Oncological Safety of Prepectoral Implant-Based Breast Reconstruction After Conservative Mastectomy: Insights from 842 Consecutive Breast Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Procedures
2.3. Follow-Up and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Oncological Staging
3.2. Surgical Technique
3.3. Neoadjuvant and Adjuvant Treatments
3.4. Surgical Complications
3.5. Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBBR | Implant-based breast reconstruction |
| ROME | Radiological and oncoplastic multidisciplinary evaluation |
| LRFS | Locoregional recurrence-free survival |
| DDFS | Distant disease-free survival |
| OS | Overall survival |
References
- Nava, M.B.; Catanuto, G.; Pennati, A.; Garganese, G.; Spano, A. Conservative mastectomies. Aesthetic Plast. Surg. 2009, 33, 681–686. [Google Scholar] [CrossRef] [PubMed]
- American Society of Plastic Surgeons. 2020 Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics?sub=2020+Plastic+Surgery+Statistics (accessed on 4 March 2025).
- Woo, A.; Harless, C.; Jacobson, S.R. Revisiting an Old Place: Single-Surgeon Experience on Post-Mastectomy Subcutaneous Implant-Based Breast Reconstruction. Breast J. 2017, 23, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Vidya, R.; Berna, G.; Sbitany, H.; Nahabedian, M.; Becker, H.; Reitsamer, R.; Rancati, A.; Macmillan, D.; Cawthorn, S. Prepectoral implant-based breast reconstruction: A joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019, 13, 927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scarabosio, A.; Contessi Negrini, F.; Pisano, G.; Beorchia, Y.; Castriotta, L.; De Francesco, F.; Riccio, M.; Parodi, P.C.; Zingaretti, N. Prepectoral Direct-To-Implant One-Stage Reconstruction With ADMs: Safety and Outcome in “Thin Patients”. Clin. Breast Cancer 2023, 23, e507–e514. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Bannier, M.; Bouteille, C.; Tallet, C.; Sabiani, L.; Charavil, A.; Bertrand, A.; Van Troy, A.; Buttarelli, M.; Teyssandier, C.; et al. Postoperative Outcomes of Pre-Pectoral Versus Sub-Pectoral Implant Immediate Breast Reconstruction. Cancers 2024, 16, 1129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chopra, S.; Rehnke, R.D.; Raghavan, V. Implant-based Prepectoral Breast Reconstruction: The Importance of Oncoplastic Plane, its Blood Supply and Assessment Methods. World J. Plast. Surg. 2021, 10, 108–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubenstein, R.N.; Kim, M.; Plotsker, E.L.; Chu, J.J.; Bell, T.; McGriff, D.V.; Allen, R., Jr.; Dayan, J.H.; Stern, C.S.; Coriddi, M.; et al. Early Complications in Prepectoral Tissue Expander-Based Breast Reconstruction. Ann. Surg. Oncol. 2024, 31, 2766–2776. [Google Scholar] [CrossRef] [PubMed]
