Perspective on Immunoglobulin N-Glycosylation Status in Follicular Lymphoma: Uncovering BCR-Dependent and Independent Mechanisms Driving Subclonal Evolution
Simple Summary
Abstract
1. Introduction
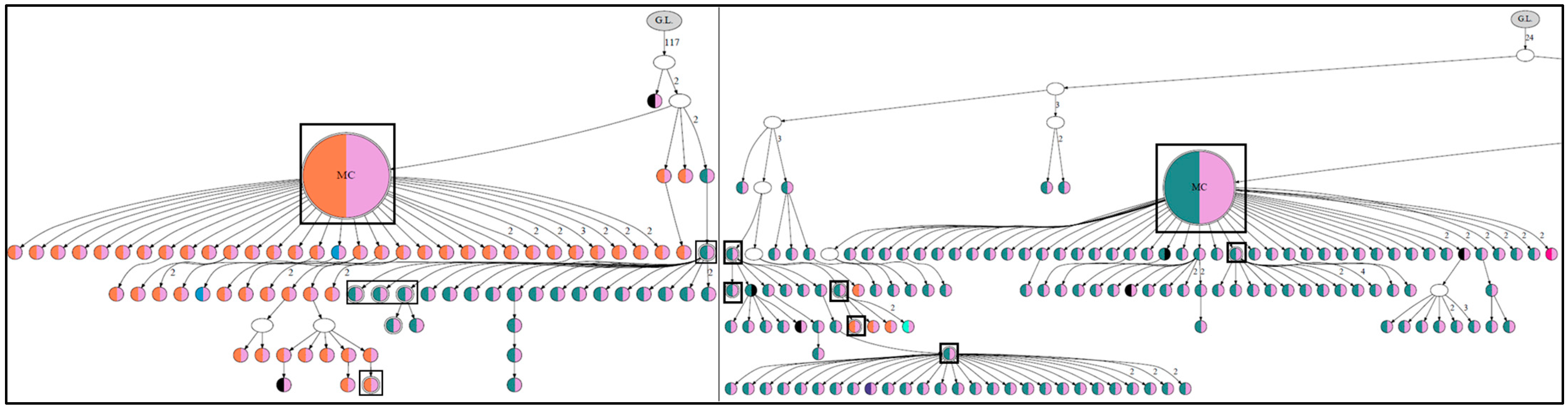
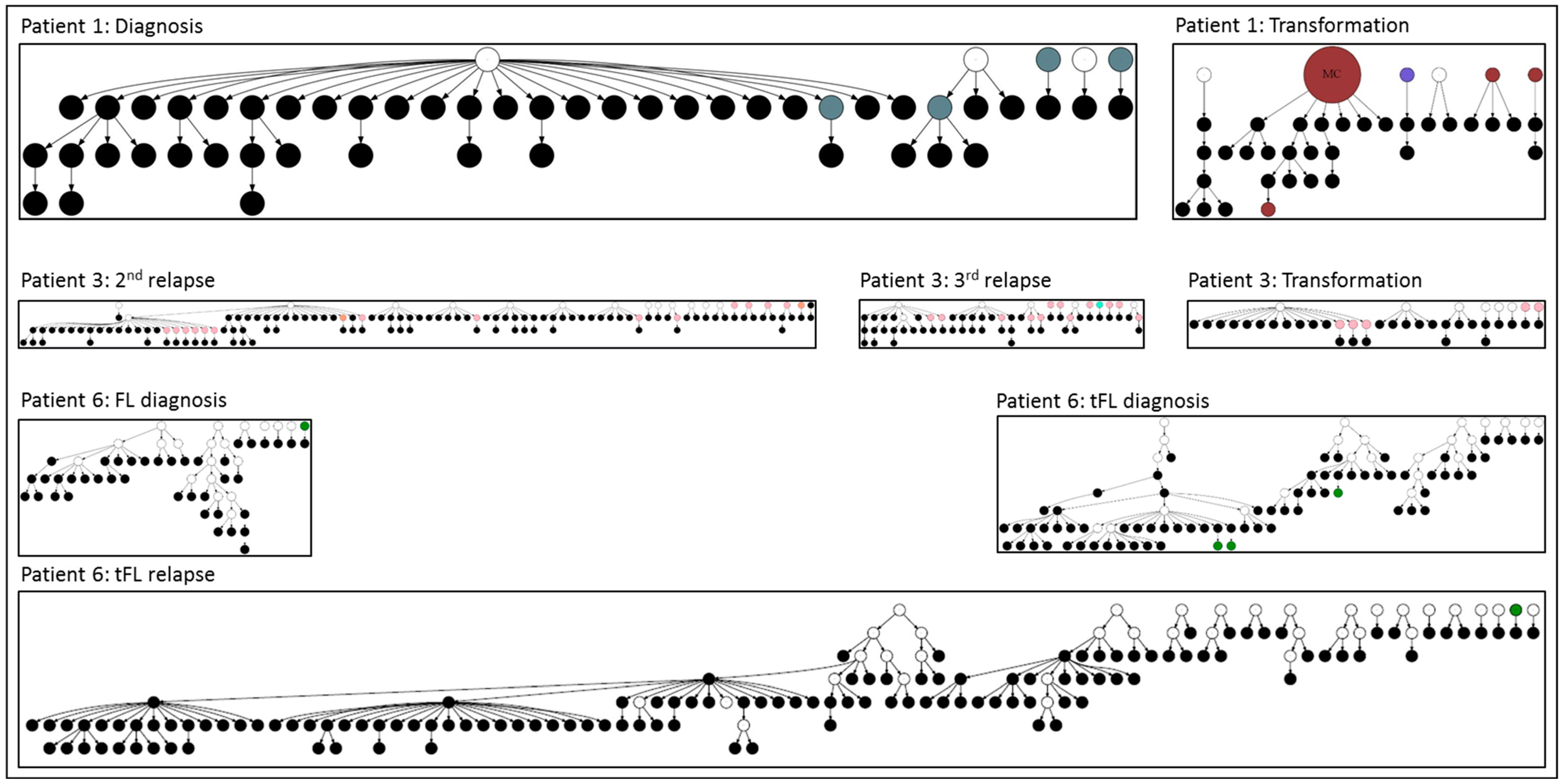
2. Follicular Lymphoma Pathogenesis and Clonal Evolution
3. Role of N-Glycosylation Motifs in Follicular Lymphoma Clonal Evolution
4. Outcomes of N-Glycosylation Loss in Follicular Lymphoma Immunoglobulin
5. N-Gly-Positive Non-Proliferating Subclones
6. Other B-Cell Lymphomas
7. Conclusions
8. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FL | Follicular Lymphoma |
| N-gly | N-Glycosylation |
| BCR | B-Cell Receptor |
| IgV | Immunoglobulin Variable Region |
| SHM | Somatic Hypermutation |
| NHL | Non-Hodgkin Lymphoma |
| LN | Lymph Node |
| BM | Bone Marrow |
| tFL | Transformed Follicular Lymphoma |
| DLBCL | Diffuse Large B-Cell Lymphoma |
| TME | Tumor Microenvironment |
| CDR | Complementarity-Determining Region |
| FR | Framework Region |
| DC-SIGN | Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-Integrin |
| NGS | Next-Generation Sequencing |
| IGHV | Immunoglobulin Heavy Chain Variable Region |
| ISFN | In Situ Follicular Neoplasia |
| t(14; 18) (q32; q21) | Chromosomal Translocation (14;18) |
| BCL2 | B-Cell Lymphoma 2 |
| VDJ | Variable, Diversity, and Joining Gene Segments |
| scRNA | Single-Cell RNA Sequencing |
| LDHA | Lactate Dehydrogenase A |
| COX5A | Cytochrome C Oxidase Subunit 5A |
| SLC1A4 | Solute Carrier Family 1 Member 4 |
| LZ Light | Zone (of Germinal Centers) |
| DZ Dark | Zone (of Germinal Centers) |
| GC | Germinal Center |
| MALT | Mucosa-Associated Lymphoid Tissue |
| BL | Burkitt’s Lymphoma |
| GCB-DLBCL | Germinal Center B-Cell Diffuse Large B-Cell Lymphoma |
| ABC-DLBCL | Activated B-Cell Diffuse Large B-Cell Lymphoma |
| MC | Major Clone |
| CHOP | Cyclophosphamide, Hydroxydaunorubicin, Oncovin, Prednisone |
| R | Rituximab |
| EPIC | European Prospective Investigation Into Cancer and Nutrition |
| CPS | Cancer Prevention Study, American Cancer Society |
References
- Jacobsen, E. Follicular Lymphoma: 2023 Update on Diagnosis and Management. Am. J. Hematol. 2022, 97, 1638–1651. [Google Scholar] [CrossRef]
