Implementation of a Traceback Testing Program for Ovarian Cancer: Findings from the FACTS Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Eligibility
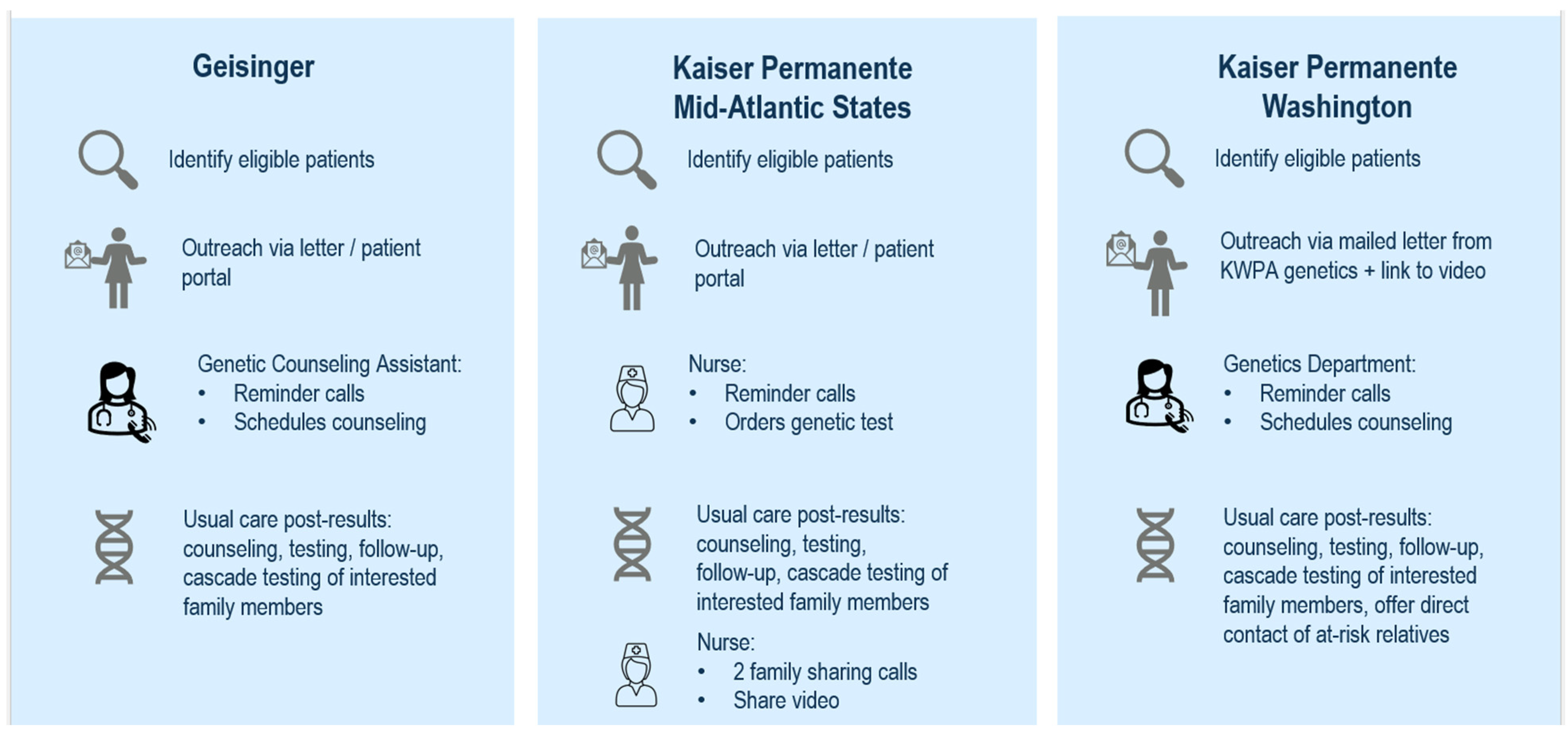
2.2. Intervention
2.3. Data Collection and Analysis
3. Results
3.1. Traceback Participant Experience
3.2. Implementer Perspective
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Eccles, D.M.; Balmaña, J.; Clune, J.; Ehlken, B.; Gohlke, A.; Hirst, C.; Potter, D.; Schroeder, C.; Tyczynski, J.E.; Gomez Garcia, E.B. Selecting Patients with Ovarian Cancer for Germline BRCA Mutation Testing: Findings from Guidelines and a Systematic Literature Review. Adv. Ther. 2016, 33, 129–150. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar]
- Daly, M.B.; Pal, T.; Maxwell, K.N.; Churpek, J.; Kohlmann, W.; AlHilli, Z.; Arun, B.; Buys, S.S.; Cheng, H.; Domchek, S.M.; et al. NCCN Guidelines(R) Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2024. J. Natl. Compr. Canc Netw. 2023, 21, 1000–1010. [Google Scholar] [PubMed]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009, 101, 80–87. [Google Scholar]
- Samimi, G.; Bernardini, M.Q.; Brody, L.C.; Caga-Anan, C.F.; Campbell, I.G.; Chenevix-Trench, G.; Couch, F.J.; Dean, M.; de Hullu, J.A.; Domchek, S.M.; et al. Traceback: A proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J. Clin. Oncol. 2017, 35, 2329–2337. [Google Scholar] [CrossRef]
- Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Rhodes, D.; Wick, M.; Okuno, S.; Kunze, K.L.; Golafshar, M.; Uson, P.L.S., Jr.; Mountjoy, L.; et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients with Hereditary Cancer Syndrome. JAMA Oncol. 2021, 7, 230–237. [Google Scholar] [PubMed]
- Roberts, M.C.; Dotson, W.D.; DeVore, C.S.; Bednar, E.M.; Bowen, D.J.; Ganiats, T.G.; Green, R.F.; Hurst, G.M.; Philp, A.R.; Ricker, C.N.; et al. Delivery of cascade screening for hereditary conditions: A scoping review of the literature. Health Aff. 2018, 37, 801–808. [Google Scholar]
- DiNucci, A.; Henrikson, N.B.; Jonas, M.C.; Basra, S.; Blasi, P.; Brown, J.; Esplin, E.D.; Hassen, D.; Hao, J.; Hu, Y.; et al. Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program. J. Pers. Med. 2021, 11, 543. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Reardon, C.M.; Widerquist, M.A.O.; Lowery, J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement. Sci. 2022, 17, 75. [Google Scholar]
- Brothers, K.B.; Bennett, R.L.; Cho, M.K. Taking an antiracist posture in scientific publications in human genetics and genomics. Genet. Med. 2021, 23, 1004–1007. [Google Scholar] [PubMed]
- Romagnoli, K.M.; Kulchak Rahm, A.; Jonas, M.C.; Schwiter, R.; Klinger, T.; Ladd, I.; Salvati, Z.; DiNucci, A.; Blasi, P.R.; Sheridan, L.; et al. Human-Centered Design Study to Inform Traceback Cascade Genetic Testing Programs at Three Integrated Health Systems. Public Health Genom. 2023, 26, 45–57. [Google Scholar]
- Bonini, K.E.; Smith, H.S.; Bonkowski, E.S.; Berkman, B.E.; Jamal, L. Modern Family: An Ethical Justification for System-Led Contact of Relatives Eligible for Cascade Screening in the United States. Public Health Genom. 2025, 28, 19–33. [Google Scholar]
