Impact of Surgical Resection After Induction Gemcitabine Plus S-1-Based Chemoradiotherapy in Patients with Locally Advanced Pancreatic Ductal Adenocarcinoma: A Focus on UR-LA Cases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
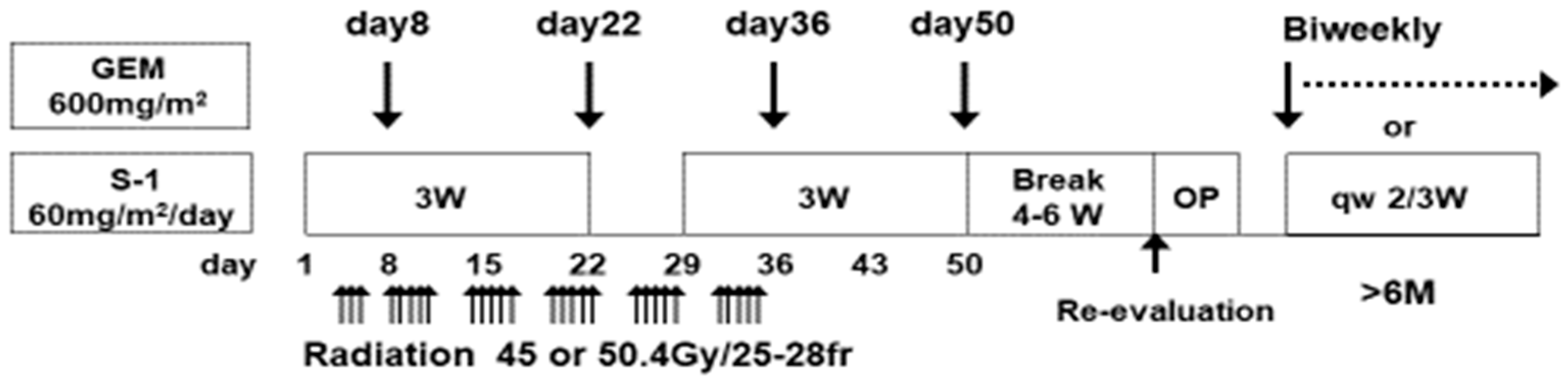
2.1. Study Design
2.2. Treatment Protocol
2.3. Indications of Surgical Resection and Post-Operative Complications
2.3.1. Indications and Contraindications of Surgical Resection
2.3.2. Surgical Procedure
2.3.3. Post-Operative Complications
2.4. Adjuvant Chemotherapy and Post-Operative Follow-Up
2.5. Pathological Evaluation of Histological Response to Chemoradiotherapy
2.6. Statistical Analyses
3. Results
3.1. Adverse Events During Induction GS-CRT
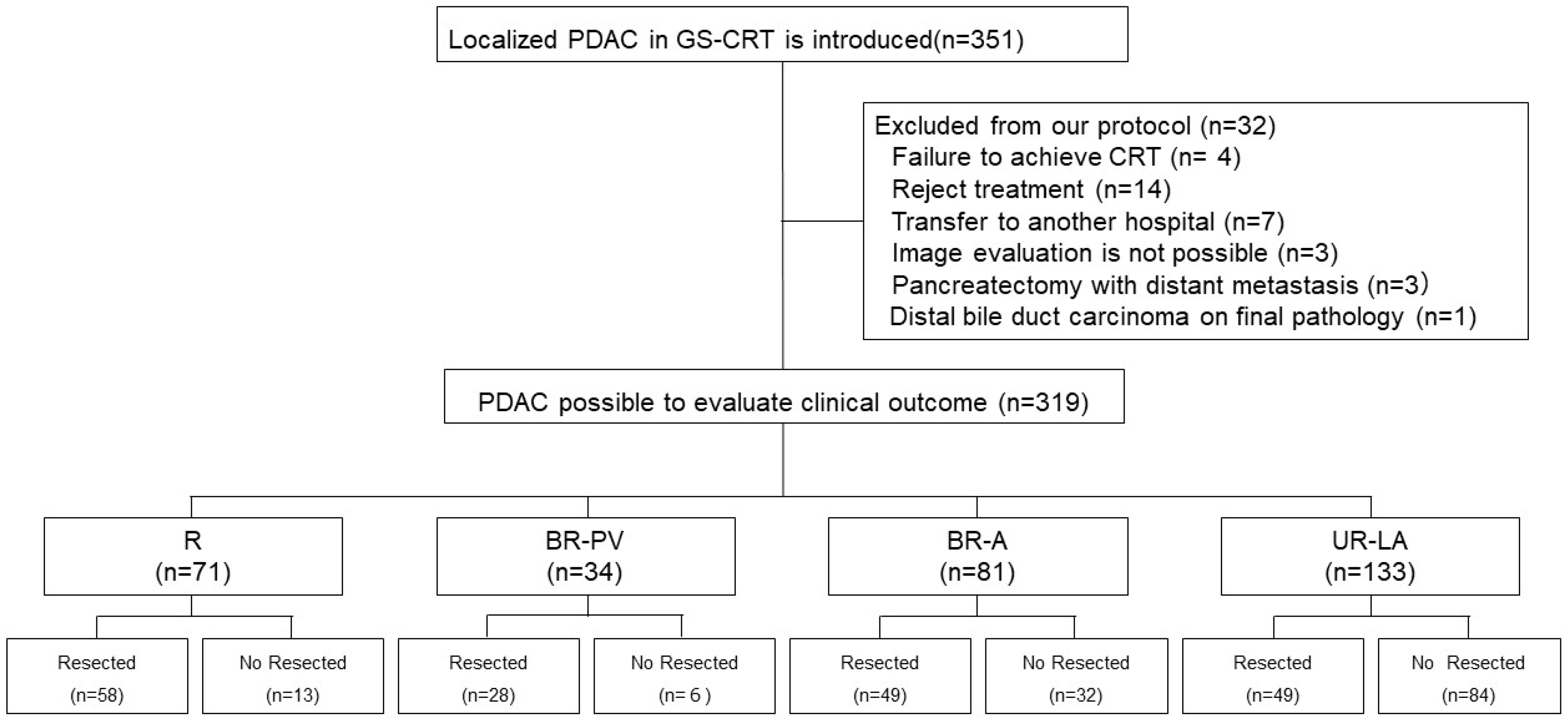
3.2. Characteristics of Patients with Localized Pancreatic Ductal Adenocarcinoma
