Annexin A1 Is Involved in the Antitumor Effects of 5-Azacytidine in Human Oral Squamous Carcinoma Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemicals and Reagents
2.3. Cell Blocks
2.4. Immunohistochemistry
2.5. Confocal Microscopy
2.6. Wound-Healing Assay
2.7. Invasion Assay
2.8. MTT Assay
2.9. Flow Cytometry for Cell Cycle and Cell Death
2.10. Western Blotting
2.11. RNA Extraction and Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.12. siRNAs Transfection Death
2.13. Statistical Analysis
3. Results
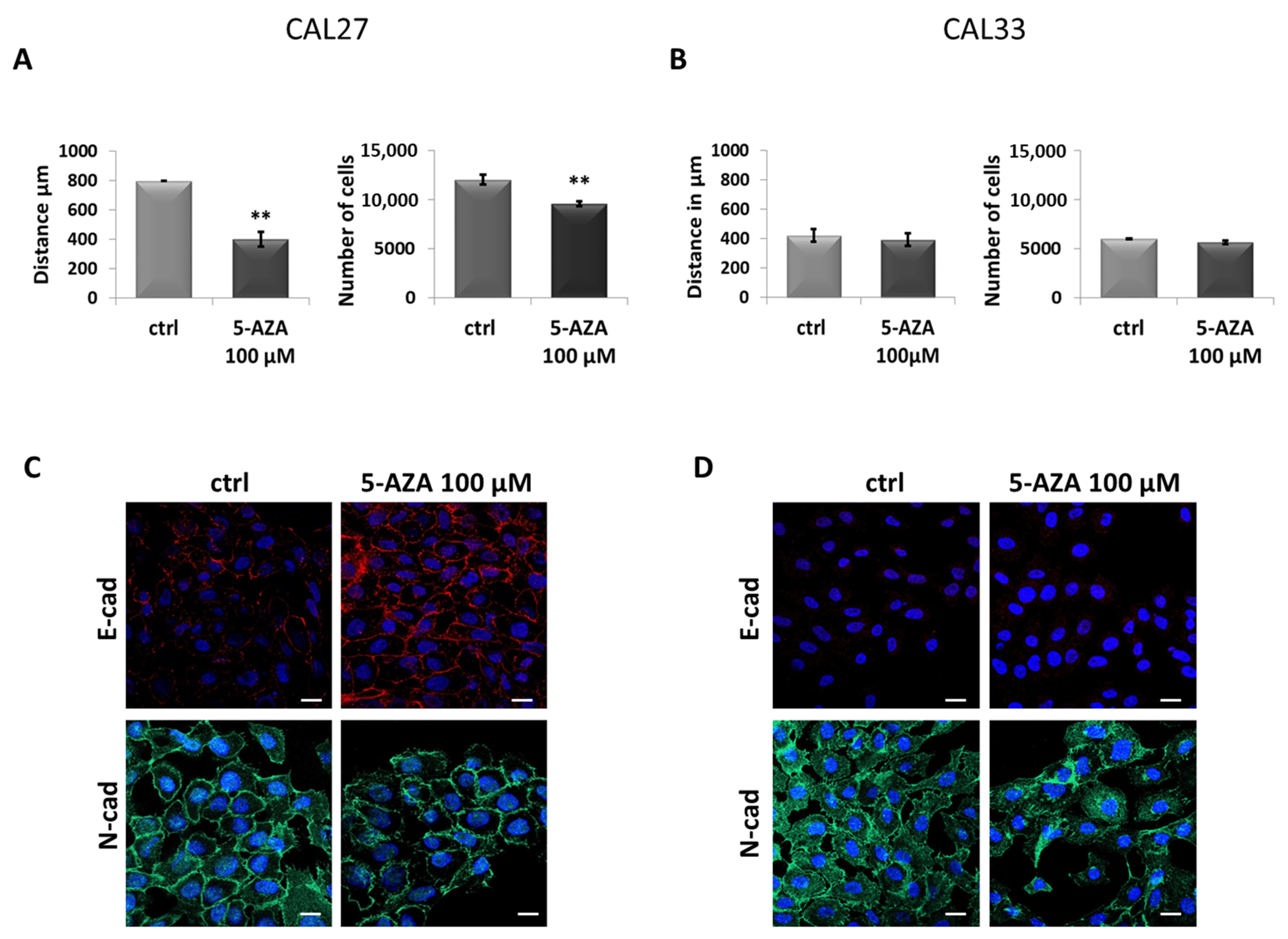
3.1. Different Phenotypes of CAL27 and CAL33 Cells in Presence of 5-AZA Treatment
3.2. In CAL27, 5-AZA Reduces Cell Motility and Induces MET
3.3. 5-AZA Induces Increased Expression of ANXA1 in CAL27 Cells
3.4. siRNA-Mediated ANXA1 Downmodulation Restores the CAL27 Aggressive Phenotype
3.5. Exogenous Ac2-26 Reduces CAL27 Motility by Acquiring a Less Aggressive Phenotype
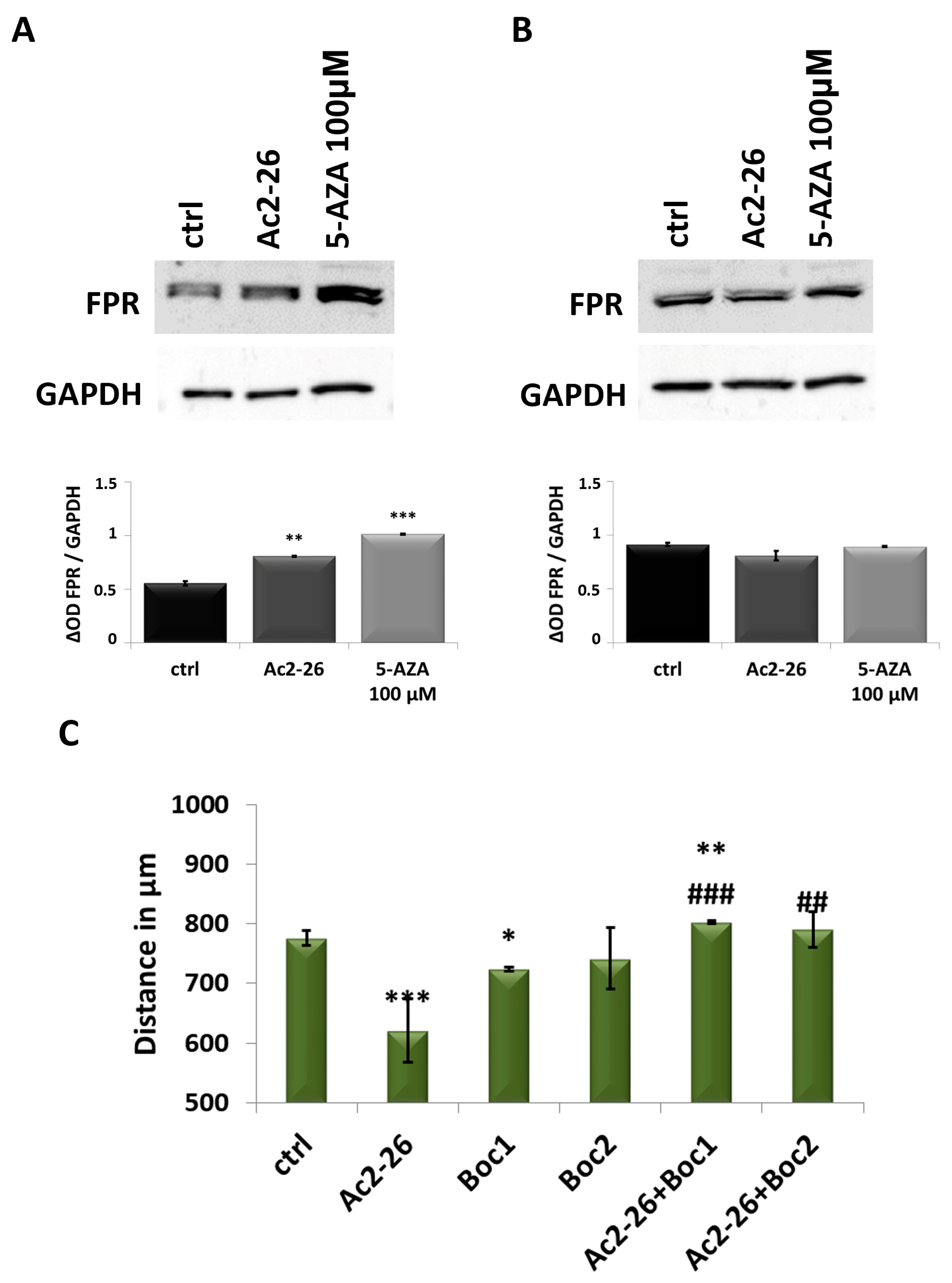
3.6. ANXA1 Effects Are Triggered by FPR
3.7. ANXA1 Action Is Highlighted by Ac2-26 Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 203. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Brenner, H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef]
- Kałafut, J.; Czerwonka, A.; Anameriç, A.; Przybyszewska-Podstawka, A.; Misiorek, J.O.; Rivero-Müller, A.; Nees, M. Shooting at Moving and Hidden Targets-Tumour Cell Plasticity and the Notch Signalling Pathway in Head and Neck Squamous Cell Carcinomas. Cancers 2021, 13, 6219. [Google Scholar] [CrossRef]
- Feller, G.; Khammissa, R.A.G.; Ballyram, R.; Beetge, M.M.; Lemmer, J.; Feller, L. Tumour Genetic Heterogeneity in Relation to Oral Squamous Cell Carcinoma and Anti-Cancer Treatment. Int. J. Environ. Res. Public Health 2023, 20, 2392. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Zerp, S.F.; Stoter, T.R.; Hoebers, F.J.; van den Brekel, M.W.; Dubbelman, R.; Kuipers, G.K.; Lafleur, M.V.; Slotman, B.J.; Verheij, M. Targeting anti-apoptotic Bcl-2 by AT-101 to increase radiation efficacy: Data from in vitro and clinical pharmacokinetic studies in head and neck cancer. Radiat. Oncol. 2015, 10, 158. [Google Scholar] [CrossRef]
- Adamska, A.; Elaskalani, O.; Emmanouilidi, A.; Kim, M.; Abdol Razak, N.B.; Metharom, P.; Falasca, M. Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv. Biol. Regul. 2018, 68, 77–87. [Google Scholar] [CrossRef]
- Taylor, S.M. 5-Aza-2′-deoxycytidine: Cell differentiation and DNA methylation. Leukemia 1993, 7 (Suppl. S1), 3–8. [Google Scholar]
- Abbey, D.; Seshagiri, P.B. Aza-induced cardiomyocyte differentiation of P19 EC-cells by epigenetic co-regulation and ERK signaling. Gene 2013, 526, 364–373. [Google Scholar] [CrossRef]