- Henrikson, N.B.; Wagner, J.K.; Hampel, H.; DeVore, C.; Shridhar, N.; Williams, J.L.; Donohue, K.E.; Kullo, I.; Prince, A.E.R. What guidance does HIPAA offer to providers considering familial risk notification and cascade genetic testing? J. Law Biosci. 2020, 7, lsaa071. [Google Scholar]
- Crabtree, B.F.; Miller, W.L. Using codes and code manuals: A template organizing style of interpretation. In Doing Qualitative Research, 2nd ed.; Crabtree, B.F., Miller, W.L., Eds.; Sage Publications: Thousand Oaks, CA, USA, 1999; pp. 163–177. [Google Scholar]
- Brooks, J.; McCluskey, S.; Turley, E.; King, N. The Utility of Template Analysis in Qualitative Psychology Research. Qual. Res. Psychol. 2015, 12, 202–222. [Google Scholar] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar]
- Weiner, B.J.; Lewis, C.C.; Stanick, C.; Powell, B.J.; Dorsey, C.N.; Clary, A.S.; Boynton, M.H.; Halko, H. Psychometric assessment of three newly developed implementation outcome measures. Implement. Sci. 2017, 12, 108. [Google Scholar]
- White, L.L.; Sawyer, J.K.; Zepp, J.M.; Prado, Y.K.; Reyes, A.A.; Maiyani, M.; Shuster, E.; Zucker, R.; Henrikson, N.B.; Rope, A.F.; et al. Genetic Testing Uptake among Ovarian Cancer Survivors in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study. Cancers 2024, 16, 2563. [Google Scholar] [CrossRef]
- Agrawal, N.Y.; Thawani, R.; Edmondson, C.P.; Chen, E.Y. Estimating the Time Toxicity of Contemporary Systemic Treatment Regimens for Advanced Esophageal and Gastric Cancers. Cancers 2023, 15, 5677. [Google Scholar] [CrossRef]
- Makhnoon, S.; Levin, B.; Ensinger, M.; Mattie, K.; Volk, R.J.; Zhao, Z.; Mendoza, T.; Shete, S.; Samiian, L.; Grana, G.; et al. A multicenter study of clinical impact of variant of uncertain significance reclassification in breast, ovarian and colorectal cancer susceptibility genes. Cancer Med. 2023, 12, 2875–2884. [Google Scholar]
- Delahunty, R.; Nguyen, L.; Craig, S.; Creighton, B.; Ariyaratne, D.; Garsed, D.W.; Christie, E.; Fereday, S.; Andrews, L.; Lewis, A.; et al. TRACEBACK: Testing of Historical Tubo-Ovarian Cancer Patients for Hereditary Risk Genes as a Cancer Prevention Strategy in Family Members. J. Clin. Oncol. 2022, 40, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Augustinsson, A.; Loman, N.; Ehrencrona, H. Retrospective genetic testing (Traceback) in women with early-onset breast cancer after revised national guidelines: A clinical implementation study. Breast Cancer Res. Treat. 2024, 205, 599–607. [Google Scholar] [PubMed]
- Frey, M.K.; Ahsan, M.D.; Bergeron, H.; Lin, J.; Li, X.; Fowlkes, R.K.; Narayan, P.; Nitecki, R.; Rauh-Hain, J.A.; Moss, H.A.; et al. Cascade Testing for Hereditary Cancer Syndromes: Should We Move Toward Direct Relative Contact? A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2022, 40, 4129–4143. [Google Scholar] [PubMed]
- Jones, L.K.; Campbell-Salome, G.; Walters, N.L.; Brangan, A.; Morgan, K.M.; Tricou, E.P.; Lindsey Mills, Z.T.; McGowan, M.P.; Gidding, S.S.; Johns, A.M.; et al. IMPACT-FH Study for Implementing Innovative Family Communication and Cascade Testing Strategies for Familial Hypercholesterolemia. JACC Adv. 2024, 3, 101198. [Google Scholar]
- Wagner, J.K.; Tanniru, J.K.; Chane, C.A.; Meyer, M.N. Exploring access to genomic risk information and the contours of the HIPAA public health exception. J. Law Biosci. 2022, 9, lsac034. [Google Scholar]
