Real-World Data on the Associations of Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors with Gynecologic Cancer Risk
Simple Summary
Abstract
1. Introduction
2. Methods
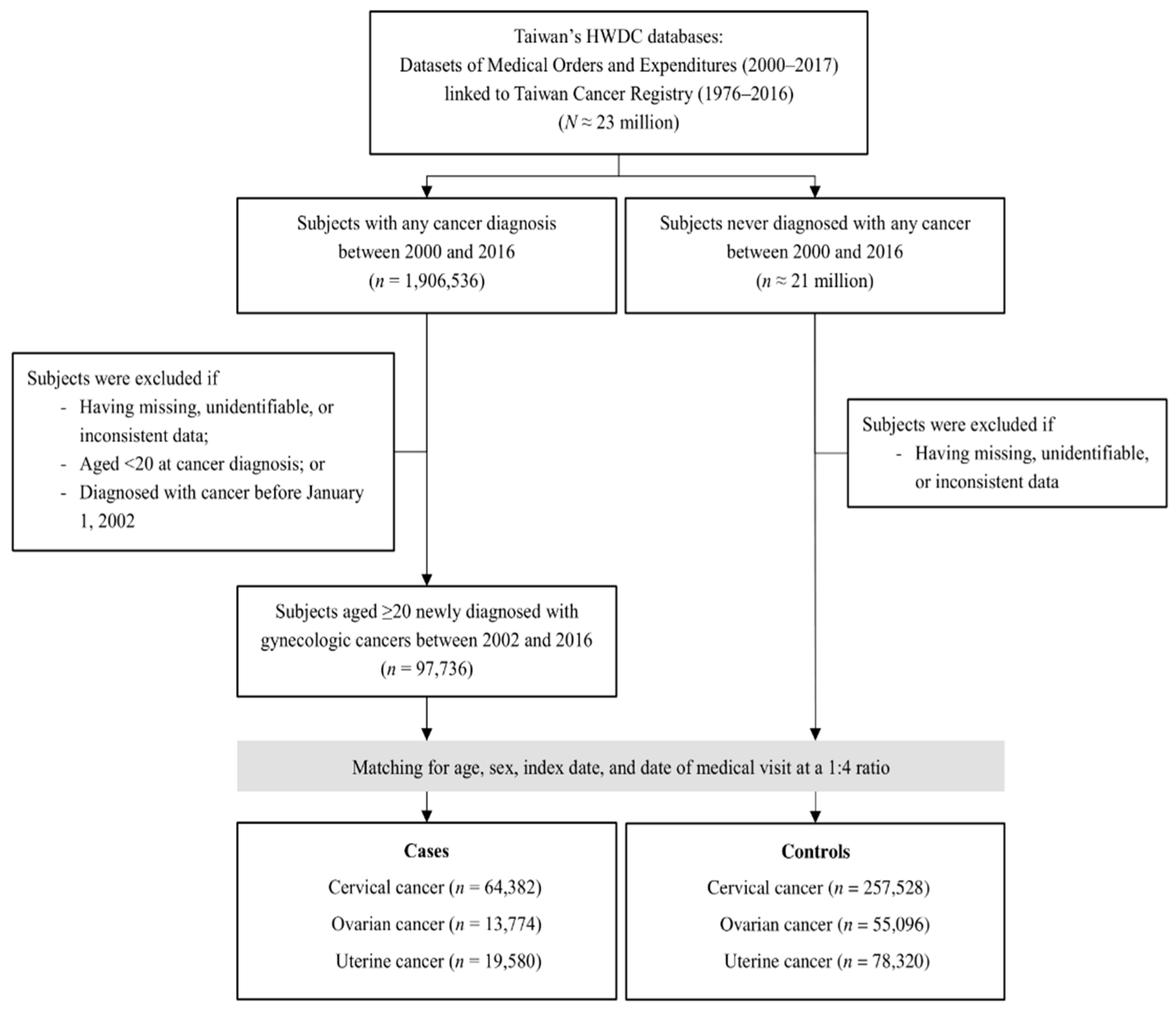
2.1. Study Design and Population
2.2. Data Sources
2.3. Cases and Controls
2.4. Definitions of Exposure to Antidepressants
2.5. Potential Confounding Factors
2.6. Statistical Analysis
2.7. Ethics Approval Statement
3. Results
3.1. Participant Characteristics
3.2. Associations of Gynecologic Cancers with the TCA and SSRI Classes
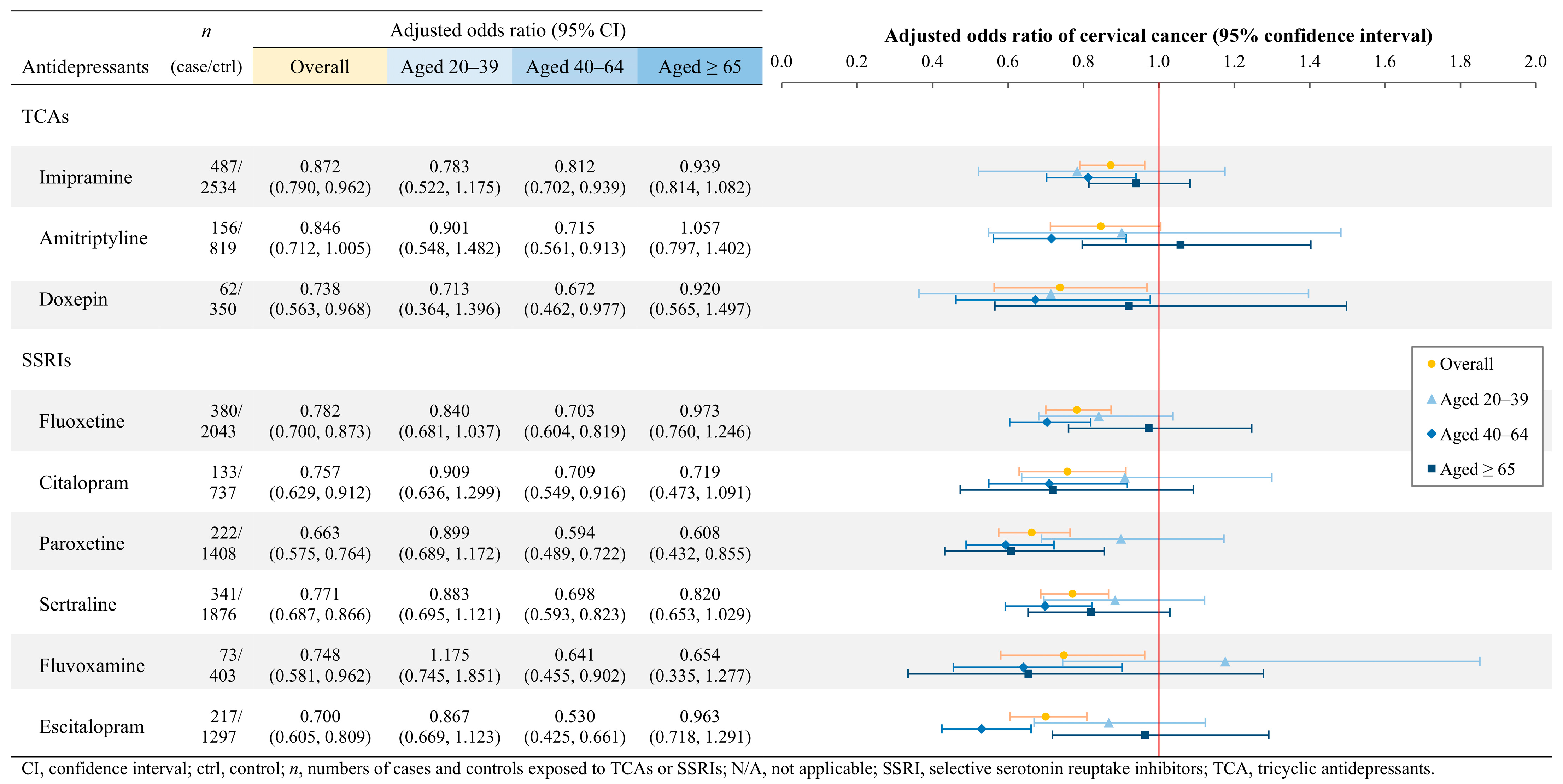
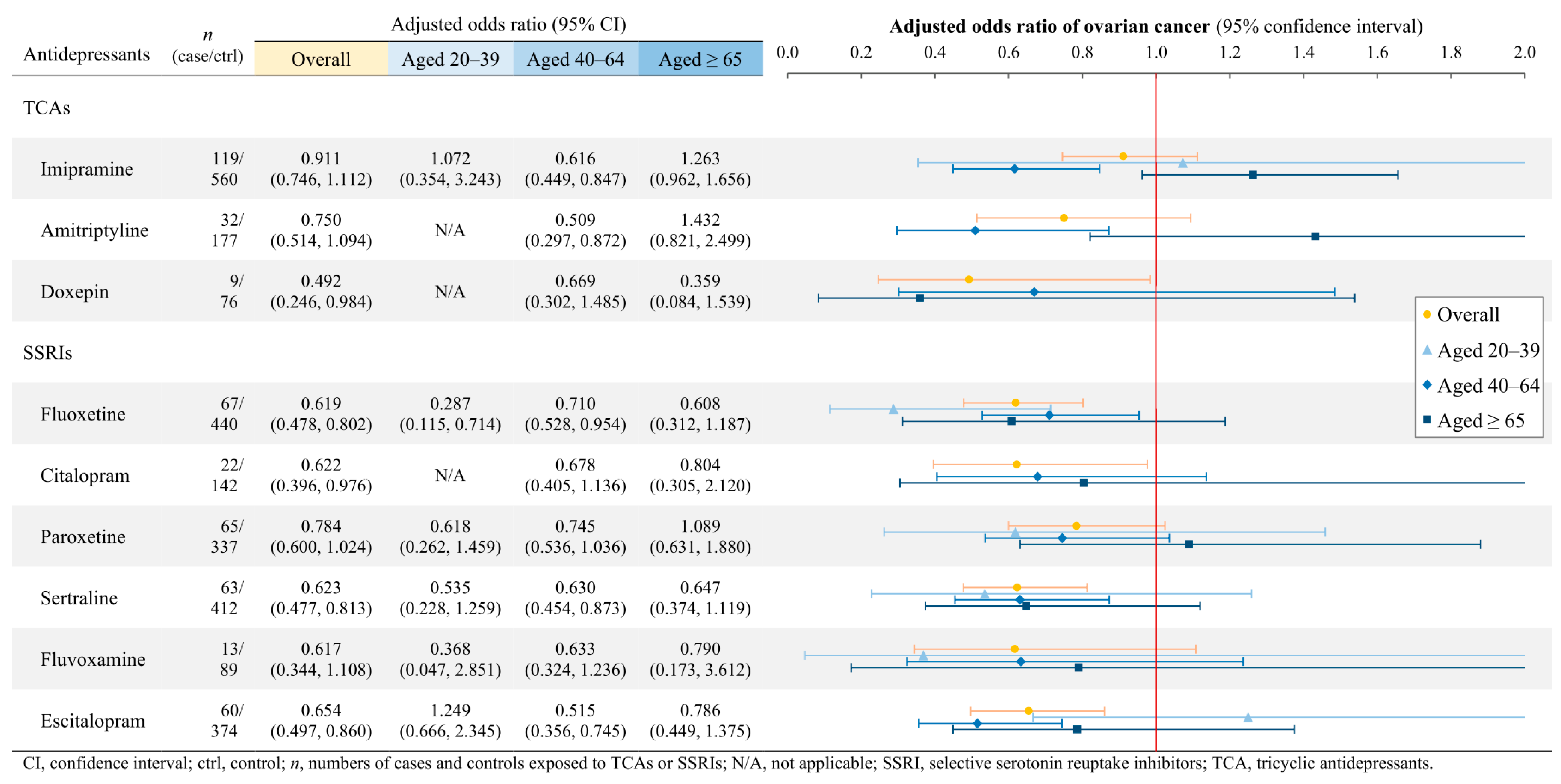
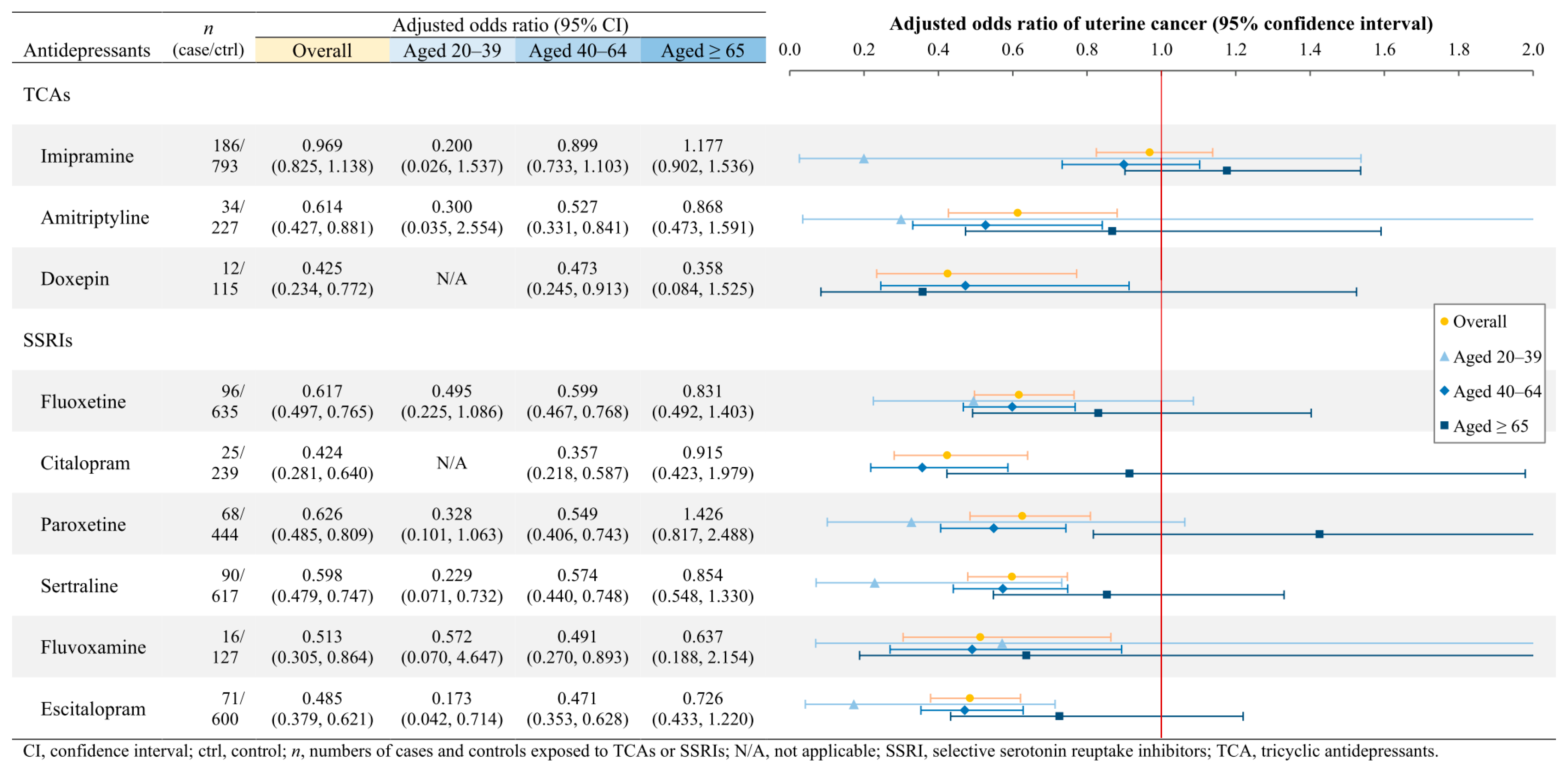
3.3. Associations of Gynecologic Cancers with Individual TCAs and SSRIs
4. Discussion
4.1. Comparison with Relevant Studies and Possible Mechanisms
4.1.1. Cervical Cancer
4.1.2. Ovarian Cancer
4.1.3. Uterine Cancer
4.1.4. Elderly Population