- Hervouet, E.; Cheray, M.; Vallette, F.M.; Cartron, P.F. DNA methylation and apoptosis resistance in cancer cells. Cells 2013, 2, 545–573. [Google Scholar] [CrossRef] [PubMed]
- Soengas, M.S.; Capodieci, P.; Polsky, D.; Mora, J.; Esteller, M.; Opitz-Araya, X.; McCombie, R.; Herman, J.G.; Gerald, W.L.; Lazebnik, Y.A.; et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 2001, 409, 207–211. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Zhou, G.; Zhang, Q.; Zhang, T.; Li, J.; Zhang, J.; Hou, J.; Liew, C.T.; Yin, D. Transcriptional silencing of the TMS1/ASC tumour suppressor gene by an epigenetic mechanism in hepatocellular carcinoma cells. J. Pathol. 2007, 212, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Cihák, A.; Vesely, J.; Skoda, J. Azapyrimidine nucleosides: Metabolism and inhibitory mechanisms. Adv. Enzym. Regul. 1985, 24, 335–354. [Google Scholar] [CrossRef]
- Hodjat, M.; Jourshari, P.B.; Amirinia, F.; Asadi, N. 5-Azacitidine and Trichostatin A induce DNA damage and apoptotic responses in tongue squamous cell carcinoma: An in vitro study. Arch. Oral Biol. 2022, 133, 105296. [Google Scholar] [CrossRef]
- Biktasova, A.; Hajek, M.; Sewell, A.; Gary, C.; Bellinger, G.; Deshpande, H.A.; Bhatia, A.; Burtness, B.; Judson, B.; Mehra, S.; et al. Demethylation Therapy as a Targeted Treatment for Human Papillomavirus-Associated Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 7276–7287. [Google Scholar] [CrossRef]
- Pan, C.; Issaeva, N.; Yarbrough, W.G. HPV-driven oropharyngeal cancer: Current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck 2018, 3, 12. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Zhu, D.W.; Yang, X.; Yang, C.Z.; Ma, J.; Liu, Y.; Yan, M.; Wang, L.Z.; Li, J.; Zhang, C.P.; Zhang, Z.Y.; et al. Annexin A1 down-regulation in oral squamous cell carcinoma correlates to pathological differentiation grade. Oral Oncol. 2013, 49, 542–550. [Google Scholar] [CrossRef]
- Lin, C.Y.; Jeng, Y.M.; Chou, H.Y.; Hsu, H.C.; Yuan, R.H.; Chiang, C.P.; Kuo, M.Y. Nuclear localization of annexin A1 is a prognostic factor in oral squamous cell carcinoma. J. Surg. Oncol. 2008, 97, 544–550. [Google Scholar] [CrossRef]
- Wan, Y.M.; Tian, J.; Qi, L.; Li, L.M.; Xu, N. ANXA1 affects cell proliferation, invasion and epithelial-mesenchymal transition of oral squamous cell carcinoma. Exp. Ther. Med. 2017, 14, 5214–5218. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-A1—A Blessing or a Curse in Cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wang, L.; Guo, J.; Sun, D.; Wang, Y.; Liu, W.; Xu, H.E.; Zhang, C. Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2. Nat. Commun. 2022, 13, 1054. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef]