- Kauffman, T.L.; Prado, Y.K.; Reyes, A.A.; Zepp, J.M.; Sawyer, J.; White, L.L.; Martucci, J.; Salas, S.B.; Vertrees, S.; Rope, A.F.; et al. Feasibility of a Traceback Approach for Using Pathology Specimens to Facilitate Genetic Testing in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study Protocol. J. Pers. Med. 2021, 11, 1194. [Google Scholar] [CrossRef]
| Total, n (%) | KPWA n (%) | KPMAS n (%) | Geisinger n (%) | p Value, Difference Between Sites | |
|---|---|---|---|---|---|
| Eligible | 597 | 149 | 224 | 224 | |
| Participated in testing | 133 | 37 | 74 | 18 | |
| Age at eligibility assessment, mean (SD) | 69.8 (10.9) | 73.8 (9.1) | 68.0 (12.0) | 69.8 (10.9) | 0.029 |
| Age at diagnosis, mean years (SD) | 57.4 (10.3) | 58.1 (10.5) | 56.6 (9) | 58.3 (11.8) | 0.821 |
| Years between eligibility assessment and age at diagnosis | 13.2 (8.2) | 13.2 (8.2) | 13.2 (8.2) | 13.2 (8.2) | 13.2 (8.2) |
| Female | 133 (100) | 41(100) | 74 (100) | 18 (100) | NA |
| Race | <0.001 | ||||
| Asian | 12 (9.0) | 2 (4.9) | 9 (12.2) | 1 (5.6) | |
| Black | 28 (21.1) | 2 (4.9) | 26 (35.1) | 0 (0.0) | |
| Other race | 7 (5.3) | 2 (4.9) | 5 (6.8) | 0 (0.0) | |
| White | 86 (64.7) | 35 (85.4) | 34 (45.9) | 17 (94.4) | |
| Hispanic ethnicity | 7 (5) | 1 (2) | 6 (8) | 0 | 0.24 |
| Ovarian cancer diagnosis | 122 (92) | 34 (83) | 72 (99) | 16 (89) | 0.008 |
| Peritoneal or fallopian cancer diagnosis | 9 (8) | 7 (17) | 2 (1) | 2 (11) |
| Total n | % | GE | % | KPWA | % | KPMAS | % | |
|---|---|---|---|---|---|---|---|---|
| Potentially eligible individuals identified through administrative data, n | 6386 | 1173 | 2274 | 2939 | ||||
| Potentially eligible, alive and age 18+ at data pull, chart reviewed 6 | 1568 | 450 | 240 | 878 | ||||
| Confirmed eligible for Traceback testing and attempted to reach, n | 597 | 20% 1 | 224 | 50% | 149 | 62% | 224 | 10% |
| Eligible individuals reached, n 7 | 354 | 59% 2 | 129 | 58% | 72 | 48% | 153 | 68% |
| Interested in genetic counseling/testing, n (KPWA and Geisinger only) | 74 | 33 | 41 | NA | ||||
| Attended genetic counseling appointment | 73 | 30 | 41 | 2 | ||||
| Consented to genetic testing (no pretest counseling) (KPMAS only) | 123 | NA | NA | 123 | ||||
| Completed Traceback genetic testing, n | 133 | 22% 2 | 18 | 8% | 37 | 25% | 74 | 33% |
| 38% 3 | 14% | 51% | 48% | |||||
| Number of participants receiving positive result | 10 | 2% 2 | 1 | 0.4% | 2 | 1% | 7 | 3% |
| 8% 4 | 5.6% | 5% | 9% | |||||
| Variant of unknown significance (VUS) result | 36 | 6% 2 | 5 | 2% | 10 | 7% | 21 | 9% |
| 28% 4 | 28% | 28% | ||||||
| Total number of at-risk relatives identified | 58 | NA 5 | 21 | 37 | ||||
| At-risk relatives completing cascade genetic testing, n | 0 | 0 | 0 |
| Genes with positive result 1 (Number of participants receiving result) (n = 10 participants) | Strong/definitive ovarian cancer association 3: ATM (2), BRCA2 (2), RAD51D (2), BRCA1 No evidence of ovarian cancer association 3: CHEK2 (3), TP53 |
| Variant of unknown significance (VUS) result genes 2 (n = 36 participants) | Strong/definitive ovarian cancer association 3: ATM 4 (3), BRCA2 (3), BRIP1(2), PALB2, RAD51D, SMARCA4, No evidence of ovarian cancer association 3: NBN (3), POLE (3), BARD1(2), CDH1 (2), SDHA (2), APC, CHEK2, HOXB1, HOXB13, KIF1B, KIT, MSH3, NF1, NTHL1, PDGFRA, POLE/HOXB1, RAD50, RET, SDHC, TSC2 |