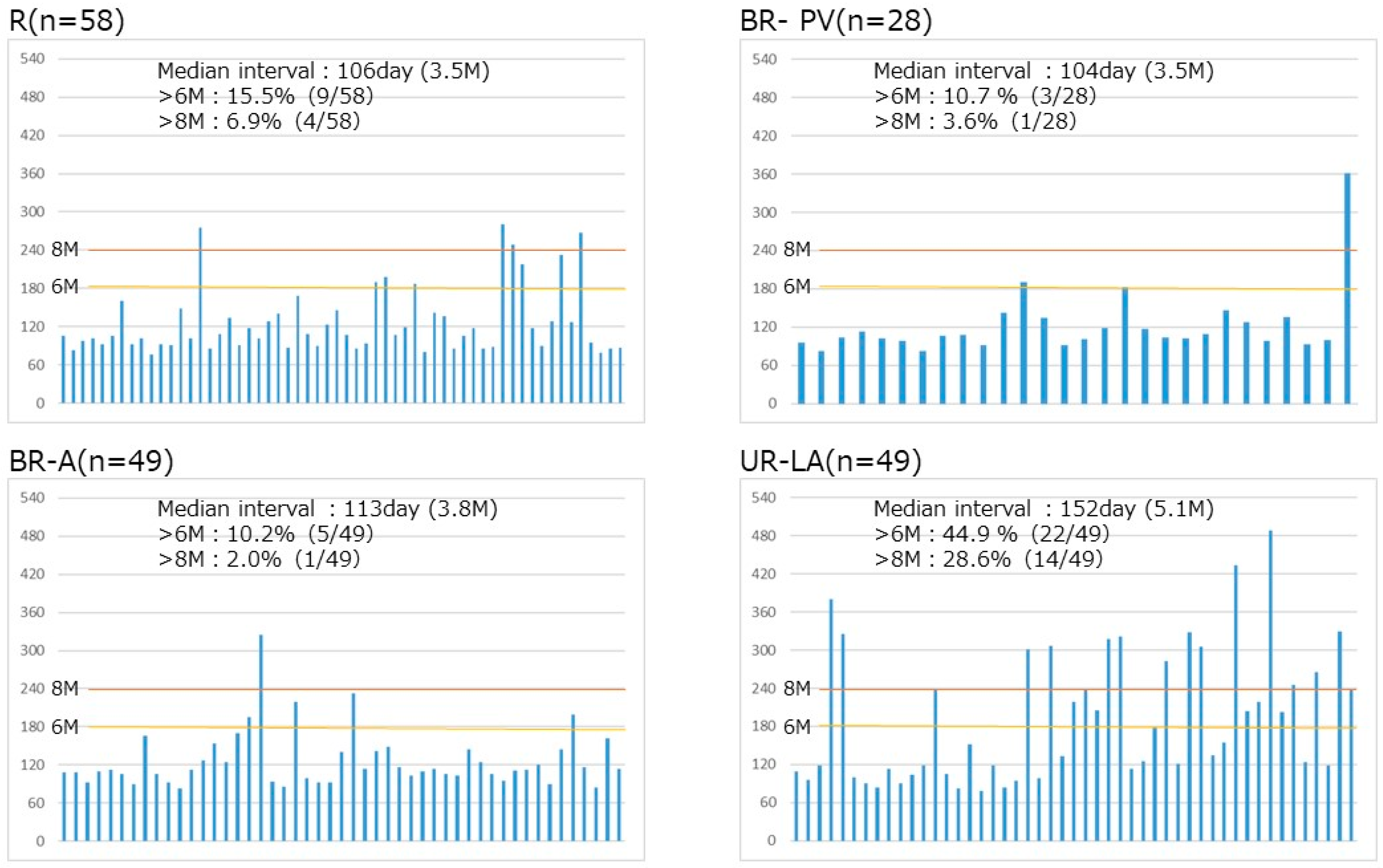
3.3. Timing of Surgical Resection Based on Tumor Resectability Classification
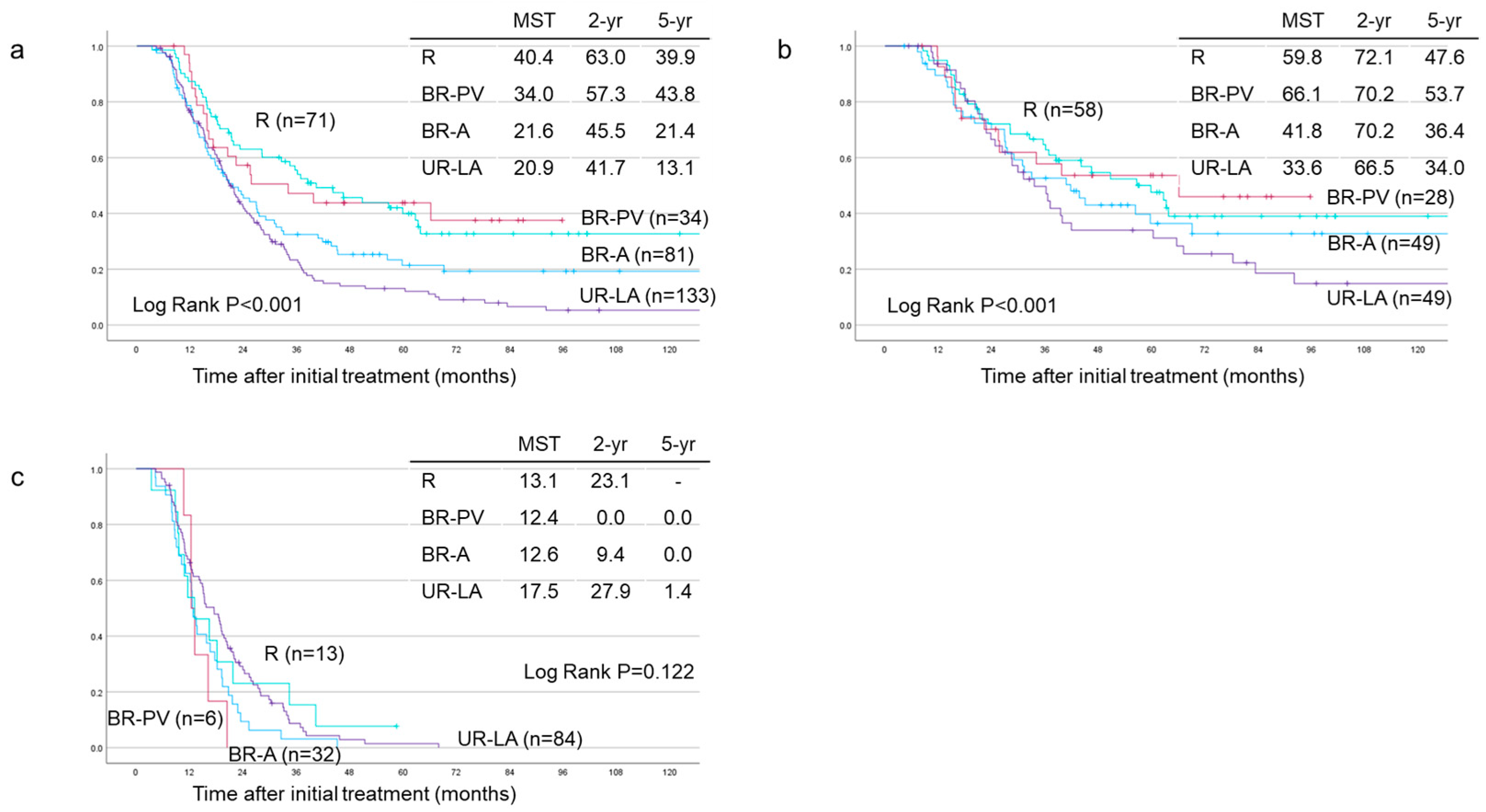
3.4. Survival Analyses Based on Tumor Resectability
3.5. Identifying Significant Prognostic Predictors of Disease-Specific Survival
3.5.1. Among the 319 Re-Evaluated Cases
3.5.2. Among the 184 Patients Who Underwent Surgical Resection
3.5.3. Among the 49 Patients with Unresectable–Locally Advanced Tumors Who Underwent Surgical Resection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| GS-CRT | Gemcitabine plus S-1-based chemoradiotherapy |
| NAC | Neoadjuvant chemotherapy |
| DSS | Disease-specific survival |
| DFS | Disease-free survival |
| OS | Overall survival |
| CA | Celiac artery |
| CHA | Common hepatic artery |
