Assessing the Effect of Cytoreduction on Solitary, Resectable Lesions in Primary Central Nervous System Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
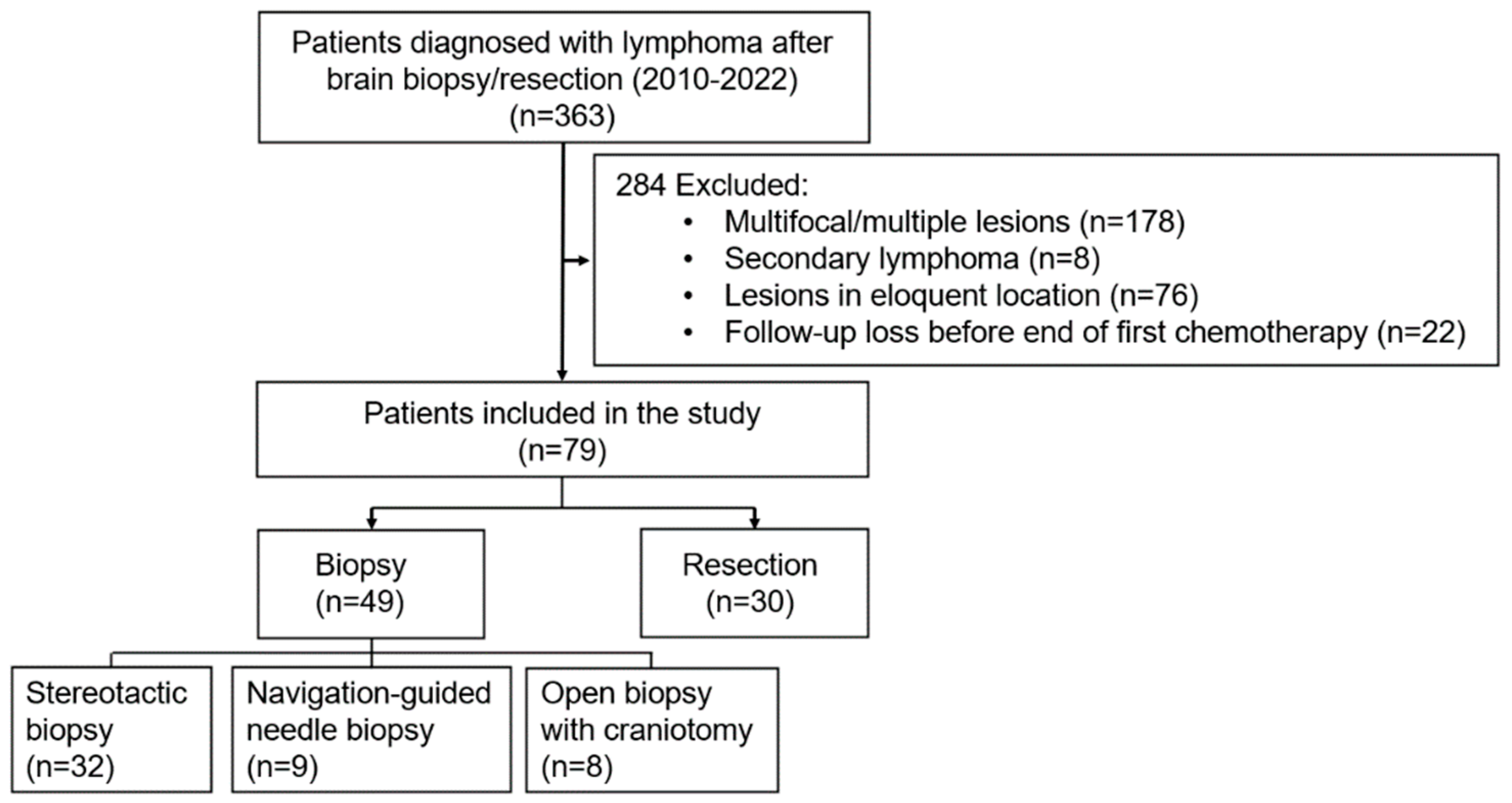
2.1. Patient Selection and Data Collection
2.2. Therapeutic Modality
2.3. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Surgical Outcomes and Complications
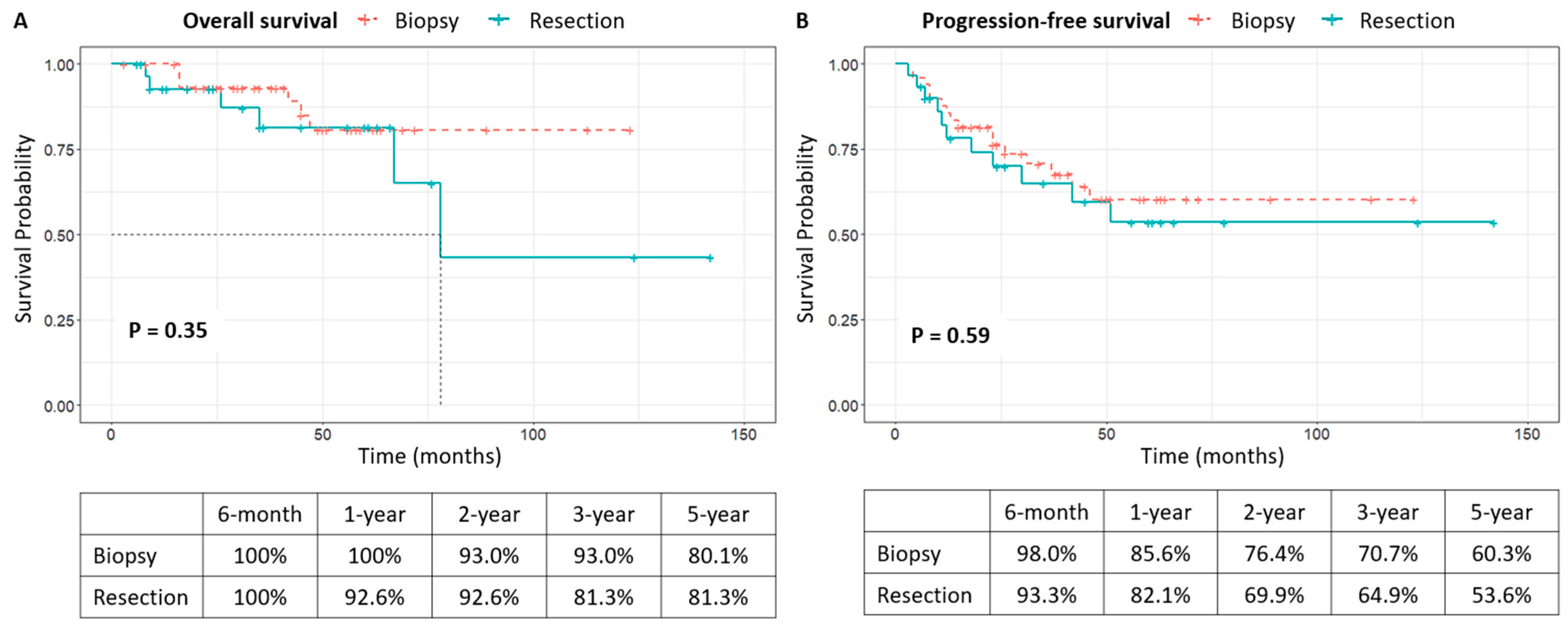
3.3. Clinical Course After Diagnosis and Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| CR | Complete response |
| CTx | Chemotherapy |
| GTR | Gross total resection |
| HD-MTX | High-dose methotrexate |
| MRI | Magnetic resonance imaging |
| MPV | Methotrexate, procarbazine, vincristine |
| OS | Overall survival |
| PCNSL | Primary central nervous system lymphoma |
| PD | Progressive disease |
| PFS | Progression-free survival |
| R-MPV | Rituximab, methotrexate, procarbazine, vincristine |
| RTx | Radiotherapy |
| STR | Subtotal resection |
| TTC | Time to chemotherapy |
| TTR | Time to remission |
| WBRT | Whole-brain radiotherapy |
References
- Schlegel, U. Primary CNS lymphoma. Ther. Adv. Neurol. Disord. 2009, 2, 93–104. [Google Scholar] [CrossRef]
- Han, C.H.; Batchelor, T.T. Diagnosis and management of primary central nervous system lymphoma. Cancer 2017, 123, 4314–4324. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Harlay, V.; Chinot, O.; Tabouret, E. Primary central nervous system lymphoma (PCNSL) in older patients. Curr. Opin. Oncol. 2023, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Calimeri, T.; Steffanoni, S.; Gagliardi, F.; Chiara, A.; Ferreri, A.J.M. How we treat primary central nervous system lymphoma. ESMO Open 2021, 6, 100213. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Deckert, M.; Ferreri, A.J.M.; Furtner, J.; Gallego Perez-Larraya, J.; Henriksson, R.; Hottinger, A.F.; Kasenda, B.; Lefranc, F.; Lossos, A.; et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol. 2023, 25, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Du, H.; Ye, X.; Zhang, L.; Xiao, H. High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: Meta-analysis of clinical trials. Sci. Rep. 2021, 11, 2125. [Google Scholar] [CrossRef]