| N | % or Range | |
|---|---|---|
| Total interviewees | 47 | 100% |
| Geisinger | 18 | 38.3% |
| KPMAS | 13 | 27.7% |
| KPWA | 16 | 34.0% |
| Median age | 69 | (48–90) |
| Female | 47 | 100% |
| Race/ethnicity | ||
| Asian | 4 | 8.5% |
| Black | 6 | 12.8% |
| White | 36 | 76.6% |
| Hispanic ethnicity | 0 | 0.0% |
| 4-year college graduate or more | 27 | 57.4% |
| Annual income $50K or less | 11 | 23.4% |
| Currently working for pay | 8 | 17.0% |
| Currently married | 26 | 55.3% |
| Completed Traceback genetic testing | 30 | 63.8% |
| Category | Them | Example Quotes |
|---|---|---|
| Reaction to initial outreach | Positive reaction | My reaction is really positive. Because after like you looking out for me, you know, when you invite me to do genetic testing you’re not only looking out for me, you’re looking out for my family. So I feel good, you know, taking genetic testing is not only for me. KPWA00032 (Acceptor) |
| I didn’t have any qualms about it. I was happy that it was my… you know my Kaiser doctor. I trust them. I don’t know how I would’ve felt had I gotten it from some other program. I don’t know, but it was all through Kaiser so I didn’t have any… any, any hesitation about it. KPMAS0008 (Acceptor) | ||
| Negative reaction | First off I had ovarian cancer in 1973. I’ve been with multiple entities of what is now Kaiser forever and you’re just now asking me this?… If you’re going to tell me what you’re looking for, if you’re going to tell me how this is going to benefit and don’t tell me it’s free. Because Kaiser doesn’t do nothing for free… To say it’s free you’re getting my DNA which is mine and ask me to give it to you for free, so that’s about the only free there is. KPMAS0001 (Decliner) | |
| Well, what was going through my head was if you want me to come down there and get another needle you are nuts, that was going through my head before she even said anything. GE90692 (Decliner) | ||
| Surprised by outreach | I was somewhat surprised, and I guess I felt as though oh what is it about me that makes them want me to do this? GE00345 (Decliner) | |
| Did not remember | I’m sorry, but I don’t know if my husband threw that away. I don’t remember seeing a letter if he answered the phone. GE00185 (Decliner) | |
| Genetic testing and counseling experience | Easy, straightforward testing process | It was pretty straightforward. I talked with the nurse and that was simple. The testing kit came as I was told it would and did the test. In an era of COVID testing this just seemed like one more set of swabs and containers. So it didn’t really seem very exotic. KPMAS0002 (Acceptor) |
| Informative conversations with genetic testing staff | I think it was great. Everybody was really nice and really informative and answered all the questions. And explained what was going in really clear terms so that I completely understood it. KPWA00059 (Acceptor) | |
| Benefits of program | Prompt to get genetic testing that otherwise would not have pursued | I received the letter and I dismissed it at first because I thought I don’t feel I need to do it. …Then one day I thought you know I have a child, I have a daughter. And I thought perhaps I should do this and gather more information for her. KPMAS0005 (Acceptor) |