4.2. Interpretation of Our Findings for Future Research
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 8, 2861. [Google Scholar] [CrossRef]
- Health at a Glance 2019: OECD Indicators. Pharmaceutical Consumption. 7 November 2019. Available online: https://www.oecd-ilibrary.org/sites/43146d4b-en/index.html?itemId=/content/component/43146d4b-en (accessed on 13 April 2021).
- Belmaker, R.H.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef]
- ATC/DDD Index 2021. 2021. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 18 January 2021).
- Steingart, A.B.; Cotterchio, M. Do antidepressants cause, promote, or inhibit cancers? J. Clin. Epidemiol. 1995, 48, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.L.; Qiao, J.M.; Gao, S. Association between antidepressant medication use and epithelial ovarian cancer risk: A systematic review and meta-analysis of observational studies. Br. J. Clin. Pharmacol. 2018, 84, 649–658. [Google Scholar] [CrossRef]
- Radin, D.P.; Patel, P. A current perspective on the oncopreventive and oncolytic properties of selective serotonin reuptake inhibitors. Biomed. Pharmacother. 2017, 87, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Mørch, L.S.; Dehlendorff, C.; Baandrup, L.; Friis, S.; Kjaer, S.K. Use of antidepressants and risk of epithelial ovarian cancer. Int. J. Cancer 2017, 141, 2197–2203. [Google Scholar] [CrossRef]
- Sperling, C.D.; Aalborg, G.L.; Dehlendorff, C.; Friis, S.; Mørch, L.S.; Kjaer, S.K. Use of antidepressants and endometrial-cancer risk: A nationwide nested case-control study. Int. J. Epidemiol. 2021, 51, 799–806. [Google Scholar] [CrossRef]
- Cosgrove, L.; Shi, L.; Creasey, D.E.; Anaya-McKivergan, M.; Myers, J.A.; Huybrechts, K.F. Antidepressants and breast and ovarian cancer risk: A review of the literature and researchers’ financial associations with industry. PLoS ONE 2011, 6, e18210. [Google Scholar] [CrossRef]
- Chan, H.L.; Hsieh, Y.H.; Lin, C.F.; Liang, H.Y.; Huang, K.Y.; Chiu, W.C.; Lee, Y.; McIntyre, R.S.; Chen, V.C. Invasive Cervical Cancer and Antidepressants: A Nationwide Population-Based Study. Medicine 2015, 94, e1866. [Google Scholar] [CrossRef]
- Cancer Registry Annual Report, 2019, Taiwan. 13 January 2022. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269 (accessed on 16 February 2022).
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Universal Health Coverage in Taiwan. 2018. Available online: https://www.nhi.gov.tw/English/Content_List.aspx?n=4D7051840BF42F52&topn=ED4A30E51A609E49 (accessed on 20 April 2021).
- ICD-9-CM and ICD-10-CM/PCS Mapping Table. 1 May 2020. Available online: https://www.nhi.gov.tw/Content_List.aspx?n=C1C92AB9ED30A9FD&topn=23C660CAACAA159D (accessed on 18 January 2021).