- Li, Q.; Ma, J.; Ma, Y.; Lin, Z.; Kang, H.; Chen, B. Improvement of outcomes of an escalated high-dose methotrexate-based regimen for patients with newly diagnosed primary central nervous system lymphoma: A real-world cohort study. Cancer Manag. Res. 2021, 13, 6115–6122. [Google Scholar] [CrossRef]
- Niparuck, P.; Boonsakan, P.; Sutthippingkiat, T.; Pukiat, S.; Chantrathammachart, P.; Phusanti, S.; Boonyawat, K.; Puavilai, T.; Angchaisuksiri, P.; Ungkanont, A.; et al. Treatment outcome and prognostic factors in PCNSL. Diagn. Pathol. 2019, 14, 56. [Google Scholar] [CrossRef]
- Bobillo, S.; Khwaja, J.; Ferreri, A.J.M.; Cwynarski, K. Prevention and management of secondary central nervous system lymphoma. Haematologica 2022, 108, 673–689. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Blay, J.Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef]
- Abrey, L.E.; Batchelor, T.T.; Ferreri, A.J.; Gospodarowicz, M.; Pulczynski, E.J.; Zucca, E.; Smith, J.R.; Korfel, A.; Soussain, C.; DeAngelis, L.M.; et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J. Clin. Oncol. 2005, 23, 5034–5043. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; Rosee, P.L.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; Celico, C.; Falautano, M.; Nonis, A.; La Rosee, P.; Binder, M.; et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia 2022, 36, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Rubenstein, J.L.; DeAngelis, L.M.; Ferreri, A.J.M.; Batchelor, T.T. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019, 21, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Soussain, C.; Ghesquieres, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef]
- Bellinzona, M.; Roser, F.; Ostertag, H.; Gaab, R.M.; Saini, M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: A series of 33 cases. Eur. J. Surg. Oncol. 2005, 31, 100–105. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, H.; Wang, J.; Wang, G.; Ma, X.; Zhao, K.; Wang, J.; Shu, K. Clinical Features, Diagnosis, and Treatment of Primary Intraventricular Lymphoma: Insights From a Monocentric Case Series. Front. Neurol. 2022, 13, 920505. [Google Scholar] [CrossRef]