| Provided relevant risk information to family members | I encouraged them both to get tested. You know, and like I say, one daughter did, she did not have that genetic mutation. And hopefully the other one doesn’t have it either, but she has not completed the genetic testing. KPWA00131 (Acceptor) | |
| Motivation to pursue preventive health actions | I’m going to get yearly mammograms and yearly breast exams. And just, you know, do what we can to catch it early if it’s going to happen. Hopefully it won’t. KPWA00059 (Acceptor) | |
| Suggestions for improvement | Personalize outreach to proband’s health history | So I think the only confusion that I had was that I already had the genetic testing, so I wasn’t quite sure why they were reaching out to me again. KPWA00208 (Acceptor) |
| I don’t recall that there was any… any indication of awareness of how long in the past my cancer diagnosis was which you know it’s kind of historical information. KPMAS0002 (Acceptor). | ||
| The only question I have is what took them so long to decide they needed to do a genetic testing… Because a lot of people have died from ovarian cancer in 20 some years. KPWA00089 (Decliner) | ||
| Explicitly explain benefits and potential impacts of testing | I would have appreciated maybe some more up-front information about how this can affect peoples’ lives if they discover things in their genes that are--could be passed on. So, you know, I think just a little more education with why you’re--why we need to do this. KPWA00037 (Acceptor) | |
| I think they could have identified, you know, these are the things that we might find out that would be helpful. You know, whether you could pass this along, whether somebody else in your family or immediate family might already have this, or they should get a particular test, or things like that. I have a couple of nieces out there, grandnieces, but because they’re not direct genetically, I just assumed that that wouldn’t affect them. So, if there is something that could be useful to them, then that would be good for me to pass along. KPWA00035 (Decliner) | ||
| Well, people always want to contribute, you know. If somehow taking my blood would be greatly helpful in say prevention or detection, then that’s fine. So I’d have to be convinced that it’s worth my time, worth my effort. KPWA00066 (Decliner) | ||
| Provide more information about cost and coverage of testing | I had no idea what it would cost, so I couldn’t decide whether I wanted to go ahead and do it anyway because nobody could help me and tell me what out of pocket costs I would be looking at. So, therefore, I did not do the genetic testing. GE00345 (Decliner) | |
| Improve readability and clarity | Well, the letters were sort of wordy and vague. I mean, I understood what was going on, but I could see how they could confuse people who are less scientifically literate. KPWA00070 (Acceptor) | |
| Offer ways to address logistical barriers | I did read through it, and I found it a little difficult on how I could possibly participate. I have no means of getting, you know, to where I can do bloodwork or anything. KPWA00089 (Decliner) |
| Theme | Exemplar Quotes |
|---|---|
