2014, 20, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- He, K.; Liu, X.; Hoffman, R.D.; Shi, R.Z.; Lv, G.Y.; Gao, J.L. G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors. FEBS Open Bio 2022, 12, 1268–1285. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Shirota, H.; Klinman, D.M. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J. Immunol. 2012, 188, 1592–1599. [Google Scholar] [CrossRef]
- Michels, T.; Shurin, G.V.; Naiditch, H.; Sevko, A.; Umansky, V.; Shurin, M.R. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J. Immunotoxicol. 2012, 9, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef]
- Wang, G.; Lu, X.; Dey, P.; Deng, P.; Wu, C.C.; Jiang, S.; Fang, Z.; Zhao, K.; Konaparthi, R.; Hua, S.; et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016, 6, 80–95. [Google Scholar] [CrossRef]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5(+) Myeloid-Derived Suppressor Cells Are Enriched and Activated in Melanoma Lesions. Cancer Res. 2018, 78, 157–167. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Molecular predictors of gemcitabine response in pancreatic cancer. World J. Gastrointest. Oncol. 2011, 3, 153–164. [Google Scholar] [CrossRef]
- Eriksson, E.; Wenthe, J.; Irenaeus, S.; Loskog, A.; Ullenhag, G. Gemcitabine reduces MDSCs, tregs and TGFβ-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J. Transl. Med. 2016, 14, 282. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Sevko, A.; Michels, T.; Vrohlings, M.; Umansky, L.; Beckhove, P.; Kato, M.; Shurin, G.V.; Shurin, M.R.; Umansky, V. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J. Immunol. 2013, 190, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Yao, W.; Wang, S.; Zhang, G.; Chen, J.; Hou, Q.; Li, S.; Li, H.; Ye, C.; et al. A randomized, multicenter phase III Study of once-per-cycle administration of efbemalenograstim alfa (F-627), a novel long-acting rhG-CSF, for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer. BMC Cancer 2024, 24, 1143. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausovà, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Guo, C.; Sharp, A.; Vogl, U.; Colombo, I.; Stathis, A.; Jain, S.; Chandran, K.; Tiu, C.; Paschalis, A.; Matthews, R.E.; et al. 454O A phase (Ph) I/II trial of the CXCR2 antagonist AZD5069 in combination with enzalutamide (ENZA) in patients (pts) with metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol. 2022, 33, S745. [Google Scholar] [CrossRef]
- Shah, M.A.; Yoshino, T.; Tebbutt, N.C.; Grothey, A.; Tabernero, J.; Xu, R.H.; Cervantes, A.; Oh, S.C.; Yamaguchi, K.; Fakih, M.; et al. Napabucasin Plus FOLFIRI in Patients with Previously Treated Metastatic Colorectal Cancer: Results From the Open-Label, Randomized Phase III CanStem303C Study. Clin. Color. Cancer 2023, 22, 100–110. [Google Scholar] [CrossRef]
- Yamazaki, T.; Gunderson, A.J.; Gilchrist, M.; Whiteford, M.; Kiely, M.X.; Hayman, A.; O’Brien, D.; Ahmad, R.; Manchio, J.V.; Fox, N.; et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: A single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 1189–1200. [Google Scholar] [CrossRef]
- Bennouna, J.; Touchefeu, Y.; Ghiringhelli, F.; Isambert, N.; Barlesi, F.; Tomasini, P.; Cassier, P.; Edeline, J.; Le Sourd, S.M.; Tosi, D.; et al. 15P STELLAR-001: A phase I study of the anti-C5aR avdoralimab in combination with the anti-PD-L1 durvalumab in advanced solid tumors. Ann. Oncol. 2022, 33, S9. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H., Jr.; Hansen, A.R.; Lee, J.S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Wu, W.; Gao, F.; Li, R.Q.; Song, W.; Zhuang, Z.N.; Liu, C.J.; Zhang, X.Z. Artificial super neutrophils for inflammation targeting and HClO generation against tumors and infections. Adv. Mater. 2019, 31, 1901179. [Google Scholar]