- Guo, C.; Liu, S.; Sun, M.Z. Potential role of Anxa1 in cancer. Future Oncol. 2013, 9, 1773–1793. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, T.; Kondo, J.; Endo, H.; Ohue, M.; Sakai, Y.; Inoue, M. Hydrodynamic stress stimulates growth of cell clusters via the ANXA1/PI3K/AKT axis in colorectal cancer. Sci. Rep. 2019, 9, 20027. [Google Scholar] [CrossRef]
- Raulf, N.; Lucarelli, P.; Thavaraj, S.; Brown, S.; Vicencio, J.M.; Sauter, T.; Tavassoli, M. Annexin A1 regulates EGFR activity and alters EGFR-containing tumour-derived exosomes in head and neck cancers. Eur. J. Cancer 2018, 102, 52–68. [Google Scholar] [CrossRef]
- Gioanni, J.; Fischel, J.L.; Lambert, J.C.; Demard, F.; Mazeau, C.; Zanghellini, E.; Ettore, F.; Formento, P.; Chauvel, P.; Lalanne, C.M.; et al. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: Establishment, characterization and response to cytotoxic treatment. Eur. J. Cancer Clin. Oncol. 1988, 24, 1445–1455. [Google Scholar] [CrossRef]
- Novizio, N.; Belvedere, R.; Morretta, E.; Tomasini, R.; Monti, M.C.; Morello, S.; Petrella, A. Role of Intracellular and Extracellular Annexin A1 in MIA PaCa-2 Spheroids Formation and Drug Sensitivity. Cancers 2022, 14, 4764. [Google Scholar] [CrossRef]
- Dalli, J.; Montero-Melendez, T.; McArthur, S.; Perretti, M. Annexin A1 N-terminal derived Peptide ac2-26 exerts chemokinetic effects on human neutrophils. Front. Pharmacol. 2012, 3, 28. [Google Scholar] [CrossRef]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef] [PubMed]
- Proto, M.C.; Fiore, D.; Piscopo, C.; Franceschelli, S.; Bizzarro, V.; Laezza, C.; Lauro, G.; Feoli, A.; Tosco, A.; Bifulco, G.; et al. Inhibition of Wnt/β-Catenin pathway and Histone acetyltransferase activity by Rimonabant: A therapeutic target for colon cancer. Sci. Rep. 2017, 7, 11678. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Pessolano, E.; Tosco, A.; Eletto, D.; Porta, A.; Campiglia, P.; Perretti, M.; Filippelli, A.; Petrella, A. Heparan sulfate binds the extracellular Annexin A1 and blocks its effects on pancreatic cancer cells. Biochem. Pharmacol. 2020, 182, 114252. [Google Scholar] [CrossRef]
- Novizio, N.; Belvedere, R.; Pessolano, E.; Tosco, A.; Porta, A.; Perretti, M.; Campiglia, P.; Filippelli, A.; Petrella, A. Annexin A1 Released in Extracellular Vesicles by Pancreatic Cancer Cells Activates Components of the Tumor Microenvironment, through Interaction with the Formyl-Peptide Receptors. Cells 2020, 9, 2719. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.K.; Dover, G.; Young, N.S.; Moore, J.G.; Charache, S.; Ley, T.; Nienhuis, A.W. 5-Azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin. J. Clin. Investig. 1985, 75, 547–557. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Ganavi, B.S. Mesenchymal-Epithelial Transition in Oral Cancer. J. Int. Oral Health 2015, 7, i–ii. [Google Scholar]