| Perceptions of the traceback program | I think the benefits of a program like this, something that comes to mind is we often, in my opinion, miss this specific patient population group for many reasons. They either are diagnosed a bit later in life, and they pass away fairly after their diagnosis or even if they do seek treatment, sometimes they are way too overwhelmed and busy to fit genetics in or maybe their physicians are not recommending genetics for whatever reason, so I think there’s many reasons why they don’t come to see us. So, I think a program like this pulls in this specific patient group where genetic testing is really beneficial for them. Genetic Counselor, Geisinger I think if we find better paths to go back and revisit some of these patients that have had really difficult diseases, that’s part of our mission. Geneticist, KPWA |
| Traceback program value | I think a lot of people who are diagnosed with cancer, they don’t realize that this [genetic testing] is an option for them, especially because they meet guidelines and that this could be a really great thing for their family and for kind of getting an answer. Maybe for future screening and future preventative care as well. So I think knowledge is power. So, you know, even if it’s late knowledge in there, in some people’s minds, it might be kind of late finding out. But I think any knowledge at any point is great…So I just think it’s a great thing overall if we can explain it in a way that suits them and let them kind of decide what they want to do. Genetic Counseling Assistant, Geisinger I think the cascade testing which we talked about was the biggest benefit…I think that’s why most people wanted to do testing and there was a huge benefit. Genetic Counselor, KPWA |
| Acceptability of outreach timing | …When someone is diagnosed with cancer and facing a cancer diagnosis, there’s so many people contacting them, there are so many things they have to do, and then this was great because things had died down, it was much later, and they could focus on it, and possibly hear it more, so I really like this approach…it could really help. There may be people who decline, that’s fine, but I think it could really help. Genetic Counselor, Geisinger |
| Perceptions of patient motivation to test | I ended up seeing a lot of people who had had an outdated genetic test, like the BRCA only or maybe a smaller panel than what we now have. I think though, overall, the most common reason that people decided to go forward with testing is to if they had children or other at-risk relatives, that was their strongest motivation. Genetic Counselor, KPWA Both of the patients I saw were elderly…so their ovarian cancer diagnoses were between 25 and 30 years prior, when genetic testing wasn’t necessarily on the market, as widespread, their oncology team at the time might not have known that’s something that can be done, what insurance and cost look like…But, I think for both of them, their main benefit of testing for them was their children and their family. Both had children, males and females, they were wanting [a] great opportunity to impact their care, both recognizing it probably didn’t mean too much for them at their current age and health status. Genetic Counselor, Geisinger |