| SMA | Superior mesenteric artery |
| SMV | Superior mesenteric vein |
| PV | Portal vein |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| Hb | Hemoglobin |
| WBC | White blood cell |
| PS | Performance status |
| PNI | Prognostic nutritional index (PNI) |
| NCCN | National Comprehensive Cancer Network |
| AHPBA | American Hepatopancreatobiliary Association |
| MDACC | University of Texas MD Anderson Cancer Center |
| ECOG | Eastern Cooperative Oncology Group |
| SSO | Society of Surgical Oncology |
| R0 | Negative resection margins |
| R1 | Positive resection margins |
| GnP | Gemcitabine/nab-paclitaxel |
| EUS-FNA | Endoscopic ultrasound-guided fine-needle aspiration |
| MDCT | Multi-dynamic computed tomography |
| R | Resecteable |
| BR-PV | Borderline resectable (superior mesenteric vein/portal vein invasion alone) |
| BR-A | Borderline resectable (arterial invasion) |
| UR-LA | Unresectable–locally advanced |
| JPS | Japan Pancreas Society |
| CTCAE | Common Terminology Criteria for Adverse Event |
| MST | Median survival time |
| NLR | Neutrophils/lymphocytes ratio |
| PD | Pancreaticoduodenenctomy |
| DP | Distal pancreatectomy |
| TP | Total pancreatectomy |
| CSC | Cancer stem cell |
| PDX | Patient-derived xenograft |
| CAFs | Cancer-associated fibroblasts |
| DOX | Doxorubicin |
| BMI | Body Mass Index |
| FDA | Food and Drug Administration |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Ministry of Health, Labour and Welfare. Overview of the Vital Statistics (Finalized Data) for 2023. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei23/index.html (accessed on 5 January 2025).