- Perretti, M.; Dalli, J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br. J. Pharmacol. 2009, 158, 936–946. [Google Scholar] [CrossRef]
- Cardin, L.T.; Sonehara, N.M.; Mimura, K.K.; Ramos Dinarte Dos Santos, A.; da Silva WAJunior Sobral, L.M.; Leopoldino, A.M.; da Cunha, B.R.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. ANXA1Ac2-26 peptide, a possible therapeutic approach in inflammatory ocular diseases. Gene 2017, 614, 26–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Yen, Y.; Brown, J.; Ta, J.Q.; Le, A.D. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010, 289, 151–160. [Google Scholar] [CrossRef]
- Rodini, C.O.; Lopes, N.M.; Lara, V.S.; Mackenzie, I.C. Oral cancer stem cells—Properties and consequences. J. Appl. Oral Sci. 2017, 25, 708–715. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Shu, M.; Hu, W. Unravelling the heterogeneity of oral squamous cell carcinoma by integrative analysis of single-cell and bulk transcriptome data. J. Cell Mol. Med. 2024, 28, e18108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yan, M.; Zhang, J.; Xu, Q.; Qi, S.; Wang, X.; Chen, W. Cancer stem-like cell related protein CD166 degrades through E3 ubiquitin ligase CHIP in head and neck cancer. Exp. Cell Res. 2017, 353, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yang, X.; Wang, L.; Clark, D.; Zuo, H.; Ye, D.; Chen, W.; Zhang, P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol. Cell. Proteom. 2013, 12, 3271–3284. [Google Scholar] [CrossRef]
- Ihnen, M.; Kress, K.; Kersten, J.F.; Kilic, E.; Choschzick, M.; Zander, H.; Müller, V.; Mahner, S.; Jänicke, F.; Woelber, L.; et al. Relevance of activated leukocyte cell adhesion molecule (ALCAM) in tumor tissue and sera of cervical cancer patients. BMC Cancer 2012, 12, 140. [Google Scholar] [CrossRef]
- Hein, S.; Müller, V.; Köhler, N.; Wikman, H.; Krenkel, S.; Streichert, T.; Schweizer, M.; Riethdorf, S.; Assmann, V.; Ihnen, M.; et al. Biologic role of activated leukocyte cell adhesion molecule overexpression in breast cancer cell lines and clinical tumor tissue. Breast Cancer Res. Treat. 2011, 129, 347–360. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, J.H.; Longmire, M.; Wang, H.; Kohrt, H.E.; Chang, H.Y.; Sunwoo, J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin. Cancer Res. 2016, 22, 3571–3581. [Google Scholar] [CrossRef]