- Grimes, D.A.; Schulz, K.F. Compared to what? Finding controls for case-control studies. Lancet 2005, 365, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, P.H.; Chen, P.N.; Yang, S.F.; Hsiao, Y.H. Molecular and Cellular Mechanisms of Metformin in Cervical Cancer. Cancers 2021, 13, 2545. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Biglia, N.; Tana, R.; Cosio, S.; Gallo, M. Metformin use and gynecological cancers: A novel treatment option emerging from drug repositioning. Crit. Rev. Oncol. Hematol. 2016, 105, 73–83. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, T.S. Associations between metabolic syndrome and gynecologic cancer. Obstet. Gynecol. Sci. 2020, 63, 215–224. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Chen, X.; Zhang, F.; Li, X. Aspirin use and endometrial cancer risk: A meta-analysis and systematic review. Ann. Transl. Med. 2020, 8, 461. [Google Scholar] [CrossRef]
- Zhang, D.; Bai, B.; Xi, Y.; Wang, T.; Zhao, Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016, 142, 368–377. [Google Scholar] [CrossRef]
- Joharatnam-Hogan, N.; Cafferty, F.H.; Macnair, A.; Ring, A.; Langley, R.E. The role of aspirin in the prevention of ovarian, endometrial and cervical cancers. Womens Health 2020, 16, 1745506520961710. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals 2020, 13, 422. [Google Scholar] [CrossRef]
- Cordero, M.D.; Sánchez-Alcázar, J.A.; Bautista-Ferrufino, M.R.; Carmona-López, M.I.; Illanes, M.; Ríos, M.J.; Garrido-Maraver, J.; Alcudia, A.; Navas, P.; de Miguel, M. Acute oxidant damage promoted on cancer cells by amitriptyline in comparison with some common chemotherapeutic drugs. Anticancer Drugs 2010, 21, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Hariharan, R.; Nair, S.A.; Pillai, M.R. Fluoxetine mediates G0/G1 arrest by inducing functional inhibition of cyclin dependent kinase subunit (CKS)1. Biochem. Pharmacol. 2008, 75, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Lu, M.L.; Liao, Y.T.; Lee, C.T.; Chen, V.C. Ovarian cancer and antidepressants. Psychooncology 2015, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Berchuck, A.; Calingaert, B.; Halabi, S.; Schildkraut, J.M. Antidepressant medication use [corrected] and risk of ovarian cancer. Obstet. Gynecol. 2005, 105, 725–730. [Google Scholar] [CrossRef]
- Dublin, S.; Rossing, M.A.; Heckbert, S.R.; Goff, B.A.; Weiss, N.S. Risk of epithelial ovarian cancer in relation to use of antidepressants, benzodiazepines, and other centrally acting medications. Cancer Causes Control 2002, 13, 35–45. [Google Scholar] [CrossRef]
- Harlow, B.L.; Cramer, D.W.; Baron, J.A.; Titus-Ernstoff, L.; Greenberg, E.R. Psychotropic medication use and risk of epithelial ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 697–702. [Google Scholar]
- Haukka, J.; Sankila, R.; Klaukka, T.; Lonnqvist, J.; Niskanen, L.; Tanskanen, A.; Wahlbeck, K.; Tiihonen, J. Incidence of cancer and antidepressant medication: Record linkage study. Int. J. Cancer 2010, 126, 285–296. [Google Scholar] [CrossRef]
- Asher, V.; Warren, A.; Shaw, R.; Sowter, H.; Bali, A.; Khan, R. The role of Eag and HERG channels in cell proliferation and apoptotic cell death in SK-OV-3 ovarian cancer cell line. Cancer Cell Int. 2011, 11, 6. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, Y.J.; Jang, E.R.; Kim, W.; Myung, S.C. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic. Clin. Pharmacol. Toxicol. 2010, 106, 446–453. [Google Scholar] [CrossRef]
- Nguyen, T.M.D.; Klett, D.; Combarnous, Y. Fluoxetine affects cytosolic cAMP, ATP, Ca(2+) responses to forskolin, and survival of human ovarian granulosa tumor COV434 cells. Korean J. Physiol. Pharmacol. 2021, 25, 189–195. [Google Scholar] [CrossRef]
- Lin, C.F.; Chan, H.L.; Hsieh, Y.H.; Liang, H.Y.; Chiu, W.C.; Huang, K.Y.; Lee, Y.; McIntyre, R.S.; Chen, V.C. Endometrial cancer and antidepressants: A nationwide population-based study. Medicine 2016, 95, e4178. [Google Scholar] [CrossRef] [PubMed]
- Pula, G.; Pistilli, A.; Montagnoli, C.; Stabile, A.M.; Rambotti, M.G.; Rende, M. The tricyclic antidepressant amitriptyline is cytotoxic to HTB114 human leiomyosarcoma and induces p75(NTR)-dependent apoptosis. Anticancer Drugs 2013, 24, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Birnbaum, R.J. Tricyclic and Tetracyclic Drugs: Pharmacology, Administration, and Side Effects. 11 December 2023. Available online: https://www.uptodate.com/contents/tricyclic-and-tetracyclic-drugs-pharmacology-administration-and-side-effects (accessed on 25 April 2025).