- Stifano, V.; Della Pepa, G.M.; Offi, M.; Montano, N.; Carcagni, A.; Pallini, R.; Lauretti, L.; Olivi, A.; D’Alessandris, Q.G. Resection versus biopsy for management of primary central nervous system lymphoma: A meta-analysis. Neurosurg. Rev. 2023, 46, 37. [Google Scholar] [CrossRef]
- van der Meulen, M.; Postma, A.A.; Smits, M.; Bakunina, K.; Minnema, M.C.; Seute, T.; Cull, G.; Enting, R.H.; van der Poel, M.; Stevens, W.B.C.; et al. Extent of radiological response does not reflect survival in primary central nervous system lymphoma. Neurooncol Adv. 2021, 3, vdab007. [Google Scholar] [CrossRef]
- Cloney, M.B.; Sonabend, A.M.; Yun, J.; Yang, J.; Iwamoto, F.; Singh, S.; Bhagat, G.; Canoll, P.; Zanazzi, G.; Bruce, J.N.; et al. The safety of resection for primary central nervous system lymphoma: A single institution retrospective analysis. J. Neurooncol 2017, 132, 189–197. [Google Scholar] [CrossRef]
- Yun, J.; Yang, J.; Cloney, M.; Mehta, A.; Singh, S.; Iwamoto, F.M.; Neugut, A.I.; Sonabend, A.M. Assessing the Safety of Craniotomy for Resection of Primary Central Nervous System Lymphoma: A Nationwide Inpatient Sample Analysis. Front. Neurol. 2017, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Labak, C.M.; Holdhoff, M.; Bettegowda, C.; Gallia, G.L.; Lim, M.; Weingart, J.D.; Mukherjee, D. Surgical Resection for Primary Central Nervous System Lymphoma: A Systematic Review. World Neurosurg. 2019, 126, e1436–e1448. [Google Scholar] [CrossRef]
- Rae, A.I.; Mehta, A.; Cloney, M.; Kinslow, C.J.; Wang, T.J.C.; Bhagat, G.; Canoll, P.D.; Zanazzi, G.J.; Sisti, M.B.; Sheth, S.A.; et al. Craniotomy and Survival for Primary Central Nervous System Lymphoma. Neurosurgery 2019, 84, 935–944. [Google Scholar] [CrossRef]
