Resection vs. Ligation vs. Preservation of the Thoracic Duct During Esophagectomy for Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Statistical Analysis
3. Results
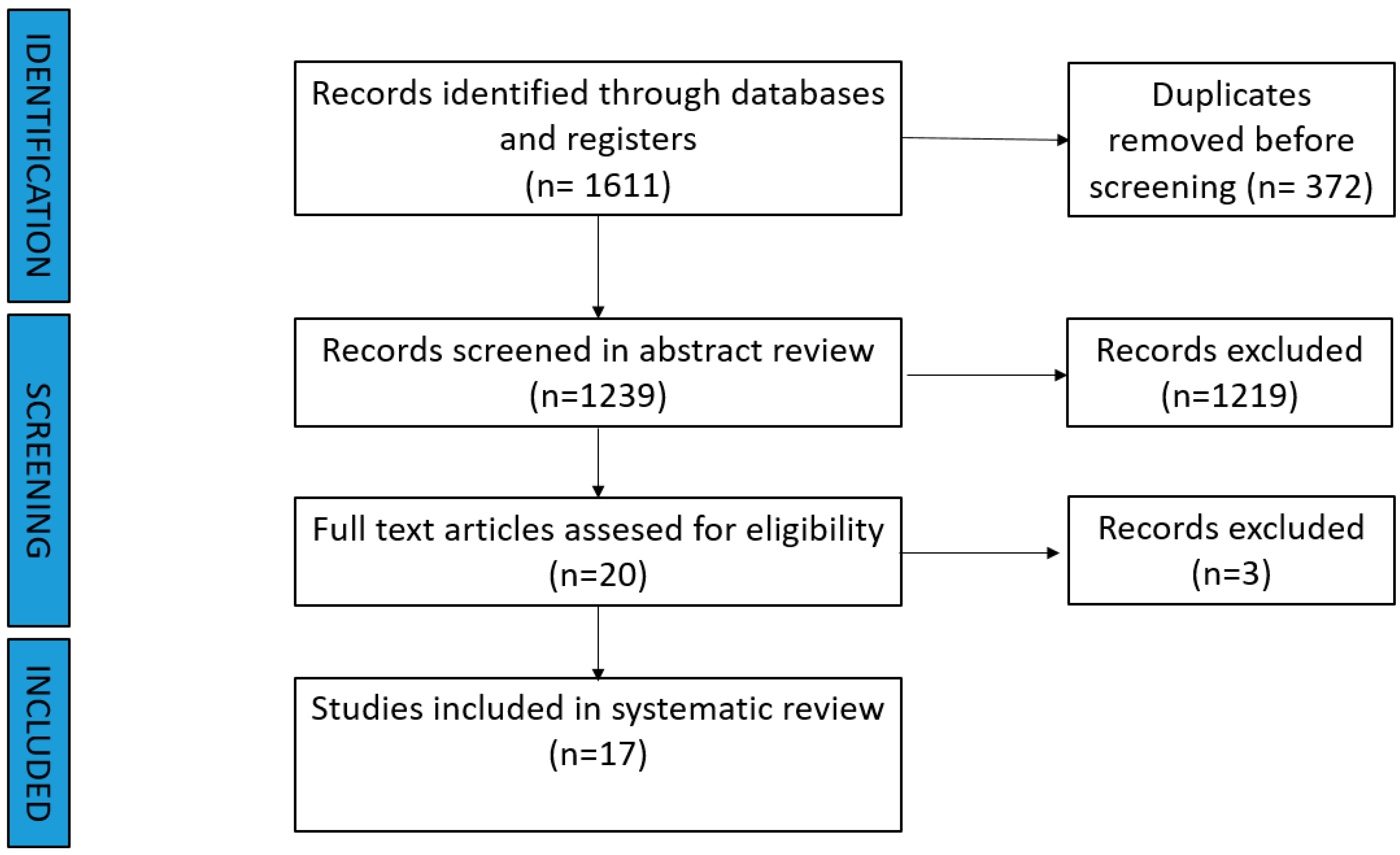
3.1. Systematic Review
3.2. Data Review and Meta-Analysis: Resection vs. Preservation of the Thoracic Duct
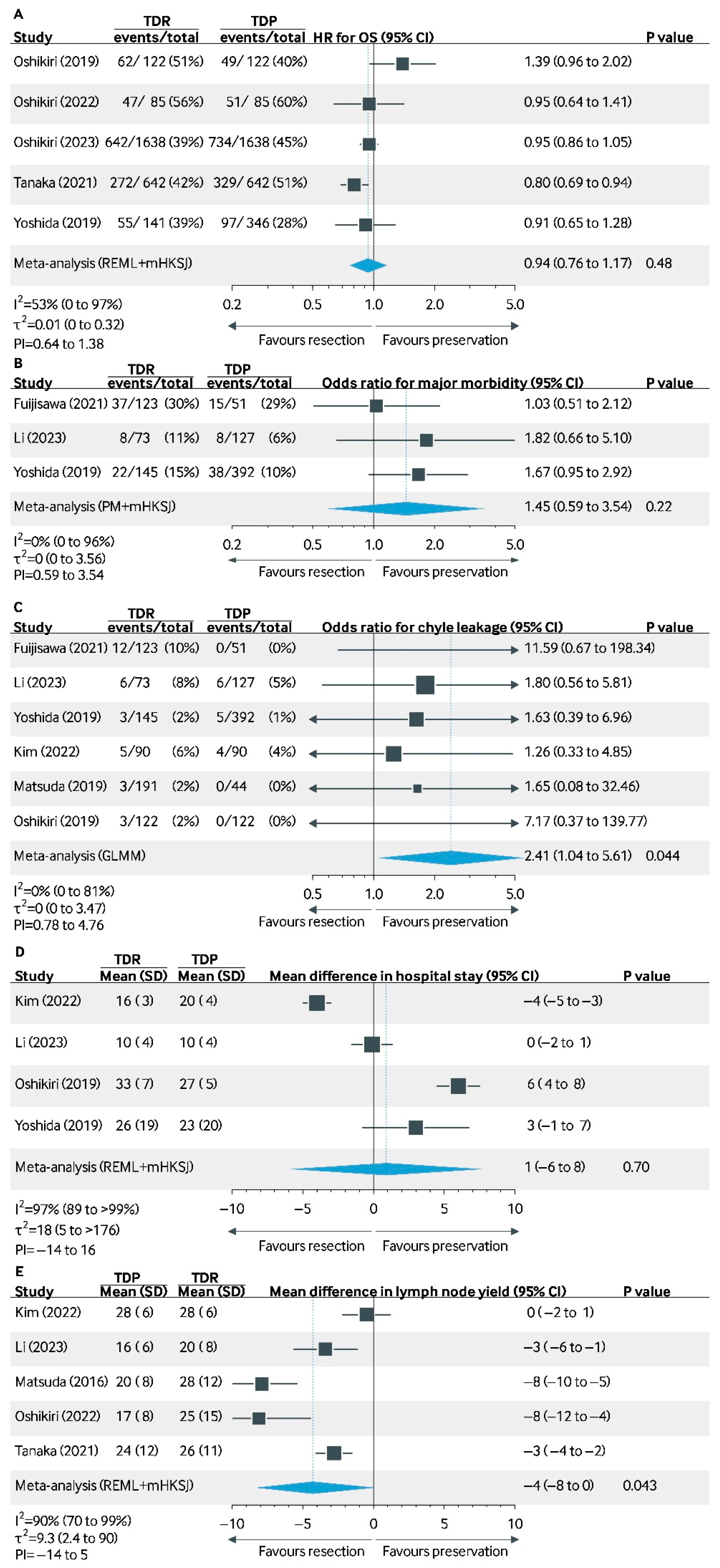
3.2.1. 5-Year Overall Survival
3.2.2. Chyle Leakage
3.2.3. Incidence of Postoperative Complications
3.2.4. Major Morbidity (Clavien–Dindo ≥ 3)
3.2.5. Length of Stay
3.2.6. Lymph Node Yield
3.3. Data Review and Meta-Analysis: Ligation vs. Preservation of the Thoracic Duct
3.3.1. 5-Year Overall Survival
3.3.2. Chyle Leakage
3.3.3. Incidence of Postoperative Complications
3.3.4. Length of Stay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Wang, F.; Hallemeier, C.L.; Lerut, T.; Fu, J. Oesophageal cancer. Lancet 2024, 404, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Defize, I.L.; Gorgels, S.M.C.; Mazza, E.; Schurink, B.; Strignano, P.; Catalano, G.; Brosens, L.A.A.; Chiusa, L.; Bleys, R.L.A.W.; Mook, S.; et al. The Presence of Metastatic Thoracic Duct Lymph Nodes in Western Esophageal Cancer Patients: A Multinational Observational Study. Ann. Thorac. Surg. 2022, 113, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Phang, K.; Bowman, M.; Phillips, A.; Windsor, J. Review of thoracic duct anatomical variations and clinical implications. Clin. Anat. 2014, 27, 637–644. [Google Scholar] [CrossRef]
- Plutecki, D.; Bonczar, M.; Wilk, J.; Necka, S.; Joniec, M.; Elsaftawy, A.; Matuszyk, A.; Walocha, J.; Koziej, M.; Ostrowski, P. The Anatomy of the Thoracic Duct and Cisterna Chyli: A Meta-Analysis with Surgical Implications. J. Clin. Med. 2024, 13, 4285. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Siddaiah-Subramanya, M.; Parente, A.; Evans, R.P.T.; Adeyeye, A.; Ainsworth, A.; Takahashi, A.M.L.; Charalabopoulos, A.; Chang, A.; Eroglue, A.; et al. Risk Factors, Diagnosis and Management of Chyle Leak Following Esophagectomy for Cancers: An International Consensus Statement. Ann. Surg. Open 2022, 3, e192. [Google Scholar] [CrossRef]
- Voeten, D.M.; Busweiler, L.A.D.; van der Werf, L.R.; Wijnhoven, B.P.L.; Verhoeven, R.H.A.; van Sandick, J.W.; van Hillegersberg, R.; van Berge Henegouwen, M.I. Outcomes of Esophagogastric Cancer Surgery During Eight Years of Surgical Auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 2021, 274, 866–873. [Google Scholar] [CrossRef]