- Hirsch, M.; Birnbaum, R.J. Selective Serotonin Reuptake Inhibitors: Pharmacology, Administration, and Side Effects. 31 July 2023. Available online: https://www.uptodate.com/contents/selective-serotonin-reuptake-inhibitors-pharmacology-administration-and-side-effects (accessed on 25 April 2025).
- Emiliano, A.B.; Fudge, J.L. From galactorrhea to osteopenia: Rethinking serotonin-prolactin interactions. Neuropsychopharmacology 2004, 29, 833–846. [Google Scholar] [CrossRef]
- Monroy, J.; Cortés, O.D.; Domínguez, R.; Mendoza-Garrido, M.E.; Gallegos, E.; Cárdenas, M.; Aragón, A.; Ayala, M.E. The differential sensitivity of the hypothalamic-hypophysial-ovarian axis to 5-hydroxytryptophan alters the secretion of estradiol. Exp. Physiol. 2024, 109, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef]
- Clendenen, T.V.; Arslan, A.A.; Lokshin, A.E.; Liu, M.; Lundin, E.; Koenig, K.L.; Berrino, F.; Hallmans, G.; Idahl, A.; Krogh, V.; et al. Circulating prolactin levels and risk of epithelial ovarian cancer. Cancer Causes Control 2013, 24, 741–748. [Google Scholar] [CrossRef]
- Levina, V.V.; Nolen, B.; Su, Y.; Godwin, A.K.; Fishman, D.; Liu, J.; Mor, G.; Maxwell, L.G.; Herberman, R.B.; Szczepanski, M.J.; et al. Biological significance of prolactin in gynecologic cancers. Cancer Res. 2009, 69, 5226–5233. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Ramírez-de-Arellano, A.; Villegas-Pineda, J.C.; Hernández-Silva, C.D.; Pereira-Suárez, A.L. The Relevant Participation of Prolactin in the Genesis and Progression of Gynecological Cancers. Front. Endocrinol. 2021, 12, 747810. [Google Scholar] [CrossRef]
- Pop, A.; Lupu, D.I.; Cherfan, J.; Kiss, B.; Loghin, F. Estrogenic/antiestrogenic activity of selected selective serotonin reuptake inhibitors. Clujul Med. 2015, 88, 381–385. [Google Scholar] [CrossRef]
- Lupu, D.; Varshney, M.K.; Mucs, D.; Inzunza, J.; Norinder, U.; Loghin, F.; Nalvarte, I.; Rüegg, J. Fluoxetine Affects Differentiation of Midbrain Dopaminergic Neurons In Vitro. Mol. Pharmacol. 2018, 94, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, P.; Kokras, N.; Dalla, C. Antidepressants’ effects on testosterone and estrogens: What do we know? Eur. J. Pharmacol. 2021, 899, 173998. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.A.; Heeb, L.; Beffinger, M.M.; Pantelyushin, S.; Linecker, M.; Roth, L.; Lehmann, K.; Ungethüm, U.; Kobold, S.; Graf, R.; et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med. 2021, 13, eabc8188. [Google Scholar] [CrossRef]
- Rácz, B.; Spengler, G. Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases. Antibiotics 2023, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, X.; Yu, B. Repurposing antidepressants for anticancer drug discovery. Drug Discov. Today 2022, 27, 1924–1935. [Google Scholar] [CrossRef]
- Yang, H.C.; Islam, M.M.; Nguyen, P.A.A.; Wang, C.H.; Poly, T.N.; Huang, C.W.; Li, Y.J. Development of a Web-Based System for Exploring Cancer Risk With Long-term Use of Drugs: Logistic Regression Approach. JMIR Public. Health Surveill. 2021, 7, e21401. [Google Scholar] [CrossRef]
- Wu, C.C.; Islam, M.M.; Nguyen, P.A.; Poly, T.N.; Wang, C.H.; Iqbal, U.; Li, Y.J.; Yang, H.C. Risk of cancer in long-term levothyroxine users: Retrospective population-based study. Cancer Sci. 2021, 112, 2533–2541. [Google Scholar] [CrossRef]