- Scardina, L.; Di Leone, A.; Biondi, E.; Carnassale, B.; Sanchez, A.M.; D’Archi, S.; Franco, A.; Moschella, F.; Magno, S.; Terribile, D.; et al. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J. Pers. Med. 2022, 12, 1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocco, N.; Rispoli, C.; Moja, L.; Amato, B.; Iannone, L.; Testa, S.; Spano, A.; Catanuto, G.; Accurso, A.; Nava, M.B. Different types of implants for reconstructive breast surgery. Cochrane Database Syst. Rev. 2016, 2016, CD010895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casella, D.; Kaciulyte, J.; Lo Torto, F.; Mori, F.L.; Barellini, L.; Fausto, A.; Fanelli, B.; Greco, M.; Ribuffo, D.; Marcasciano, M. “To Pre or Not to Pre”: Introduction of a Prepectoral Breast Reconstruction Assessment Score to Help Surgeons Solving the Decision-Making Dilemma. Retrospective Results of a Multicenter Experience. Plast. Reconstr. Surg. 2021, 147, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Scardina, L.; Di Leone, A.; Terribile, D.A.; Sanchez, A.M.; Magno, S.; D’Archi, S.; Franco, A.; Mason, E.J.; Carnassale, B.; et al. Immediate Prosthetic Breast Reconstruction after Nipple-Sparing Mastectomy: Traditional Subpectoral Technique versus Direct-to-Implant Prepectoral Reconstruction without Acellular Dermal Matrix. J. Pers. Med. 2021, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, N.; Piana, M.; Battellino, L.; Galvano, F.; De Francesco, F.; Riccio, M.; Beorchia, Y.; Castriotta, L.; Parodi, P.C. Pre-pectoral Breast Reconstruction: Surgical and Patient-Reported Outcomes of Two-Stages vs Single-Stage Implant-Based Breast Reconstruction. Aesthetic Plast. Surg. 2023, 48, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Bernini, M.; Meattini, I.; Saieva, C.; Becherini, C.; Salvestrini, V.; Visani, L.; Stocchi, G.; Bellini, C.; Lorenzetti, V.; Sordi, S.; et al. Pre-pectoral breast reconstruction: Early and long-term safety evaluation of 146 unselected cases of the early pre-pectoral era of a single-institution, including cases with previous breast irradiation and post-mastectomy radiation therapy. Breast Cancer 2022, 29, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Morigi, C.; Bagnardi, V.; Corso, G.; Vicini, E.; Fontana, S.K.; Naninato, P.; Ratini, S.; Magnoni, F.; Toesca, A.; et al. Oncological outcomes of nipple-sparing mastectomy: A single-center experience of 1989 patients. Ann. Surg. Oncol. 2018, 25, 3849–3857. [Google Scholar] [CrossRef]