- Kridel, R.; Sehn, L.H.; Gascoyne, R.D. Pathogenesis of Follicular Lymphoma. J. Clin. Investig. 2012, 122, 3424. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, A.; Hujo, M.; Sund, R.; Prusila, R.E.I.; Kuusisto, M.E.L.; Kuitunen, H.; Jantunen, E.; Mercadal, S.; Sorigue, M.; Sancho, J.-M.; et al. Mortality among patients with low-grade follicular lymphoma: A binational retrospective analysis. Cancer 2022, 128, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A.; Eric, J. Follicular Lymphoma: 2020 Update on Diagnosis and Management. Am. J. Hematol. 2019, 95, 316–325. [Google Scholar] [CrossRef]
- Mozessohn, L.; Cheung, M.C.; Crump, M.; Buckstein, R.; Berinstein, N.; Imrie, K.; Kuruvilla, J.; Piliotis, E.; Kukreti, V. Chemoimmunotherapy Resistant Follicular Lymphoma: Predictors of Resistance, Association with Transformation and Prognosis. Leuk. Lymphoma 2014, 55, 2502–2507. [Google Scholar] [CrossRef] [PubMed]
- Al-Tourah, A.J.; Gill, K.K.; Chhanabhai, M.; Hoskins, P.J.; Klasa, R.J.; Savage, K.J.; Sehn, L.H.; Shenkier, T.N.; Gascoyne, R.D.; Connors, J.M. Population-Based Analysis of Incidence and Outcome of Transformed Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2008, 26, 5165–5169. [Google Scholar] [CrossRef]
- Montoto, S.; Davies, A.J.; Matthews, J.; Calaminici, M.; Norton, A.J.; Amess, J.; Vinnicombe, S.; Waters, R.; Rohatiner, A.Z.S.; Lister, T.A. Risk and Clinical Implications of Transformation of Follicular Lymphoma to Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2007, 25, 2426–2433. [Google Scholar] [CrossRef]
- Okosun, J.; Bödör, C.; Wang, J.; Araf, S.; Yang, C.Y.; Pan, C.; Boller, S.; Cittaro, D.; Bozek, M.; Iqbal, S.; et al. Integrated Genomic Analysis Identifies Recurrent Mutations and Evolution Patterns Driving the Initiation and Progression of Follicular Lymphoma. Nat. Genet. 2013, 46, 176–181. [Google Scholar] [CrossRef]
- Schroers-Martin, J.G.; Soo, J.; Brisou, G.; Scherer, F.; Kurtz, D.M.; Sworder, B.J.; Khodadoust, M.S.; Jin, M.C.; Bru, A.; Liu, C.L.; et al. Tracing founder mutations in circulating and tissue-resident follicular lymphoma precursors. Cancer Discov. 2023, 13, 1310–1323. [Google Scholar] [CrossRef]
- Laurent, C.; Dietrich, S.; Tarte, K. Cell Cross Talk within the Lymphoma Tumor Microenvironment: Follicular Lymphoma as a Paradigm. Blood 2024, 143, 1080–1090. [Google Scholar] [CrossRef]
- Marshall, R.D. Glycoproteins. Annu. Rev. Biochem. 1972, 41, 673–702. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Karaveg, K.; Moremen, K.W. Substrate Recognition and Catalysis by GH47 α-Mannosidases Involved in Asn-Linked Glycan Maturation in the Mammalian Secretory Pathway. Proc. Natl. Acad. Sci. USA 2016, 113, E7890–E7899. [Google Scholar] [CrossRef]