- Wang, H.; Unternaehrer, J.J. Epithelial-mesenchymal Transition and Cancer Stem Cells: At the Crossroads of Differentiation and Dedifferentiation. Dev. Dyn. 2019, 248, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Gailhouste, L.; Liew, L.C.; Hatada, I.; Nakagama, H.; Ochiya, T. Epigenetic reprogramming using 5-azacytidine promotes an anti-cancer response in pancreatic adenocarcinoma cells. Cell Death Dis. 2018, 9, 468. [Google Scholar] [CrossRef]
- Parker, W.B.; Thottassery, J.V. 5-Aza-4′-thio-2′-deoxycytidine, a New Orally Bioavailable Nontoxic “Best-in-Class”: DNA Methyltransferase 1-Depleting Agent in Clinical Development. J. Pharmacol. Exp. Ther. 2021, 379, 211–222. [Google Scholar] [CrossRef]
- Cihak, A.; Vesely, J.; Hynie, S. Transformation and metabolic effects of 5-aza-2′-deoxycytidine in mice. Biochem. Pharmacol. 1980, 29, 2929–2932. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Tokumaru, Y.; Mukhopadhyay, S.; Yan, L.; Matsuyama, R.; Endo, I.; Takabe, K. Annexin A1 Expression Is Associated with Epithelial-Mesenchymal Transition (EMT), Cell Proliferation, Prognosis, and Drug Response in Pancreatic Cancer. Cells 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Rondepierre, F.; Bouchon, B.; Papon, J.; Bonnet-Duquennoy, M.; Kintossou, R.; Moins, N.; Maublant, J.; Madelmont, J.C.; D’Incan, M.; Degoul, F. Proteomic studies of B16 lines: Involvement of annexin A1 in melanoma dissemination. Biochim. Biophys. Acta. 2009, 1794, 61–69. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Wu, M.S.; Lin, J.T.; Lin, M.T.; Shun, C.T.; Huang, H.Y.; Hua, K.T.; Kuo, M.L. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer 2012, 118, 5757–5767. [Google Scholar] [CrossRef]
- Maschler, S.; Gebeshuber, C.A.; Wiedemann, E.M.; Alacakaptan, M.; Schreiber, M.; Custic, I.; Beug, H. Annexin A1 attenuates EMT and metastatic potential in breast cancer. EMBO Mol. Med. 2010, 2, 401–414. [Google Scholar] [CrossRef]
- Paweletz, C.P.; Ornstein, D.K.; Roth, M.J.; Bichsel, V.E.; Gillespie, J.W.; Calvert, V.S.; Vocke, C.D.; Hewitt, S.M.; Duray, P.H.; Herring, J.; et al. Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000, 60, 6293–6297. [Google Scholar] [PubMed]
- Garcia Pedrero, J.M.; Fernandez, M.P.; Morgan, R.O.; Herrero Zapatero, A.; Gonzalez, M.V.; Suarez Nieto, C.; Rodrigo, J.P. Annexin A1 down-regulation in head and neck cancer is associated with epithelial differentiation status. Am. J. Pathol. 2004, 164, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Zhong, L.P.; Zhou, X.J.; Pan, H.Y.; Wei, K.J.; Li, J.; Chen, W.T.; Zhang, Z.Y. Decreased expression of Annexin A1 correlates with pathologic differentiation grade in oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 362–370. [Google Scholar] [CrossRef]