- Weber, W.P.; Haug, M.; Kurzeder, C.; Bjelic-Radisic, V.; Koller, R.; Reitsamer, R.; Fitzal, F.; Biazus, J.; Brenelli, F.; Urban, C.; et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res. Treat. 2018, 172, 523–527. [Google Scholar] [CrossRef]
- Fu, M.; Chen, Q.; Zeng, L.; Hong, T.; Zou, Q.; Yuan, Y.; Yi, W. Prognosis comparison between nipple-sparing mastectomy and total mastectomy in breast cancer: A case-control study after propensity score matching. Ann. Surg. Oncol. 2022, 29, 2221–2230. [Google Scholar] [CrossRef]
- Salibian, A.H.; Harness, J.K. Oncologic Safety of Staged Prepectoral Implant Reconstruction following Nipple-Sparing Mastectomy: A Mean 9-Year Follow-Up. Plast. Reconstr. Surg. 2022, 150, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Scardina, L.; Di Leone, A.; Sanchez, A.M.; D’Archi, S.; Biondi, E.; Franco, A.; Mason, E.J.; Magno, S.; Terribile, D.; Barone-Adesi, L.; et al. Nipple sparing mastectomy with prepectoral immediate prosthetic reconstruction without acellular dermal matrices: A single center experience. Minerva Surg. 2021, 76, 498–505. [Google Scholar] [CrossRef]
- Piccolo, P.P.; Venturi, M.; Mesbahi, A.N.; Nahabedian, M.Y. Current status prepectoral and subpectoral breast reconstruction in the USA. Gland. Surg. 2023, 12, 1794–1805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taj, S.; Chandavarkar, R.; Vidya, R. Current Global Trends in Prepectoral Breast Reconstruction. Medicina 2024, 60, 431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rancati, A.; Angrigiani, C.; Lamas, G.; Rancati, A.; Berrino, V.; Barbosa, K.; Dorr, J.; Irigo, M. Current status of prepectoral breast reconstruction in Argentina. Gland. Surg. 2024, 13, 1552–1560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caputo, G.G.; Zingaretti, N.; Kiprianidis, I.; Zanfisi, C.; Domenici, L.; Parodi, P.C.; Governa, M. Quality of Life and Early Functional Evaluation in Direct-to-Implant Breast Reconstruction After Mastectomy: A Comparative Study Between Prepectoral Versus Dual-Plane Reconstruction. Clin. Breast Cancer 2021, 21, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Johnson, L.; Sinai, P.; Mills, N.; White, P.; Holcombe, C.; Potter, S.; Pre-BRA Feasibility Study Steering Group. Patient-reported outcomes 3 and 18 months after mastectomy and immediate prepectoral implant-based breast reconstruction in the UK Pre-BRA prospective multicentre cohort study. Br. J. Surg. 2025, 112, znaf032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nolan, I.T.; Farajzadeh, M.M.; Bekisz, J.M.; Boyd, C.J.; Gibson, E.G.; Salibian, A.A. Prepectoral versus Subpectoral Breast Reconstruction after Nipple-sparing Mastectomy: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostapenko, E.; Nixdorf, L.; Devyatko, Y.; Exner, R.; Wimmer, K.; Fitzal, F. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann. Surg. Oncol. 2023, 30, 126–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franceschini, G.; Scardina, L.; Masetti, R. Precision surgery for breast cancer: Current trends and future perspectives. Updates Surg. 2023, 75, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Alessandri Bonetti, M.; Carbonaro, R.; Borelli, F.; Amendola, F.; Cottone, G.; Mazzocconi, L.; Mastroiacovo, A.; Zingaretti, N.; Parodi, P.C.; Vaienti, L. Outcomes in Hybrid Breast Reconstruction: A Systematic Review. Medicina 2022, 58, 1232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zingaretti, N.; Miotti, G.; Maronese, C.A.; Isola, M.; Guarneri, G.F.; Albanese, R.; De Francesco, F.; Riccio, M.; Cereser, L.; Zuiani, C.; et al. A Prospective Investigation of Predictive Parameters for Preoperative Volume Assessment in Breast Reconstruction. J. Clin. Med. 2021, 10, 5216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rancati, A.O.; Angrigiani, C.H.; Hammond, D.C.; Nava, M.B.; Gonzalez, E.G.; Dorr, J.C.; Gercovich, G.F.; Rocco, N.; Rostagno, R.L. Direct to Implant Reconstruction in Nipple Sparing Mastectomy: Patient Selection by Preoperative Digital Mammogram. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1369. [Google Scholar] [CrossRef]
- Gentile, D.; Sagona, A.; Spoto, R.; Franceschin, D.; Vaccari, S.; Vinci, V.; Biondi, E.; Scardina, L.; Tinterri, C. Salvage Mastectomy Is not the Treatment of Choice for Aggressive Subtypes of Ipsilateral Breast Cancer Recurrence: A Single-Institution Retrospective Study. Eur. J. Breast Health 2022, 18, 315–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bogdan, R.G.; Helgiu, A.; Cimpean, A.M.; Ichim, C.; Todor, S.B.; Iliescu-Glaja, M.; Bodea, I.C.; Crainiceanu, Z.P. Assessing Fat Grafting in Breast Surgery: A Narrative Review of Evaluation Techniques. J. Clin. Med. 2024, 13, 7209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barone Adesi, L.; Taraschi, F.; Macrì, G.; Scardina, L.; Di Leone, A.; Franceschini, G.; Salgarello, M. Fat Grafting and Prepectoral Prosthetic Reconstruction with Polyurethane-Covered Implants: Protective Role against Adjuvant Radiotherapy. J. Clin. Med. 2024, 13, 4982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristics of Overall Sample (N = 842) | Prepectoral (N = 648; 77.0%) | Subpectoral (N = 194; 23.0%) | Univariate Analysis | Multivariable Analysis |
|---|---|---|---|---|
| p-Value | p-Value; OR (95% CI) | |||
| Demographics | ||||