- Wu, Q.; He, Z.; Wang, X.; Zhang, Q.; Wei, Q.; Ma, S.; Ma, C.; Li, J.; Wang, Q. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef]
- Rao, L.; Wu, L.; Liu, Z.; Tian, R.; Yu, G.; Zhou, Z.; Yang, K.; Xiong, H.G.; Zhang, A.; Yu, G.T.; et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 2020, 11, 4909. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Lu, Q.; Zhao, J.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; He, Z.; Sun, J. Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition. Biomaterials 2020, 242, 119932. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Shen, Y.; Hassan, M.; Shen, J.; Jiang, W.; Su, Y.; Chen, J.; Bai, L.; Zhou, W.; et al. Pseudoneutrophil Cytokine Sponges Disrupt Myeloid Expansion and Tumor Trafficking to Improve Cancer Immunotherapy. Nano Lett. 2020, 20, 242–251. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, Y.; Qin, G.; Wei, Y.; Yang, J.; Huang, Y.; Ren, J.; Qu, X. A neutrophil mimicking metal-porphyrin-based nanodevice loaded with porcine pancreatic elastase for cancer therapy. Nat. Commun. 2023, 14, 1974. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, D.; Sun, X.; Mo, Q.; Chen, S.; Chen, W.; Gui, R.; Ma, X. Biomimetic nanotherapy: Core-shell structured nanocomplexes based on the neutrophil membrane for targeted therapy of lymphoma. J. Nanobiotechnol. 2021, 19, 179. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Ouyang, Y.; Chu, L.; Xu, M.; Wang, K.; Tong, X. Engineering of Neutrophil Membrane Camouflaging Nanoparticles Realizes Targeted Drug Delivery for Amplified Antitumor Therapy. Int. J. Nanomed. 2021, 16, 1175–1187. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Cao, Y.; Wang, Y.; Wang, F.; Zhang, F.; Zheng, S. Biodegradable Hypocrellin B nanoparticles coated with neutrophil membranes for hepatocellular carcinoma photodynamics therapy effectively via JUNB/ROS signaling. Int. Immunopharmacol. 2021, 99, 107624. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X.; et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Z.; Zhao, C.; Liu, L.; Zhang, K.; Lai, J.; Meng, Q.F.; Yao, G.; Huang, Q.; Zhao, X.Z.; et al. Neutrophil membrane-coated immunomagnetic nanoparticles for efficient isolation and analysis of circulating tumor cells. Biosens. Bioelectron. 2022, 213, 114425. [Google Scholar] [CrossRef]
- Zheng, J.; Qi, R.; Dai, C.; Li, G.; Sang, M. Enzyme Catalysis Biomotor Engineering of Neutrophils for Nanodrug Delivery and Cell-Based Thrombolytic Therapy. ACS Nano 2022, 16, 2330–2344. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Ding, J.; Sui, D.; Liu, M.; Su, Y.; Wang, Y.; Liu, M.; Luo, X.; Liu, X.; Deng, Y.; Song, Y. Sialic acid conjugate-modified liposomes enable tumor homing of epirubicin via neutrophil/monocyte infiltration for tumor therapy. Acta Biomater. 2021, 134, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lu, Y.; Shi, Y.; Jiang, M.; Shan, X.; Li, X.; Zhang, J.; Qin, B.; Liu, X.; Guo, X.; et al. Neutrophil hitchhiking for drug delivery to the bone marrow. Nat. Nanotechnol. 2023, 18, 647–656. [Google Scholar] [CrossRef]
- Ju, C.; Wen, Y.; Zhang, L.; Wang, Q.; Xue, L.; Shen, J.; Zhang, C. Neoadjuvant Chemotherapy Based on Abraxane/Human Neutrophils Cytopharmaceuticals with Radiotherapy for Gastric Cancer. Small 2019, 15, e1905688. [Google Scholar] [CrossRef]