- Schellekes, N.; Barbotti, A.; Abramov, Y.; Sitt, R.; Di Meco, F.; Ram, Z.; Grossman, R. Resection of primary central nervous system lymphoma: Impact of patient selection on overall survival. J. Neurosurg. 2021, 135, 1016–1025. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Liu, W.; Hu, F.; Zhao, K.; Jiang, W.; Lei, T.; Shu, K. The role of surgical resection in primary central nervous system lymphoma: A single-center retrospective analysis of 70 patients. BMC Neurol. 2021, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Chojak, R.; Kozba-Gosztyla, M.; Polanska, K.; Rojek, M.; Chojko, A.; Bogacz, R.; Skorupa, N.; Wieclaw, J.; Czapiga, B. Surgical resection versus biopsy in the treatment of primary central nervous system lymphoma: A systematic review and meta-analysis. J. Neurooncol. 2022, 160, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Antoniou, E.; Kwan, E.; Gregory, G.; Lai, L.T. Cytoreductive Surgery for Primary Central Nervous System Lymphoma: Is it time to consider extent of resection? J. Clin. Neurosci. 2022, 106, 110–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.W.; Shu, H.S. Outcome of Primary Central Nervous System Lymphoma Treated with Combined Surgical Resection and High- Dose Methotrexate Chemotherapy: A Single-Institution Retrospective Study. Turk. Neurosurg. 2022, 32, 1–5. [Google Scholar] [CrossRef]
- Ouyang, T.; Wang, L.; Zhang, N.; Zhang, Z.; Xiong, Y.; Li, M.; Hong, T. Clinical Characteristics, Surgical Outcomes, and Prognostic Factors of Intracranial Primary Central Nervous System Lymphoma. World Neurosurg. 2020, 139, e508–e516. [Google Scholar] [CrossRef]
- Weller, M.; Martus, P.; Roth, P.; Thiel, E.; Korfel, A. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro-Oncology 2012, 14, 1481–1484. [Google Scholar] [CrossRef]

| Characteristics | Biopsy (N = 49) | Resection (N = 30) | p-Value |
|---|---|---|---|
| Sex, n (%) | 0.087 | ||
| Male | 29 (59.2) | 11 (36.7) | |
| Female | 20 (40.8) | 19 (63.3) | |
| Age, years, n (%) | 0.586 | ||
| ≥60 | 24 (49.0) | 12 (40.0) | |
| <60 | 25 (51.0) | 18 (60.0) | |
| Comorbidities, n (%) | |||
| Hypertension | 18 (36.7) | 9 (30.0) | 0.713 |
| Diabetes | 9 (18.4) | 4 (13.3) | 0.785 |
| Cardiovascular disease | 5 (10.2) | 0 (0.0) | 0.183 |
| Others | 5 (10.2) | 2 (6.7) | 0.897 |
| IELSG score, n (%) | 0.491 | ||
| 0–1 | 15 (30.6) | 13 (43.3) | |
| 2–3 | 31 (63.3) | 15 (50.0) | |