| Characteristics | Cervical Cancer | Ovarian Cancer | Uterine Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 64,382) | Controls (n = 257,528) | Cases (n = 13,774) | Controls (n = 55,096) | Cases (n = 19,580) | Controls (n = 78,320) | |||||||
| Age | ||||||||||||
| Mean ± SD | 49.74 | ± 14.48 | 49.74 | ± 14.48 | 51.72 | ± 13.96 | 51.72 | ± 13.95 | 53.68 | ± 10.93 | 53.68 | ± 10.92 |
| 20–39 years, n (%) | 17,861 | (27.74) | 71,444 | (27.74) | 2547 | (18.49) | 10,188 | (18.49) | 1905 | (9.73) | 7620 | (9.73) |
| 40–64 years, n (%) | 34,581 | (53.71) | 138,324 | (53.71) | 8698 | (63.15) | 34,792 | (63.15) | 14,783 | (75.50) | 59,132 | (75.50) |
| ≥65 years, n (%) | 11,940 | (18.55) | 47,760 | (18.55) | 2529 | (18.36) | 10,116 | (18.36) | 2892 | (14.77) | 11,568 | (14.77) |
| Comorbid conditions, n (%) | ||||||||||||
| Myocardial infarction | 108 | (0.17) | 520 | (0.20) | 39 | (0.28) | 112 | (0.20) | 47 | (0.24) | 142 | (0.18) |
| Congestive heart failure | 896 | (1.39) | 4222 | (1.64) | 235 | (1.71) | 914 | (1.66) | 342 | (1.76) | 1195 | (1.53) |
| Peripheral vascular disease | 377 | (0.59) | 1884 | (0.73) | 97 | (0.70) | 405 | (0.74) | 128 | (0.65) | 643 | (0.82) |
| Cerebrovascular disease | 2347 | (3.65) | 11,388 | (4.42) | 545 | (3.96) | 2560 | (4.65) | 831 | (4.24) | 3518 | (4.49) |
| Dementia | 437 | (0.68) | 2409 | (0.94) | 102 | (0.74) | 497 | (0.90) | 88 | (0.45) | 521 | (0.67) |
| Chronic pulmonary disease | 1766 | (2.74) | 8427 | (3.27) | 370 | (2.69) | 1760 | (3.19) | 544 | (2.78) | 2478 | (3.16) |
| Rheumatic disease | 898 | (1.39) | 4141 | (1.61) | 219 | (1.59) | 935 | (1.70) | 246 | (1.26) | 1396 | (1.78) |
| Peptic ulcer disease | 6348 | (9.86) | 31,919 | (12.39) | 1814 | (13.17) | 7020 | (12.74) | 2083 | (10.64) | 10,536 | (13.45) |
| Liver disease | 2909 | (4.52) | 15,551 | (6.04) | 893 | (6.48) | 3420 | (6.21) | 1256 | (6.41) | 5337 | (6.81) |
| Diabetes | 6452 | (10.02) | 34,379 | (13.35) | 1664 | (12.08) | 8312 | (15.09) | 3506 | (17.91) | 12,780 | (16.32) |
| Hemiplegia or paraplegia | 103 | (0.16) | 481 | (0.19) | 30 | (0.22) | 140 | (0.25) | 24 | (0.12) | 149 | (0.19) |
| Renal disease | 1272 | (1.98) | 5543 | (2.15) | 281 | (2.04) | 1322 | (2.40) | 466 | (2.38) | 2015 | (2.57) |
| AIDS/HIV infection | 38 | (0.06) | 42 | (0.02) | 0 | (0.00) | 9 | (0.02) | 0 | (0.00) | 13 | (0.02) |
| CCI scores | ||||||||||||
| Mean (SD) * | 0.40 | (0.87) | 0.50 | (0.92) | 0.49 | (0.94) | 0.53 | (0.94) | 0.53 | (0.94) | 0.56 | (0.94) |
| Other drugs, n (%) | ||||||||||||
| Metformin | 3990 | (6.20) | 23,451 | (9.11) | 1192 | (8.65) | 6186 | (11.23) | 2408 | (12.30) | 10,291 | (13.14) |
| Aspirin | 3775 | (5.86) | 19,109 | (7.42) | 906 | (6.58) | 4436 | (8.05) | 1545 | (7.89) | 6401 | (8.17) |
| Statin | 3708 | (5.76) | 20,402 | (7.92) | 943 | (6.85) | 5125 | (9.30) | 2162 | (11.04) | 7891 | (10.08) |
| Cervical Cancer (64,382 Cases; 257,528 Controls) | Ovarian Cancer (13,774 Cases; 55,096 Controls) | Uterine Cancer (19,580 Cases; 78,320 Controls) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Antidepressants | n (Case/ Ctrl) | Crude OR (95% CI) | Adjusted OR a (95% CI) | n (Case/ Ctrl) | Crude OR (95% CI) | Adjusted OR a (95% CI) | n (Case/ Ctrl) | Crude OR (95% CI) | Adjusted OR a (95% CI) |
| TCAs | |||||||||
| Overall | 1249/ 6800 | 0.729 (0.686, 0.775) | 0.799 (0.751, 0.850) | 282/ 1521 | 0.736 (0.647, 0.837) | 0.775 (0.681, 0.882) | 431/ 2177 | 0.787 (0.709, 0.874) | 0.813 (0.732, 0.903) |