- Lagarde, S.M.; Omloo, J.M.; de Jong, K.; Busch, O.R.; Obertop, H.; van Lanschot, J.J.B. Incidence and management of chyle leakage after esophagectomy. Ann. Thorac. Surg. 2005, 80, 449–454. [Google Scholar] [CrossRef]
- Weijs, T.J.; Ruurda, J.P.; Broekhuizen, M.E.; Bracco Gartner, T.C.L.; van Hillegersberg, R. Outcome of a Step-Up Treatment Strategy for Chyle Leakage After Esophagectomy. Ann. Thorac. Surg. 2017, 104, 477–484. [Google Scholar] [CrossRef]
- Goense, L.; van Dijk, W.A.; Govaert, J.A.; van Rossum, P.S.N.; Ruurda, J.P.; van Hillegersberg, R. Hospital costs of complications after esophagectomy for cancer. Eur. J. Surg. Oncol. EJSO 2017, 43, 696–702. [Google Scholar] [CrossRef]
- Schafrat, P.J.M.; Henckens, S.P.G.; Hagens, E.R.C.; Eshuis, W.J.; Gisbertz, S.S.; Laméris, W.; van Berge Henegouwen, M.I. Clinical implications of chyle leakage following esophagectomy. Dis. Esophagus 2022, 36, doac047. [Google Scholar] [CrossRef]
- Udagawa, H.; Ueno, M.; Shinohara, H.; Haruta, S.; Lee, S.; Momose, K.; Tsurumaru, M. Should lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 2014, 11, 204–210. [Google Scholar] [CrossRef]
- Farrow, H.; Pickering, O.J.; Gossage, J.A.; Pucher, P.H. Impact of thoracic duct resection during radical esophagectomy on oncological and survival outcomes: Systematic review. Eur. J. Surg. Oncol. 2024, 50, 107271. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Takiguchi, G.; Miura, S.; Goto, H.; Otsubo, D.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Yamashita, K.; Matsuda, T.; et al. Thoracic Duct Resection During Esophagectomy Does Not Contribute to Improved Prognosis in Esophageal Squamous Cell Carcinoma: A Propensity Score Matched-Cohort Study. Ann. Surg. Oncol. 2019, 26, 4053–4061. [Google Scholar] [CrossRef]
- Tokumaru, S.; Kitazawa, M.; Nakamura, S.; Koyama, M.; Soejima, Y. Intraoperative visualization of morphological patterns of the thoracic duct by subcutaneous inguinal injection of indocyanine green in esophagectomy for esophageal cancer. Ann. Gastroenterol. Surg. 2022, 6, 873–879. [Google Scholar] [CrossRef]
- Barnes, T.G.; MacGregor, T.; Sgromo, B.; Maynard, N.D.; Gillies, R.S. Near infra-red fluorescence identification of the thoracic duct to prevent chyle leaks during oesophagectomy. Surg. Endosc. 2022, 36, 5319–5325. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Yoshida, N.; Nagai, Y.; Baba, Y.; Miyamoto, Y.; Iwagami, S.; Iwatsuki, M.; Hiyoshi, Y.; Eto, K.; Ishimoto, T.; Kiyozumi, Y.; et al. Effect of Resection of the Thoracic Duct and Surrounding Lymph Nodes on Short- and Long-Term and Nutritional Outcomes After Esophagectomy for Esophageal Cancer. Ann. Surg. Oncol. 