| 4–5 | 3 (6.1) | 2 (6.7) | |
| Location, n (%) | 0.188 | ||
| Frontal | 13 (26.5) | 14 (46.7) | |
| Temporal | 7 (14.3) | 1 (3.3) | |
| Parietal | 10 (20.4) | 7 (23.3) | |
| Occipital | 5 (10.2) | 4 (13.3) | |
| Cerebellum | 11 (22.4) | 2 (6.7) | |
| Periventricular or suprasellar | 3 (6.1) | 2 (6.7) | |
| Tumor diameter, cm, (range) | 3.5 (1.1–6.7) | 3.6 (1.3–6.6) | 0.754 |
| Peritumoral edema, n (%) | 0.491 | ||
| Minimal | 1 (2.0) | 0 (0.0) | |
| Moderate | 6 (12.2) | 6 (20.0) | |
| Severe | 42 (85.7) | 24 (80.0) | |
| Midline shifting, n (%) | 0.048 * | ||
| No | 43 (87.8) | 20 (66.7) | |
| Yes | 6 (12.2) | 10 (33.3) | |
| Preoperative steroid use | <0.001 * | ||
| No | 45 (91.8) | 10 (33.3) | |
| Yes | 4 (8.2) | 20 (66.7) | |
| Induction CTx | 0.437 | ||
| MTX alone | 16 (32.7) | 13 (43.3) | |
| MTX + Other chemotherapeutic agents | 30 (61.2) | 14 (46.7) | |
| Others | 3 (6.1) | 3 (10.0) | |
| Consolidation CTx | 1.000 | ||
| None | 19 (38.8) | 12 (40.0) | |
| Yes | 30 (61.2) | 18 (60.0) | |
| Salvage RTx | 1.000 | ||
| None | 45 (91.8) | 28 (93.3) | |
| Yes | 4 (8.2) | 2 (6.7) | |
| Salvage WBRT | 1.000 | ||
| None | 45 (91.8) | 27 (90.0) | |
| Yes | 4 (8.2) | 3 (10.0) |
| Characteristics | Biopsy (N = 49) | Resection (N = 30) | p-Value |
|---|---|---|---|
| KPS (Preop) | 1.000 | ||
| ≥80 | 37 (75.5) | 23 (76.7) | |
| <80 | 12 (24.5) | 7 (23.3) | |
| KPS (Postop, 1 month) | 0.503 | ||
| ≥80 | 42 (85.7) | 28 (93.3) | |
| <80 | 7 (14.3) | 2 (6.7) | |
| Initial symptoms, n (%) | |||
| Cognition decline | 10 (20.4) | 7 (23.3) | 0.980 |
| Headache | 17 (34.7) | 6 (20.0) | 0.254 |
| Nausea/vomiting | 5 (10.2) | 3 (10.0) | 1.000 |
| Dysarthria | 10 (20.8) | 5 (16.7) | 0.874 |
| Hemiparesis | 9 (18.4) | 11 (36.7) | 0.121 |
| Dizziness | 9 (18.4) | 5 (16.7) | 1.000 |
| Visual disturbance | 7 (14.3) | 2 (6.7) | 0.503 |
| Others | 3 (6.1) | 0 (0) | 0.438 |
| Symptom improvement (Postop, 3 days) | <0.001 * | ||
| No change | 30 (61.2) | 6 (20.0) | |
| Improved | 10 (20.4) | 24 (80.0) | |
| Deteriorated | 9 (18.4) | 0 (0) | |
| Postop complications | 0.496 | ||
| Any complication | 5 (10.2) | 1 (3.3) | |