| Aged 20–39 | 143/ 681 | 0.839 (0.700, 1.005) | 0.876 (0.730, 1.050) | 10/ 66 | 0.605 (0.310, 1.177) | 0.605 (0.310, 1.179) | 6/ 66 | 0.362 (0.157, 0.835) | 0.327 (0.140, 0.765) |
| Aged 40–64 | 618/ 3731 | 0.656 (0.602, 0.715) | 0.740 (0.678, 0.807) | 146/ 961 | 0.601 (0.504, 0.716) | 0.655 (0.549, 0.782) | 274/ 1576 | 0.690 (0.606, 0.785) | 0.724 (0.636, 0.825) |
| Aged ≥ 65 | 488/ 2388 | 0.810 (0.733, 0.894) | 0.867 (0.784, 0.959) | 126/ 494 | 1.021 (0.836, 1.248) | 1.015 (0.830, 1.242) | 151/ 535 | 1.136 (0.944, 1.367) | 1.132 (0.940, 1.363) |
| SSRIs | |||||||||
| Overall | 1262/ 7154 | 0.700 (0.659, 0.743) | 0.736 (0.693, 0.782) | 262/ 1656 | 0.626 (0.549, 0.714) | 0.638 (0.559, 0.728) | 339/ 2412 | 0.554 (0.494, 0.622) | 0.567 (0.505, 0.636) |
| Aged 20–39 | 355/ 1650 | 0.858 (0.764, 0.963) | 0.878 (0.782, 0.987) | 28/ 202 | 0.550 (0.369, 0.818) | 0.553 (0.371, 0.825) | 15/ 194 | 0.304 (0.179, 0.515) | 0.313 (0.184, 0.530) |
| Aged 40–64 | 627/ 4068 | 0.609 (0.560, 0.663) | 0.649 (0.596, 0.707) | 176/ 1136 | 0.612 (0.521, 0.718) | 0.641 (0.545, 0.753) | 244/ 1863 | 0.516 (0.451, 0.590) | 0.531 (0.464, 0.608) |
| Aged ≥ 65 | 280/ 1436 | 0.775 (0.680, 0.882) | 0.816 (0.716, 0.930) | 58/ 318 | 0.723 (0.545, 0.960) | 0.699 (0.525, 0.930) | 80/ 355 | 0.899 (0.703, 1.149) | 0.897 (0.700, 1.149) |
| Cervical Cancer | Ovarian Cancer | Uterine Cancer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Groups | Overall | 20–39 | 40–64 | ≥65 | Overall | 20–39 | 40–64 | ≥65 | Overall | 20–39 | 40–64 | ≥65 |
| TCAs | **** | **** | ** | *** | **** | *** | ** | **** | ||||
| Imipramine | ** | ** | ** | |||||||||
| Amitriptyline | ** | * | ** | ** | ||||||||
| Doxepin | * | * | * | ** | * | |||||||
| SSRIs | **** | * | **** | ** | **** | ** | **** | * | **** | **** | **** | |
| Fluoxetine | **** | **** | *** | ** | * | **** | **** | |||||
| Citalopram | ** | ** | * | **** | **** | |||||||
| Paroxetine | **** | **** | ** | *** | *** | |||||||
| Sertraline | **** | **** | *** | ** | **** | * | **** | |||||
| Fluvoxamine | * | * | * | * | ||||||||
| Escitalopram | **** | **** | ** | *** | **** | * | **** | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Huang, C.-W.; Nguyen, N.T.H.; Lin, M.-C.; Nguyen, P.-A.; Islam, M.M.; Chien, S.-C.; Yang, H.-C. Real-World Data on the Associations of Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors with Gynecologic Cancer Risk. Cancers 2025, 17, 1616. https://doi.org/10.3390/cancers17101616
Wang C-H, Huang C-W, Nguyen NTH, Lin M-C, Nguyen P-A, Islam MM, Chien S-C, Yang H-C. Real-World Data on the Associations of Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors with Gynecologic Cancer Risk. Cancers. 2025; 17(10):1616. https://doi.org/10.3390/cancers17101616
Chicago/Turabian StyleWang, Ching-Huan, Chih-Wei Huang, Nhi Thi Hong Nguyen, Ming-Chin Lin, Phung-Anh Nguyen, Md. Mohaimenul Islam, Shuo-Chen Chien, and Hsuan-Chia Yang. 2025. "Real-World Data on the Associations of Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors with Gynecologic Cancer Risk" Cancers 17, no. 10: 1616. https://doi.org/10.3390/cancers17101616
APA StyleWang, C.-H., Huang, C.-W., Nguyen, N. T. H., Lin, M.-C., Nguyen, P.-A., Islam, M. M., Chien, S.-C., & Yang, H.-C. (2025). Real-World Data on the Associations of Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors with Gynecologic Cancer Risk. Cancers, 17(10), 1616. https://doi.org/10.3390/cancers17101616