2019, 26, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Takeuchi, H.; Kawakubo, H.; Shimada, A.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Kameyama, K.; Kitagawa, Y. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: Number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine 2016, 95, e3839. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Fu, J.H.; Wang, X.; Zhang, L.J.; Liu, Q.W.; Luo, K.J.; Lin, P.; Yang, H.X. Prophylactic thoracic duct ligation has unfavorable impact on overall survival in patients with resectable oesophageal cancer. Eur. J. Surg. Oncol. 2014, 40, 1756–1762. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Liu, Q.-W.; Zhang, S.-S.; Li, J.-B.; Yang, H.; Wen, J.; Fu, J.-H. Prophylactic thoracic duct ligation is associated with poor prognosis and regional lymph node relapse in esophageal squamous cell carcinoma. J. Surg. Oncol. 2020, 122, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Numasaki, H.; Oguma, J.; Toh, Y.; Watanabe, M.; Muto, M.; Kakeji, Y.; Doki, Y. Is Thoracic Duct Resection Necessary for Esophageal Squamous Cell Carcinoma Patients Treated with Neoadjuvant Chemoradiotherapy? A Propensity-Matched Analysis Based on the Comprehensive Registry of Esophageal Cancer in Japan. Ann. Surg. Oncol. 2023, 30, 2691–2698. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamasaki, M.; Sugimura, K.; Shiraishi, O.; Motoori, M.; Hamakawa, T.; Takeno, A.; Yamashita, K.; Makino, T.; Kimura, Y.; et al. Thoracic Duct Resection Has a Favorable Impact on Prognosis by Preventing Hematogenous Spread of Esophageal Cancer Cells: A Multi-institutional Analysis of 2269 Patients. Ann. Surg. Oncol. 2021, 28, 4402–4410. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ohkura, Y.; Ueno, M.; Yago, A.; Shimoyama, H.; Udagawa, H. Nutritional Outcomes of Thoracic Duct Resection for Radical Esophagectomy by Assessing Body Composition Changes in One Year: A Single-Center Retrospective Study. Ann. Surg. Oncol. 2021, 28, 8414–8425. [Google Scholar] [CrossRef]
- Kim, H.E.; Yang, Y.H.; Park, B.J.; Park, S.Y.; Min, I.K.; Kim, D.J. Skeletonizing En Bloc Esophagectomy Revisited: Oncologic Outcome in Association with the Presence of Thoracic Duct Lymph Nodes. Ann. Surg. Oncol. 2022, 29, 4909–4917. [Google Scholar] [CrossRef]