| Surgical site bleeding | 2 (4.1) | 0 (0) | |
| Delayed wound healing | 1 (2.0) | 0 (0) | |
| Systemic complication | 2 (4.1) | 1 (3.3) |
| Characteristics | Total (N = 79) | Biopsy (N = 49) | Resection (N = 30) | p-Value |
|---|---|---|---|---|
| Follow-up, months | 0.904 | |||
| mean (range) | 41.9 (3–142) | 41.5 (3–123) | 42.5 (6–142) | |
| Death, n (%) | 12 (15.2) | 6 (12.0) | 6 (20.0) | 0.542 |
| Recurrence, n (%) | 27 (34.1) | 16 (32.7) | 11 (36.7) | 0.904 |
| TTC, days | 0.189 | |||
| Mean (range) | 14.7 (1–47) | 13.5 (1–47) | 16.7 (1–43) | |
| TTR, months | 0.018 * | |||
| Mean (range) | 4.4 (1–20) | 4.9 (2–20) | 3.5 (1–8) | |
| Response to first induction CTx | 0.001 * | |||
| CR | 44 (55.7) | 22 (44.9) | 22 (73.3) | |
| CRu/PR | 27 (34.2) | 24 (49.0) | 3 (10.0) | |
| PD | 8 (10.1) | 3 (6.1) | 5 (16.7) |
| No. of Deaths/Total No. of Patients (%) | Univariate | Multivariate | No. of Recurrences /Total No. of Patients (%) | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At 5 Years | Log-Rank | HR | 95% CI | p Value | At 5 Years | Log-Rank | HR | 95% CI | p Value | |||
| Overall | 12/79 (15.2) | 27/79 (34.2) | ||||||||||
| Sex | 0.567 | 0.144 | ||||||||||
| Female | 6/40 (15.0) | 83.5 ± 6.9 | 11/40 (27.5) | 67.2 ± 8.4 | ||||||||
| Male | 6/39 (15.4) | 78.4 ± 9.5 | 16/39 (41.0) | 46.5 ± 9.9 | 1.458 | 0.658–3.227 | 0.353 | |||||
| Age (years) | 0.005 * | 0.007 * | ||||||||||
| <65 | 4/43 (9.3) | 92.7 ± 4.1 | 11/43 (25.6) | 70.5 ± 7.7 | ||||||||
| ≥65 | 8/36 (22.2) | 46.4 ± 16.0 | 2.076 | 0.421–10.228 | 0.369 | 16/36 (44.4) | 34.6 ± 11.6 | 1.889 | 0.732–4.870 | 0.188 | ||
| Tumor size (mm) | 0.913 | 0.578 | ||||||||||
| <30 | 5/31 (16.1) | 83.3 ± 8.0 | 12/31 (38.7) | 58.5 ± 9.3 | ||||||||
| ≥30 | 7/48 (14.6) | 79.7 ± 7.7 | 15/48 (31.3) | 56.8 ± 9.0 | ||||||||
| IELSG score | 0.479 | 0.904 | ||||||||||
| 0–1 | 3/29 (10.3) | 83.8 ± 8.8 | 9/29 (31.0) | 64.8 ± 9.8 | ||||||||
| 2–3 | 9/45 (20.0) | 78.4 ± 7.6 | 17/45 (37.8) | 53.4 ± 8.7 | ||||||||
| 4–5 | 0/5 (0.0) | 0.0 | 1/5 (20.0) | 66.7 ± 27.2 | ||||||||
| Surgery | 0.351 | 0.586 | ||||||||||