- Stevenson, F.K.; Forconi, F. The Essential Microenvironmental Role of Oligomannoses Specifically Inserted into the Antigen-Binding Sites of Lymphoma Cells. Blood 2024, 143, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Haebe, S.; Day, G.; Czerwinski, D.K.; Sathe, A.; Grimes, S.M.; Chen, T.; Long, S.R.; Martin, B.; Ozawa, M.G.; Ji, H.P.; et al. Follicular Lymphoma Evolves with a Surmountable Dependency on Acquired Glycosylation Motifs in the B-Cell Receptor. Blood 2023, 142, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.; Krysov, S.; Ghaemmaghami, A.M.; Emara, M.; Potter, K.N.; Johnson, P.; Packham, G.; Martinez-Pomares, L.; Stevenson, F.K. Glycosylation of Surface Ig Creates a Functional Bridge between Human Follicular Lymphoma and Microenvironmental Lectins. Proc. Natl. Acad. Sci. USA 2010, 107, 18587–18592. [Google Scholar] [CrossRef]
- Linley, A.; Krysov, S.; Ponzoni, M.; Johnson, P.W.; Packham, G.; Stevenson, F.K. Lectin Binding to Surface Ig Variable Regions Provides a Universal Persistent Activating Signal for Follicular Lymphoma Cells. Blood 2015, 126, 1902–1910. [Google Scholar] [CrossRef]
- Odabashian, M.; Carlotti, E.; Araf, S.; Okosun, J.; Spada, F.; Gribben, J.G.; Forconi, F.; Stevenson, F.K.; Calaminici, M.; Krysov, S. IGHV Sequencing Reveals Acquired N-Glycosylation Sites as a Clonal and Stable Event during Follicular Lymphoma Evolution. Blood 2020, 135, 834–844. [Google Scholar] [CrossRef]
- Odabashian, M.; Carlotti, E.; Araf, S.; Okosun, J.; Forconi, F.; Stevenson, F.K.; Gribben, J.G.; Calaminici, M.; Krysov, S. Immunoglobulin Variable Region Gene Sequences Reveal N-Glycosylation Motifs As an Early and Stable Event in Follicular Lymphoma Pathology. Blood 2018, 132, 4101. [Google Scholar] [CrossRef]
- van Bergen, C.A.M.; Kloet, S.L.; Quinten, E.; Sepúlveda Yáñez, J.H.; Menafra, R.; Griffioen, M.; Jansen, P.M.; Koning, M.T.; Knijnenburg, J.; Navarrete, M.A.; et al. Acquisition of a Glycosylated B-Cell Receptor Drives Follicular Lymphoma toward a Dark Zone Phenotype. Blood Adv. 2023, 7, 5812–5816. [Google Scholar] [CrossRef]
- Carbone, A.; Roulland, S.; Gloghini, A.; Younes, A.; von Keudell, G.; López-Guillermo, A.; Fitzgibbon, J. Follicular Lymphoma. Nat. Rev. Dis. Primers 2019, 5, 83. [Google Scholar] [CrossRef]