- Li, C.; Li, B.; Yang, Y.; Li, Z. Short-term clinical effects of robot-assisted esophagectomy with thoracic duct resection. J. Gastrointest. Oncol. 2023, 14, 11–21. [Google Scholar] [CrossRef]
- Oshikiri, T.; Numasaki, H.; Oguma, J.; Toh, Y.; Watanabe, M.; Muto, M.; Kakeji, Y.; Doki, Y. Prognosis of Patients with Esophageal Carcinoma After Routine Thoracic Duct Resection: A Propensity-matched Analysis of 12,237 Patients Based on the Comprehensive Registry of Esophageal Cancer in Japan. Ann. Surg. 2023, 277, e1018–e1025. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kawakubo, H.; Takeuchi, H.; Hayashi, M.; Mayanagi, S.; Takemura, R.; Irino, T.; Fukuda, K.; Nakamura, R.; Wada, N.; et al. Minimally invasive oesophagectomy with extended lymph node dissection and thoracic duct resection for early-stage oesophageal squamous cell carcinoma. Br. J. Surg. 2020, 107, 705–711. [Google Scholar] [CrossRef]
- Bao, T.; Wang, Y.J.; Li, K.K.; Liu, X.H.; Guo, W. Short- and long-term outcomes of prophylactic thoracic duct ligation during thoracoscopic-laparoscopic McKeown esophagectomy for cancer: A propensity score matching analysis. Surg. Endosc. 2020, 34, 5023–5029. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, Y.-P.; Jiang, Y.-G.; Niu, H.-J.; Liu, X.-H.; Ma, Z.; Wang, R.-W. Prevention of postoperative chylothorax with thoracic duct ligation during video-assisted thoracoscopic esophagectomy for cancer. Surg. Endosc. 2012, 26, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Wang, X.; Lu, Q.; Lu, C.; Chen, H.; Li, C. The influence of thoracic duct ligation on long-term survival of patients with esophageal cancer: A propensity score-matched analysis. J. Thorac. Dis. 2020, 12, 5532–5541. [Google Scholar] [CrossRef]
- Lai, F.-C.; Chen, L.; Tu, Y.-R.; Lin, M.; Li, X. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: A random control study. Ann. Thorac. Surg. 2011, 91, 1770–1774. [Google Scholar] [CrossRef]
- Yang, R.F.; Sui, S.J.; Yu, L.L.; Li, H.Y.; Zhang, R.Q.; Wang, P. Survival Analysis of Patients with Thoracic Duct Ligation during Thoracoscopic Esophagectomy. J. Coll. Physicians Surg. Pak. 2022, 32, 288–292. [Google Scholar] [CrossRef]
- Hagens, E.R.C.; van Berge Henegouwen, M.I.; Cuesta, M.A.; Gisbertz, S.S. The extent of lymphadenectomy in esophageal resection for cancer should be standardized. J. Thorac. Dis. 2017, 9 (Suppl. S8), S713–S723. [Google Scholar] [CrossRef]
- Ketel, M.H.M.; van der Aa, D.C.; Henckens, S.P.G.; Rosman, C.; van Berge Henegouwen, M.I.; Klarenbeek, B.R.; Gisbertz, S.S. Extent and Boundaries of Lymph Node Stations During Minimally Invasive Esophagectomy: A Survey Among Dutch Esophageal Surgeons. Ann. Surg. Oncol. 2024, 31, 5683–5696. [Google Scholar] [CrossRef]