- Zhong, J.; Shi, J.; Amundadottir, L.T. Artificial Intelligence and Improved Early Detection for Pancreatic Cancer. Innovation 2023, 4, 100457. [Google Scholar] [PubMed]
- Korfiatis, P.; Suman, G.; Patnam, N.G.; Trivedi, K.H.; Karbhari, A.; Mukherjee, S.; Cook, C.; Klug, J.R.; Patra, A.; Khasawneh, H.; et al. Automated Artificial Intelligence Model Trained on a Large Data Set Can Detect Pancreas Cancer on Diagnostic Computed Tomography Scans As Well As Visually Occult Preinvasive Cancer on Prediagnostic Computed Tomography Scans. Gastroenterology 2023, 165, 1533–1546.e4. [Google Scholar] [PubMed]
- Tripathi, S.; Tabari, A.; Mansur, A.; Dabbara, H.; Bridge, C.P.; Daye, D. From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer. Diagnostics 2024, 14, 174. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Version 1.2025. National Comprehensive Cancer Network. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 5 January 2025).
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.W.; Kishiwada, M.; et al. International Consensus on Definition and Criteria of Borderline Resectable Pancreatic Ductal Adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar]
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; Traverso, L.W.; Linehan, D.C. Pretreatment Assessment of Resectable and Borderline Resectable Pancreatic Cancer: Expert Consensus Statement. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [PubMed]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.T.; Evans, D.B.; Wolff, R.A. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar]
- Katz, M.H.G.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; de W. Marsh, R.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar]
- Japan Pancreas Society. Classification of Pancreatic Cancer, 4th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2016. (In English) [Google Scholar]
- Japan Pancreas Society. Japanese Classification of Pancreatic Carcinoma, 8th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2023. [Google Scholar]
- Ishida, M.; Fujii, T.; Kishiwada, M.; Shibuya, K.; Satoi, S.; Ueno, M.; Nakata, K.; Takano, S.; Uchida, K.; Ohike, N.; et al. Japanese classification of pancreatic carcinoma by the Japan Pancreas Society: Eighth edition. J. Hepatobiliary Pancreat. Sci. 2024, 31, 755–768. [Google Scholar] [CrossRef]
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Ben Abdelghani, M.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs. Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578. [Google Scholar] [CrossRef]
- Gemenetzis, G.; Groot, V.P.; Blair, A.B.; Laheru, D.A.; Zheng, L.; Narang, A.K.; Fishman, E.K.; Hruban, R.H.; Yu, J.; Burkhart, R.A.; et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann. Surg. 2019, 270, 340–347. [Google Scholar] [PubMed]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated with First-Line FOLFIRINOX vs. Gemcitabine and Nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [PubMed]
- Truty, M.J.; Kendrick, M.L.; Nagorney, D.M.; Smoot, R.L.; Cleary, S.P.; Graham, R.P.; Goenka, A.H.; Hallemeier, C.L.; Haddock, M.G.; Harmsen, W.S.; et al. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann. Surg. 2021, 273, 341–349. [Google Scholar] [PubMed]