- Hao, M.; Zhu, L.; Hou, S.; Chen, S.; Li, X.; Li, K.; Zhu, N.; Chen, S.; Xue, L.; Ju, C. Sensitizing tumors to immune checkpoint blockage via STING agonists delivered by tumor-penetrating neutrophil cytopharmaceuticals. ACS Nano 2023, 17, 1663–1680. [Google Scholar] [CrossRef]
- Chu, D.; Zhao, Q.; Yu, J.; Zhang, F.; Zhang, H.; Wang, Z. Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy. Adv. Health Mater. 2016, 5, 1088–1093. [Google Scholar] [CrossRef]
- Ye, B.; Zhao, B.; Wang, K.; Guo, Y.; Lu, Q.; Zheng, L.; Li, A.; Qiao, J. Neutrophils mediated multistage nanoparticle delivery for prompting tumor photothermal therapy. J. Nanobiotechnol. 2020, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Hu, G.; Wu, X.; Nie, Y.; Wu, H.; Kong, D.; Ning, X. Multistage targeted “Photoactive neutrophil” for enhancing synergistic photo-chemotherapy. Biomaterials 2021, 279, 121224. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Tie, C.; Yan, C.; Deng, Z.; Wan, Q.; Liu, X.; Yan, F.; Zheng, H. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018, 9, 4777. [Google Scholar] [CrossRef]
- Wang, J.; Mei, T.; Liu, Y.; Zhang, Y.; Zhang, Z.; Hu, Y.; Wang, Y.; Wu, M.; Yang, C.; Zhong, X.; et al. Dual-targeted and MRI-guided photothermal therapy via iron-based nanoparticles-incorporated neutrophils. Biomater. Sci. 2021, 9, 3968–3978. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, J.E.; Luo, T.; Wang, J.; Kang, L.; Wang, Y.; Luo, S.; Wang, Z.; Zhou, Z.; Zhu, J.; et al. Nanoengineered Neutrophils as a Cellular Sonosensitizer for Visual Sonodynamic Therapy of Malignant Tumors. Adv. Mater. 2022, 34, e2109969. [Google Scholar] [CrossRef]

| Breast Cancer | ||||
|---|---|---|---|---|
| Intervention/Treatment | Target | Phase | Identifier/ References | Results |
| Efbemalenograstim alfa (F-627) | G-CSF | III | NCT04174599 [126] |
|
| Reparixin (R) + paclitaxel (PTX) | CXCR1/CXCR2 | II | NCT02370238 [127] |
|
| Pancreatic Cancer | ||||
| Intervention/Treatment | Target | Phase | Identifier | Results |
| AZD9150 + MEDI4736 | STAT3 | II | NCT02983578 |
|
| NIS793 + Nab-paclitaxel/gemcitabine | TGF-β | III | NCT04935359 |
|
| SX-682 + tislelizumab | CXCR1/CXCR2 | II | NCT05604560 |
|
| Prostate Cancer | ||||
| Intervention/Treatment | Target | Phase | Identifier/ References | Results |
| AZD5069 + enzalutamide (ENZA) | CXCR1/CXCR2 | I/II | NCT03177187 [128] |
|
| Hepatocellular Carcinoma | ||||
| Intervention/Treatment | Target | Phase | Identifier | Results |
| BMS-986253 + nivolumab | IL-8 | II | NCT04050462 |
|
| Colorectal Cancer | ||||
| Intervention/Treatment | Target | Phase | Identifier/ References | Results |
| AZD9150 + MEDI4736 | STAT3 | II | NCT02983578 |
|
| BBI-608 + FOLFIRI (5-FU, leucovorin, irinotecan) | III | NCT02753127 [129] |
| |
| Head and Neck Cancer | ||||
| Intervention/Treatment | Target | Phase | Identifier | Results |
| TTI-101 + pembrolizumab | STAT3 | I/II | NCT05668949 |
|
| Melanoma | ||||
| Intervention/Treatment | Target | Phase | Identifier | Results |
| Tocilizumab + ipilimumab and nivolumab | IL-6 | II | NCT03999749 |
|
| Non-Hodgkin’s Lymphoma | ||||
| Intervention/Treatment | Target | Phase | Identifier/ References | Results |
| CC-95251 + rituximab | CD47-SIRPα | I | NCT03934814 [129] |
|
| Lemzoparlimab + rituximab | I | NCT03783403 |
| |
| Multiple Myeloma | ||||
| Intervention/Treatment | Target | Phase | Identifier | Results |
| Siltuximab | IL-6 | II | NCT01484275 |
|
| Rectal Cancer | ||||
| Intervention/Treatment | Target | Phase | Identifier/ References | Results |
| LY2157299 + neoadjuvant chemoradiation | TGF-β | II | NCT02688712 [130] |
|
| Solid Tumors | ||||
| Intervention/Treatment | Target | Phase | Identifier/ References | Results |