| Biopsy | 6/49 (12.2) | 80.7 ± 7.4 | 16/49 (32.7) | 60.3 ± 8.2 | ||||||||
| Resection | 6/30 (20.0) | 81.3 ± 8.7 | 1.900 | 0.585–6.175 | 0.286 | 11/30 (36.7) | 53.6 ±10.8 | |||||
| Induction CTx | 0.151 | 0.015 * | ||||||||||
| MTX based CTx | 10/73 (13.7) | 83.7 ± 5.5 | 22/73 (30.1) | 63.4 ± 6.5 | ||||||||
| Others | 2/6 (33.3) | 55.6 ± 24.8 | 2.503 | 0.495–12.650 | 0.267 | 5/6 (83.3) | 22.2 ± 19.2 | 2.172 | 0.782–6.034 | 0.137 | ||
| Consolidation CTx | 0.001 * | 0.002 * | ||||||||||
| None | 13/22 (59.1) | 52.2 ± 13.7 | 16/31 (51.6) | 29.6 ± 10.5 | ||||||||
| Yes | 5/27 (18.5) | 95.0 ± 3.5 | 0.237 | 0.044–1.289 | 0.096 | 11/48 (22.9) | 73.0 ± 7.3 | 0.509 | 0.198–1.306 | 0.160 | ||
| Salvage RTx | 0.372 | |||||||||||
| None | 10/73 (13.7) | 80.5 ± 6.2 | ||||||||||
| Yes | 2/6 (33.3) | 83.3 ± 15.2 | 3.499 | 0.638–19.183 | 0.149 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Byeon, Y.; Kim, G.J.; Jeon, J.; Hong, C.K.; Kim, J.H.; Kim, Y.-H.; Cho, Y.H.; Hong, S.H.; Chong, S.J.; et al. Assessing the Effect of Cytoreduction on Solitary, Resectable Lesions in Primary Central Nervous System Lymphoma. Cancers 2025, 17, 917. https://doi.org/10.3390/cancers17060917
Lee C, Byeon Y, Kim GJ, Jeon J, Hong CK, Kim JH, Kim Y-H, Cho YH, Hong SH, Chong SJ, et al. Assessing the Effect of Cytoreduction on Solitary, Resectable Lesions in Primary Central Nervous System Lymphoma. Cancers. 2025; 17(6):917. https://doi.org/10.3390/cancers17060917
Chicago/Turabian StyleLee, Chaejin, Yukyeng Byeon, Gung Ju Kim, Juhee Jeon, Chang Ki Hong, Jeong Hoon Kim, Young-Hoon Kim, Young Hyun Cho, Seok Ho Hong, Sang Joon Chong, and et al. 2025. "Assessing the Effect of Cytoreduction on Solitary, Resectable Lesions in Primary Central Nervous System Lymphoma" Cancers 17, no. 6: 917. https://doi.org/10.3390/cancers17060917
APA StyleLee, C., Byeon, Y., Kim, G. J., Jeon, J., Hong, C. K., Kim, J. H., Kim, Y.-H., Cho, Y. H., Hong, S. H., Chong, S. J., & Song, S. W. (2025). Assessing the Effect of Cytoreduction on Solitary, Resectable Lesions in Primary Central Nervous System Lymphoma. Cancers, 17(6), 917. https://doi.org/10.3390/cancers17060917