- Álvarez-Teijeiro, S.; Menéndez, S.T.; Villaronga, M.Á.; Pena-Alonso, E.; Rodrigo, J.P.; Morgan, R.O.; Granda-Díaz, R.; Salom, C.; Fernandez, M.P.; García-Pedrero, J.M. Annexin A1 down-regulation in head and neck squamous cell carcinoma is mediated via transcriptional control with direct involvement of miR-196a/b. Sci. Rep. 2017, 7, 6790. [Google Scholar] [CrossRef]
- Tian, C.; Chen, K.; Gong, W.; Yoshimura, T.; Huang, J.; Wang, J.M. The G-Protein Coupled Formyl Peptide Receptors and Their Role in the Progression of Digestive Tract Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820973280. [Google Scholar] [CrossRef]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Yang, H.; Adjemian, S.; Chaba, K.; Semeraro, M.; et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015, 350, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Gastardelo, T.S.; Cunha, B.R.; Raposo, L.S.; Maniglia, J.V.; Cury, P.M.; Lisoni, F.C.; Tajara, E.H.; Oliani, S.M. Inflammation and cancer: Role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma. PLoS ONE 2014, 9, e111317. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limón, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Cherfils-Vicini, J.; Damotte, D.; Fisson, S.; Fridman, W.H.; Cremer, I.; Dieu-Nosjean, M.C. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011, 30, 13–25. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novizio, N.; Belvedere, R.; Palazzo, M.; Varricchio, S.; Merolla, F.; Staibano, S.; Ilardi, G.; Petrella, A. Annexin A1 Is Involved in the Antitumor Effects of 5-Azacytidine in Human Oral Squamous Carcinoma Cells. Cancers 2025, 17, 1058. https://doi.org/10.3390/cancers17071058
Novizio N, Belvedere R, Palazzo M, Varricchio S, Merolla F, Staibano S, Ilardi G, Petrella A. Annexin A1 Is Involved in the Antitumor Effects of 5-Azacytidine in Human Oral Squamous Carcinoma Cells. Cancers. 2025; 17(7):1058. https://doi.org/10.3390/cancers17071058
Chicago/Turabian StyleNovizio, Nunzia, Raffaella Belvedere, Mariangela Palazzo, Silvia Varricchio, Francesco Merolla, Stefania Staibano, Gennaro Ilardi, and Antonello Petrella. 2025. "Annexin A1 Is Involved in the Antitumor Effects of 5-Azacytidine in Human Oral Squamous Carcinoma Cells" Cancers 17, no. 7: 1058. https://doi.org/10.3390/cancers17071058
APA StyleNovizio, N., Belvedere, R., Palazzo, M., Varricchio, S., Merolla, F., Staibano, S., Ilardi, G., & Petrella, A. (2025). Annexin A1 Is Involved in the Antitumor Effects of 5-Azacytidine in Human Oral Squamous Carcinoma Cells. Cancers, 17(7), 1058. https://doi.org/10.3390/cancers17071058