- Weiss, L.M.; Warnke, R.A.; Sklar, J.; Cleary, M.L. Molecular Analysis of the t(14;18) Chromosomal Translocation in Malignant Lymphomas. N. Engl. J. Med. 1987, 317, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Xiang, H.; Ma, L.; Boxer, L.M. Functional Long-Range Interactions of the IgH 3’ Enhancers with the Bcl-2 Promoter Region in t(14;18) Lymphoma Cells. Oncogene 2008, 27, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Schüler, F.; Dölken, L.; Hirt, C.; Kiefer, T.; Berg, T.; Fusch, G.; Weitmann, K.; Hoffmann, W.; Fusch, C.; Janz, S.; et al. Prevalence and Frequency of Circulating t(14;18)-MBR Translocation Carrying Cells in Healthy Individuals. Int. J. Cancer 2009, 124, 958. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Q.; Yan, H.; Yang, Y.; Wang, P.; Zhang, Y.; Deng, Z.; Yu, M.; Zhou, W.; Wang, Q.; et al. More than One Antibody of Individual B Cells Revealed by Single-Cell Immune Profiling. Cell Discov. 2019, 5, 64. [Google Scholar] [CrossRef]
- Vettermann, C.; Schlissel, M.S. Allelic Exclusion of Immunoglobulin Genes: Models and Mechanisms. Immunol. Rev. 2010, 237, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, M.; Carlotti, E.; Hazanov, H.; Gribben, J.G.; Mehr, R. Mutational Patterns along Different Evolution Paths of Follicular Lymphoma. Front. Oncol. 2022, 12, 1029995. [Google Scholar] [CrossRef]
- Kridel, R.; Chan, F.C.; Mottok, A.; Boyle, M.; Farinha, P.; Tan, K.; Meissner, B.; Bashashati, A.; McPherson, A.; Roth, A.; et al. Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study. PLoS Med. 2016, 13, e1002197. [Google Scholar] [CrossRef]
- Zhu, D.; McCarthy, H.; Ottensmeier, C.H.; Johnson, P.; Hamblin, T.J.; Stevenson, F.K. Acquisition of Potential N-Glycosylation Sites in the Immunoglobulin Variable Region by Somatic Mutation Is a Distinctive Feature of Follicular Lymphoma. Blood 2002, 99, 2562–2568. [Google Scholar] [CrossRef]
- Hirata, T.; Kizuka, Y. N-Glycosylation. In The Role of Glycosylation in Health and Disease; Springer: Cham, Switzerland, 2021; Volume 1325, pp. 3–24. [Google Scholar] [CrossRef]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, Biological Functions and Clinical Implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef]
- McCann, K.J.; Johnson, P.W.M.; Stevenson, F.K.; Ottensmeier, C.H. Universal N-Glycosylation Sites Introduced into the B-Cell Receptor of Follicular Lymphoma by Somatic Mutation: A Second Tumorigenic Event? Leukemia 2006, 20, 530–534. [Google Scholar] [CrossRef]