| Traceback workflow feedback | I didn’t really notice any differences in the patients at all or the work I was conducting. It all seemed pretty similar to other patients I would see through the cancer clinic. Genetic Counseling Assistant, Geisinger I would say that the only thing that would make this more perfect would be to have them come in, get a cheek swab in the office and leave so they don’t have to deal with sending the specimen back. Nurse Coordinator, KPMAS |
| Traceback program sustainability | I think working side by side with cancer genetics—that already has a really good process of getting these patients seen—it is perfect. I mean, this is just another way to capture a group of people that might be missed or might not know that they have this option. Genetic Counseling Assistant, Geisinger …one of the things that I learned from Traceback is that it is essential that we have training for all providers who interact with patients with this type of cancer or any type of serious cancer that we, give them the correct information. And if a provider can’t do that directly, is to know that you collaborate with other providers to do that work. Geneticist, KPWA |
| Cost of testing | I got a lot of positive feedback from patients about how happy and excited they were that this was offered to them free of charge. Nurse Coordinator, KPMAS There seemed to be a good amount of times when they expected that this should be covered because I was contacted and it should be free, so that was the big difference I saw. Genetic Counselor, Geisinger |
| Future directions | I mean, even if there were something like population screening, I think it would still be valid to go back and recontact individuals and check on how they’re doing. Genetic Counselor, KPWA I would be interested in maybe researching more into how we can make the genetic counseling model a little bit more inclusive to other groups. Genetic Counseling Assistant, KPWA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henrikson, N.B.; Jonas, M.C.; Blasi, P.R.; Buchanan, A.H.; Suwannarat, P.; Leppig, K.; Scrol, A.; Leitzel, T.; Deneal, A.N.; Canedo, D.; et al. Implementation of a Traceback Testing Program for Ovarian Cancer: Findings from the FACTS Study. Cancers 2025, 17, 1154. https://doi.org/10.3390/cancers17071154
Henrikson NB, Jonas MC, Blasi PR, Buchanan AH, Suwannarat P, Leppig K, Scrol A, Leitzel T, Deneal AN, Canedo D, et al. Implementation of a Traceback Testing Program for Ovarian Cancer: Findings from the FACTS Study. Cancers. 2025; 17(7):1154. https://doi.org/10.3390/cancers17071154
Chicago/Turabian StyleHenrikson, Nora B., M. Cabell Jonas, Paula R. Blasi, Adam H. Buchanan, Pim Suwannarat, Kathleen Leppig, Aaron Scrol, Tracey Leitzel, Adrienne N. Deneal, Daniela Canedo, and et al. 2025. "Implementation of a Traceback Testing Program for Ovarian Cancer: Findings from the FACTS Study" Cancers 17, no. 7: 1154. https://doi.org/10.3390/cancers17071154
APA StyleHenrikson, N. B., Jonas, M. C., Blasi, P. R., Buchanan, A. H., Suwannarat, P., Leppig, K., Scrol, A., Leitzel, T., Deneal, A. N., Canedo, D., Ramaprasan, A., Basra, S. S., Brown, J., Odums, M., Hu, Y., Romagnoli, K. M., Khieu, E., Balton, E., Patel, S., ... Rahm, A. K. (2025). Implementation of a Traceback Testing Program for Ovarian Cancer: Findings from the FACTS Study. Cancers, 17(7), 1154. https://doi.org/10.3390/cancers17071154