- Mine, S.; Tanaka, K.; Kawachi, H.; Shirakawa, Y.; Kitagawa, Y.; Toh, Y.; Yasuda, T.; Watanabe, M.; Kamei, T.; Oyama, T.; et al. Japanese Classification of Esophageal Cancer, 12th Edition: Part I. Esophagus 2024, 21, 179–215. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 2019, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Bona, D.; Calì, M.; Manara, M.; Rausa, E.; Bonitta, G.; Elshafei, M.; Markar, S.R.; Bonavina, L. Does Thoracic Duct Ligation at the Time of Esophagectomy Impact Long-Term Survival? An Individual Patient Data Meta-Analysis. J. Clin. Med. 2024, 13, 2849. [Google Scholar] [CrossRef] [PubMed]
- Schuring, N.; van Berge Henegouwen, M.I.; Gisbertz, S.S. History and evidence for state of the art of lymphadenectomy in esophageal cancer surgery. Dis. Esophagus 2024, 37, doad065. [Google Scholar] [CrossRef] [PubMed]
- Cavriani, G.; Domingos, H.V.; Oliveira-Filho, R.M.; Sudo-Hayashi, L.S.; Vargaftig, B.B.; de Lima, W.T. Lymphatic thoracic duct ligation modulates the serum levels of IL-1β and IL-10 after intestinal ischemia/reperfusion in rats with the involvement of tumor necrosis factor α and nitric oxide. Shock 2007, 27, 209–213. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H.; Cochrane GRADEing Methods Group (Formerly Applicability and Recommendations Methods Group); The Cochrane Statistical Methods Group. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 375–402. [Google Scholar] [CrossRef]

| Author (Year) | Country | Study Design | Time Frame | Total Included Patients (n) | Neoadjuvant Therapy (CT or CRT) | Histology Tumor SCC/AC/Other | Risk of Bias (ROBINS-I) |
|---|---|---|---|---|---|---|---|
| Oshikiri (2022) [26] | Japan | Retrospective + PSM | 2007–2012 | 170 | 170 | 170/0/0 | Moderate |
| Tanaka (2021) [27] | Japan | Retrospective + PSM | 2000–2017 | 1284 | 1032 | 1284/0/0 | Moderate |
| Fujisawa (2021) [28] | Japan | Retrospective + PSM | 2015–2019 | 174 | 98 | NR | Moderate |
| Kim (2022) [29] | Korea | Retrospective | 2013–2019 | 232 | 0 | 232/0/0 | Moderate |
| Li (2023) [30] | China | Retrospective | 2019–2020 | 200 | 68 | 200/0/0 | Moderate |
| Yoshida (2019) [22] | Japan | Retrospective | 2005–2018 | 537 | 233 | NR | Serious |
| Oshikiri (2019) [13] | Japan | Retrospective + PSM | 2010–2014 | 244 | 174 | 174 | Serious |