- Mattox, D.E.; Bailey-Kellogg, C. Comprehensive Analysis of Lectin-Glycan Interactions Reveals Determinants of Lectin Specificity. PLoS Comput. Biol. 2021, 17, e1009470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cai, M.; Xu, H.; Jiang, J.; Wang, H. A Single-Molecule Force Spectroscopy Study of the Interactions between Lectins and Carbohydrates on Cancer and Normal Cells. Nanoscale 2013, 5, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Chiodin, G.; Allen, J.D.; Bryant, D.J.; Rock, P.; Martino, E.A.; Valle-Argos, B.; Duriez, P.J.; Watanabe, Y.; Henderson, I.; Blachly, J.S.; et al. Insertion of Atypical Glycans into the Tumor Antigen-Binding Site Identifies DLBCLs with Distinct Origin and Behavior. Blood 2021, 138, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ottensmeier, C.H.; Du, M.Q.; McCarthy, H.; Stevenson, F.K. Incidence of Potential Glycosylation Sites in Immunoglobulin Variable Regions Distinguishes between Subsets of Burkitt’s Lymphoma and Mucosa-Associated Lymphoid Tissue Lymphoma. Br. J. Haematol. 2003, 120, 217–222. [Google Scholar] [CrossRef]
- Hollander, N.; Haimovich, J. Altered N-Linked Glycosylation in Follicular Lymphoma and Chronic Lymphocytic Leukemia: Involvement in Pathogenesis and Potential Therapeutic Targeting. Front. Immunol. 2017, 8, 912. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manu, G.P.; Odabashian, M.; Krysov, S. Perspective on Immunoglobulin N-Glycosylation Status in Follicular Lymphoma: Uncovering BCR-Dependent and Independent Mechanisms Driving Subclonal Evolution. Cancers 2025, 17, 1219. https://doi.org/10.3390/cancers17071219
Manu GP, Odabashian M, Krysov S. Perspective on Immunoglobulin N-Glycosylation Status in Follicular Lymphoma: Uncovering BCR-Dependent and Independent Mechanisms Driving Subclonal Evolution. Cancers. 2025; 17(7):1219. https://doi.org/10.3390/cancers17071219
Chicago/Turabian StyleManu, Gloria Pokuaa, Mariette Odabashian, and Sergey Krysov. 2025. "Perspective on Immunoglobulin N-Glycosylation Status in Follicular Lymphoma: Uncovering BCR-Dependent and Independent Mechanisms Driving Subclonal Evolution" Cancers 17, no. 7: 1219. https://doi.org/10.3390/cancers17071219
APA StyleManu, G. P., Odabashian, M., & Krysov, S. (2025). Perspective on Immunoglobulin N-Glycosylation Status in Follicular Lymphoma: Uncovering BCR-Dependent and Independent Mechanisms Driving Subclonal Evolution. Cancers, 17(7), 1219. https://doi.org/10.3390/cancers17071219