| Age (years) | <0.001 | |||
| - ≤50 | 414 (63.9%) | 161 (83.0%) | ||
| - >50 | 234 (33.1%) | 33 (17.0%) | <0.001; 0.418 (0.265–0.660) | |
| Surgery | 0.287 | |||
| - NSM | 583 (90.0%) | 178 (91.8%) | ||
| - SSM | 57 (8.8%) | 16 (8.2%) | 0.226; 0.347 (0.062–1.927) | |
| - SRM | 8 (1.2%) | 0 (0.0%) | >0.999; 0.00 (0.00-) | |
| Surgery | <0.001 | |||
| - Monolateral | 470 (72.5%) | 113 (58.3%) | ||
| - Bilateral | 178 (27.5%) | 81 (41.7%) | <0.001; 4.479 (2.746–7.306) | |
| Incision | <0.001 | |||
| - External quadrants | 555 (85.7%) | 161 (83.0%) | ||
| - Superior quadrants | 1 (0.2%) | 0 (0.0%) | >0.999; 0.00 (0.00-) | |
| - Inframammary fold | 17 (2.6%) | 2 (1.0%) | 0.187; 0.359 (0.078–1.642) | |
| - Periareolar | 65 (10.0%) | 18 (9.3%) | 0.134; 3.485 (0.681–17.834) | |
| - SRM | 8 (1.2%) | 0 (0.0%) | >0.999; 0.00 (0.00-) | |
| - Transaxillary | 2 (0.3%) | 13 (6.7%) | <0.001; 15.536 (3.310–72.915) | |
| Contralateral surgery | <0.001 | |||
| - None | 365 (56.3%) | 38 (19.6%) | ||
| - Additive implant | 9 (1.4%) | 19 (9.7%) | <0.001; 4.306 (2.784–6.660) | |
| - Reductive mastoplasty | 96 (14.8%) | 56 (28.9%) | <0.001; 21.969 (8.918–54.118) | |
| - Bilateral mastectomy | 178 (27.5%) | 81 (41.8%) | <0.001; 5.206 (3.159–8.580) | |
| Tumor sub-type | 0.747 | |||
| - CDI | 475 (73.3%) | 143 (73.7%) | ||
| - CLI | 76 (11.7%) | 26 (13.4%) | 0.158; 1.496 (0.855–2.617) | |
| - CDIS | 83 (12.8%) | 20 (10.3%) | 0.041; 0.493 (0.250–0.972) | |
| - Others | 14 (2.2%) | 5 (2.6%) | 0.827; 0.879 (0.275–2.803) | |
| T | 0.495 | |||
| - 0 | 79 (12.2%) | 29 (14.9%) | ||
| - is | 77 (11.9%) | 19 (9.8%) | ||
| - 1mi | 32 (4.9%) | 8 (4.1%) | ||
| - 1a | 51 (7.8%) | 13 (6.7%) | ||
| - 1b | 69 (10.7%) | 25 (12.4%) | ||
| - 1c | 153 (23.6%) | 57 (29.4%) | ||
| - 2 | 165 (25.5%) | 40 (20.6%) | ||
| - 3 | 21 (3.2%) | 4 (2.1%) | ||
| - 4 | 1 (0.2%) | 0 (0.0%) | ||
| N | 0.433 | |||
| - 0 | 410 (63.2%) | 114 (58.8%) | ||
| - ITC | 13 (2.0%) | 4 (2.0%) | ||
| - 1mi | 34 (5.3%) | 8 (4.2%) | ||
| - 1 | 139 (21.5%) | 52 (26.8%) | ||
| - 2 | 39 (6.0%) | 9 (4.6%) | ||
| - 3 | 13 (2.0%) | 7 (3.6%) | ||
| STAGE | 0.990 | |||
| - I | 371 (57.3%) | 112 (57.7%) | ||
| - II | 224 (34.6%) | 66 (34.0%) | 0.774; 1.067 (0.687–1.656) | |
| - III | 53 (8.1%) | 16 (8.3%) | 0.317; 1.464 (0.694–3.091) | |
| NAD | 0.669 | |||
| - Yes | 223 (34.4%) | 70 (36.1%) | 0.302; 0.793 (0.511–1.231) | |
| - No | 425 (65–6%) | 124 (63.9%) | ||
| RT | 0.192 | |||
| - Yes | 181 (27.9%) | 45 (23.2%) | 0.481; 0.836 (0.508–1.377) | |
| - No | 467 (72.1%) | 149 (76.8%) | ||
| Multifocality | 0.011 | |||
| - Yes | 216 (33.3%) | 46 (23.7%) | 0.046; 0.639 (0.411–0.993) | |