| TJ210001 | C5aR | I | NCT04947033 |
|
| IPH5401 + durvalumab | I | NCT03665129 [131] |
| |
| Navarixin + pembrolizumab | CXCR1/CXCR2 | II | NCT03473925 [132] |
|
| M7824 (bintrafusp alfa) | TGF-β | III | NCT03631706 |
|
| BI 765063 + BI 754091 | CD47-SIRPα | I | NCT03990233 |
|
| Acute Myeloid Leukemia | ||||
| Intervention/Treatment | Target | Phase | Identifier | Results |
| Magrolimab + azacitidine | CD47-SIRPα | III | NCT04778397 |
|
| Technology | Model | Concentration | Mechanisms | Reference |
|---|---|---|---|---|
| Artificial “super neutrophils” (GCZM) (Figure 4A) |
|
|
| [134] |
| Supramolecular core–shell nanogel system (SCNG) |
|
|
| [135] |
| Hybrid cellular membrane nanovesicles (hNVs) |
|
|
| [136] |
| Platelet–neutrophil hybrid membrane-coated gold nanocages (PNMAuDIs) (Figure 4B) |
|
|
| [137] |
| Pseudoneutrophil cytokine sponges (pCSs) (Figure 4C) |
|
|
| [138] |
| Neutrophil-mimicking nanodevice (FKPN) (Figure 4D) |
|
|
| [139] |
| Nm@MSNs-DOX/SM nanocomplex (Figure 4E) |
|
|
| [140] |
| Neutrophil membrane-coated nanoparticles (TNM-PN) (Figure 4F) |
|
|
| [141] |
| Neutrophil membrane-coated PLGA nanoparticles (NM-PN) (Figure 4G) |
|
| ||
| NM-HB NPs-mediated PDT |
|
|
| [142] |
| Neutrophil membrane-camouflaging nanoparticles (TNM-PN) |
|
|
| [143] |
| Neutrophil membrane-coated immunomagnetic nanoparticles (Neu-IMNs) |
|
|
| [144] |
| Urease micromotor-powered neutrophils (UM-NEs) nanodrug delivery system (Figure 5A) |
|
|
| [145] |
| Paclitaxel-loaded liposomal neutrophils (PTX-CL/NEs) (Figure 5B) |
|
|
| [146] |
| Sialic acid-modified liposomal epirubicin (EPI-SL) |
|
|
| [147] |
| CTX-NPs@NEs (cabazitaxel-loaded nanoparticles with neutrophils) (Figure 5C) |
|
|
| [148] |
| Abraxane/human neutrophils cytopharmaceuticals (Figure 5D) |
|
|
| [149] |
| Neotype neutrophil cytopharmaceutical (NEs@STING-Mal-NP) with liposomal STING agonists (Figure 5E) |
|
|
| [150] |
| Pyropheophorbide-a loaded albumin NPs (Ppa-loaded BSA NPs) |
|
|
| [151] |
| Neutrophil-based delivery system for Au nanorods (AuNR) (Figure 5F) |
|
|
| [152] |
| Photoactive neutrophils (PAN) encapsulated multifunctional nanocomplex (RA/Ce6) of RGD apoptotic peptide conjugate (RA) decorated Liposomal photosensitizer Ce6 (Figure 5G) |
|
|
| [153] |
| Inflammation-activatable engineered neutrophils: neutrophil/Dox-loaded MMSNs (ND-MMSNs) (Figure 5H) |
|
|
| [154] |
| Nanoparticle–neutrophil composites (NSNP@Ne) (Figure 5I) |
|
|
| [155] |
| Nanoengineered neutrophils (Acouscyte/O₂) |
|
|
| [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahnou, H.; El Kebbaj, R.; Hba, S.; Ouadghiri, Z.; El Faqer, O.; Pinon, A.; Liagre, B.; Limami, Y.; Duval, R.E. Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy. Cancers 2025, 17, 1232. https://doi.org/10.3390/cancers17071232
Wahnou H, El Kebbaj R, Hba S, Ouadghiri Z, El Faqer O, Pinon A, Liagre B, Limami Y, Duval RE. Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy. Cancers. 2025; 17(7):1232. https://doi.org/10.3390/cancers17071232
Chicago/Turabian StyleWahnou, Hicham, Riad El Kebbaj, Soufyane Hba, Zaynab Ouadghiri, Othman El Faqer, Aline Pinon, Bertrand Liagre, Youness Limami, and Raphaël Emmanuel Duval. 2025. "Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy" Cancers 17, no. 7: 1232. https://doi.org/10.3390/cancers17071232
APA StyleWahnou, H., El Kebbaj, R., Hba, S., Ouadghiri, Z., El Faqer, O., Pinon, A., Liagre, B., Limami, Y., & Duval, R. E. (2025). Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy. Cancers, 17(7), 1232. https://doi.org/10.3390/cancers17071232