| Oshikiri (2023) [31] | Japan | Retrospective + PSM | 2007–2012 | 3274 | 1320 | 3214/62/0 | Moderate |
| Matsuda (2019) [32] | Japan | Retrospective | 2004–2016 | 235 | NR | 235/0/0 | Moderate |
| Matsuda (2016) [23] | Japan | Retrospective | 2004–2015 | 256 | 139 | 256/0/0 | Serious |
| Author (Year) | Country | Study Design | Time Frame | Total Included Patients (n) | Neoadjuvant therapy (CT or CRT) | Histology Tumor SCC/AC/Other | Risk of Bias (ROBINS-I or ROB-2 *) |
|---|---|---|---|---|---|---|---|
| Bao (2020) [33] | China | Retrospective | 2009–2018 | 600 | 48 | 587/13/0 | Moderate |
| Guo (2011) [34] | China | Retrospective | 2009–2010 | 135 | NR | NR | Moderate |
| Hou (2014) [24] | China | Retrospective | 1996–2008 | 1793 | 116 | 1534/187/83 | Serious |
| Chen (2019) [25] | China | Retrospective + PSM | 2003–2013 | 874 | 112 | 874/0/0 | Serious |
| Fei (2020) [35] | China | Retrospective + PSM | 2012–2014 | 609 | 9 | 231/106/31 | Moderate |
| Lai (2011) [36] | China | RCT | 2004–2009 | 653 | NR | 577/65/11 | Moderate * |
| Yang (2022) [37] | China | RCT | 2016–2021 | 69 | NR | 69/0/0 | Moderate* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijssen, D.J.; van der Aa, D.C.; Ali, M.; Kazemier, G.; Jamaludin, F.S.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Resection vs. Ligation vs. Preservation of the Thoracic Duct During Esophagectomy for Cancer: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 967. https://doi.org/10.3390/cancers17060967
Nijssen DJ, van der Aa DC, Ali M, Kazemier G, Jamaludin FS, Eshuis WJ, van Berge Henegouwen MI, Gisbertz SS. Resection vs. Ligation vs. Preservation of the Thoracic Duct During Esophagectomy for Cancer: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(6):967. https://doi.org/10.3390/cancers17060967
Chicago/Turabian StyleNijssen, David J., Dillen C. van der Aa, Mahsoem Ali, Geert Kazemier, Faridi S. Jamaludin, Wietse J. Eshuis, Mark I. van Berge Henegouwen, and Suzanne S. Gisbertz. 2025. "Resection vs. Ligation vs. Preservation of the Thoracic Duct During Esophagectomy for Cancer: A Systematic Review and Meta-Analysis" Cancers 17, no. 6: 967. https://doi.org/10.3390/cancers17060967
APA StyleNijssen, D. J., van der Aa, D. C., Ali, M., Kazemier, G., Jamaludin, F. S., Eshuis, W. J., van Berge Henegouwen, M. I., & Gisbertz, S. S. (2025). Resection vs. Ligation vs. Preservation of the Thoracic Duct During Esophagectomy for Cancer: A Systematic Review and Meta-Analysis. Cancers, 17(6), 967. https://doi.org/10.3390/cancers17060967