| - No | 432 (66.7%) | 148 (76.3%) | ||
| VP | 0.462 | |||
| - NO | 539 (83.2%) | 160 (82.5%) | ||
| - BRCA1 | 51 (7.9%) | 21 (10.8%) | 0.431; 0.773 (0.408–1.465) | |
| - BRCA2 | 32 (4.9%) | 8 (4.1%) | 0.275; 0.618 (0.260–1.466) | |
| - Others | 26 (4.0%) | 5 (2.6%) | 0.070; 0.372 (0.128–1.082) |
| Any Breast-Related Complication | All pz | Prepec | Subpec | p-Value |
|---|---|---|---|---|
| Minor complications (NAC necrosis, wound dehiscence) | 9 (1.1%) | 8 (1.2%) | 1 (0.5%) | 0.393 |
| Major complications (Skin flap necrosis, hematoma, infection) | 21 (2.5%) | 16 (2.5%) | 5 (2.6%) | 0.932 |
| Overall | Prepec | Subpec | |
|---|---|---|---|
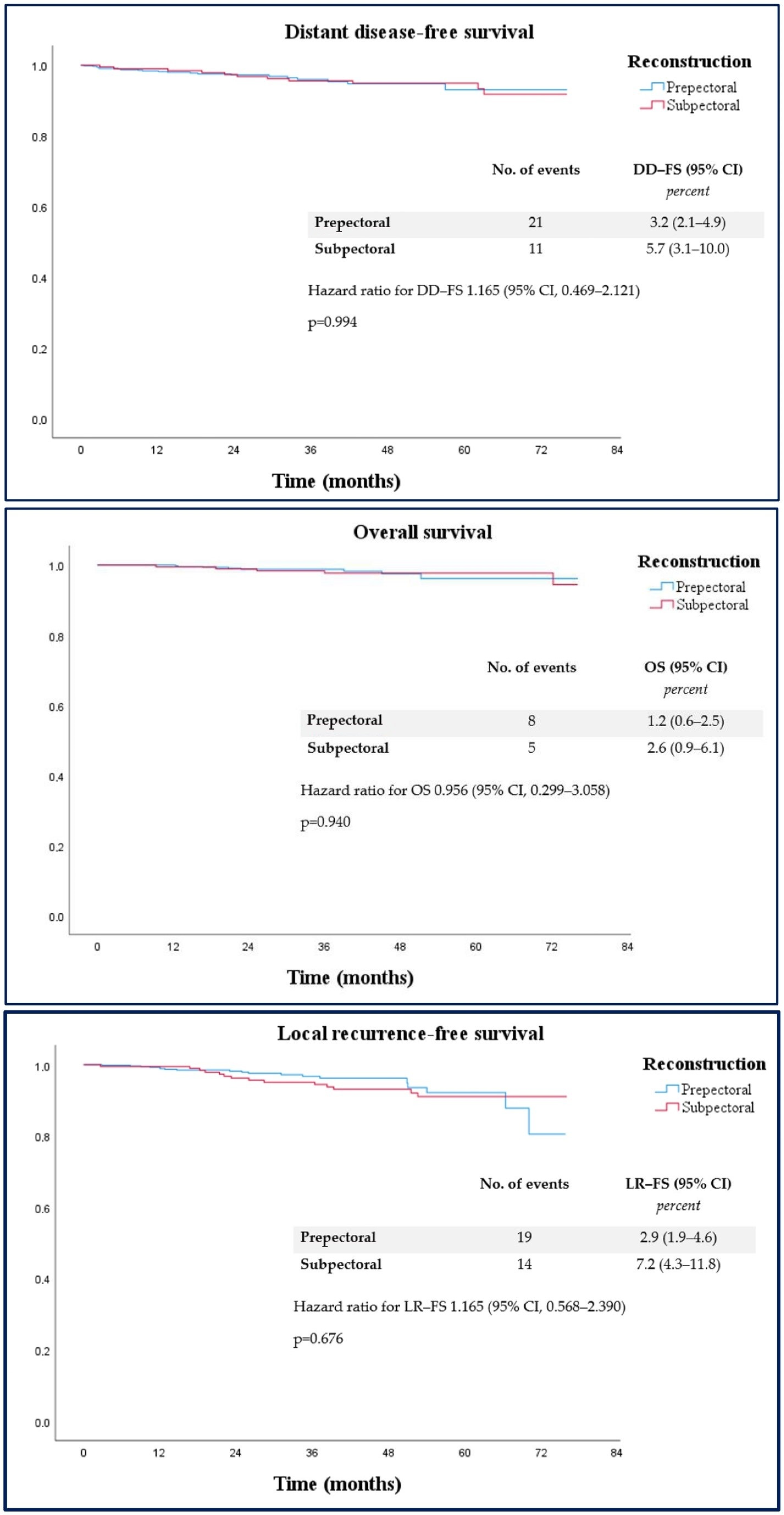
| LRFS rate | 33 (3.9%) | 19 (2.9%) | 14 (7.2%) |
| DDFS rate | 32 (3.8%) | 21 (3.2%) | 11 (5.7%) |
| OS rate | 13 (1.5%) | 8 (1.2%) | 5 (2.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scardina, L.; Di Leone, A.; Sanchez, A.M.; Accetta, C.; Barone Adesi, L.; Biondi, E.; Carnassale, B.; D’Archi, S.; De Lauretis, F.; Di Guglielmo, E.; et al. Oncological Safety of Prepectoral Implant-Based Breast Reconstruction After Conservative Mastectomy: Insights from 842 Consecutive Breast Cancer Patients. Cancers 2025, 17, 925. https://doi.org/10.3390/cancers17060925
Scardina L, Di Leone A, Sanchez AM, Accetta C, Barone Adesi L, Biondi E, Carnassale B, D’Archi S, De Lauretis F, Di Guglielmo E, et al. Oncological Safety of Prepectoral Implant-Based Breast Reconstruction After Conservative Mastectomy: Insights from 842 Consecutive Breast Cancer Patients. Cancers. 2025; 17(6):925. https://doi.org/10.3390/cancers17060925
Chicago/Turabian StyleScardina, Lorenzo, Alba Di Leone, Alejandro Martin Sanchez, Cristina Accetta, Liliana Barone Adesi, Ersilia Biondi, Beatrice Carnassale, Sabatino D’Archi, Flavia De Lauretis, Enrico Di Guglielmo, and et al. 2025. "Oncological Safety of Prepectoral Implant-Based Breast Reconstruction After Conservative Mastectomy: Insights from 842 Consecutive Breast Cancer Patients" Cancers 17, no. 6: 925. https://doi.org/10.3390/cancers17060925
APA StyleScardina, L., Di Leone, A., Sanchez, A. M., Accetta, C., Barone Adesi, L., Biondi, E., Carnassale, B., D’Archi, S., De Lauretis, F., Di Guglielmo, E., Franco, A., Magno, S., Moschella, F., Natale, M., Salgarello, M., Savia, E., Silenzi, M., Visconti, G., Masetti, R., & Franceschini, G. (2025). Oncological Safety of Prepectoral Implant-Based Breast Reconstruction After Conservative Mastectomy: Insights from 842 Consecutive Breast Cancer Patients. Cancers, 17(6), 925. https://doi.org/10.3390/cancers17060925

