- Ushida, Y.; Inoue, Y.; Oba, A.; Mie, T.; Ito, H.; Ono, Y.; Sato, T.; Ozaka, M.; Sasaki, T.; Saiura, A.; et al. Optimizing Indications for Conversion Surgery Based on Analysis of 454 Consecutive Japanese Cases with Unresectable Pancreatic Cancer Who Received Modified FOLFIRINOX or Gemcitabine Plus Nab-Paclitaxel: A Single-Center Retrospective Study. Ann. Surg. Oncol. 2022, 29, 5038–5050. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized Phase II/III Trial of Neoadjuvant Chemotherapy with Gemcitabine and S-1 Versus Up-Front Surgery for Resectable Pancreatic Cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- Eade, A.V.; Friedman, L.R.; Larrain, C.; Rainey, A.; Hernandez, J.M.; Chawla, A.; Ferrone, C.R. ALLIANCE A021806: A Phase III Trial of Perioperative Versus Adjuvant Chemotherapy for Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2024, 31, 6373–6374. [Google Scholar]
- Ahmad, S.A.; Duong, M.; Sohal, D.P.S.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., III; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Surgical Outcome Results from SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2020, 272, 481–486. [Google Scholar]
- Schwarz, L.; Vernerey, D.; Bachet, J.B.; Tuech, J.J.; Portales, F.; Michel, P.; Cunha, A.S. Resectable pancreatic adenocarcinoma neoadjuvant FOLF(IRIN)OX-based chemotherapy—A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer 2018, 18, 762. [Google Scholar]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar]
- Groot Koerkamp, B.; Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Haberkorn, B.C.M.; de Hingh, I.H.J.T.; Karsten, T.M.; van der Kolk, M.B.; et al. Neoadjuvant Chemotherapy with FOLFIRINOX versus Neoadjuvant Gemcitabine-Based Chemoradiotherapy for Borderline Resectable and Resectable Pancreatic Cancer (PREOPANC-2). In Proceedings of the ESMO 2023 Congress, Madrid, Spain, 20–24 October 2023. [Google Scholar]
- Ghaneh, P.; Palmer, D.; Cicconi, S.; Jackson, R.; Halloran, C.M.; Rawcliffe, C.; Sripadam, R.; Mukherjee, S.; Soonawalla, Z.; Wadsley, J.; et al. Immediate Surgery Compared with Short-Course Neoadjuvant Gemcitabine Plus Capecitabine, FOLFIRINOX, or Chemoradiotherapy in Patients with Borderline Resectable Pancreatic Cancer (ESPAC5): A Four-Arm, Multicentre, Randomised, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 157–168. [Google Scholar]
- Katz, M.H.G.; Shi, Q.; Meyers, J.; Herman, J.M.; Chuong, M.; Wolpin, B.M.; Ahmad, S.; Marsh, R.; Schwartz, L.; Behr, S.; et al. Efficacy of Preoperative mFOLFIRINOX vs. mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1263–1270. [Google Scholar] [PubMed]
- Mukherjee, S.; Hurt, C.N.; Bridgewater, J.; Falk, S.; Cummins, S.; Wasan, H.; Crosby, T.; Jephcott, C.; Roy, R.; Radhakrishna, G.; et al. Gemcitabine-Based or Capecitabine-Based Chemoradiotherapy for Locally Advanced Pancreatic Cancer (SCALOP): A Multicentre, Randomised, Phase 2 Trial. Lancet Oncol. 2013, 14, 317–326. [Google Scholar] [PubMed]
- Fietkau, R.; Ghadimi, M.; Grützmann, R.; Wittel, U.A.; Jacobasch, L.; Uhl, W.; Croner, R.S.; Bechstein, W.O.; Neumann, U.P.; Waldschmidt, D.; et al. Randomized Phase III Trial of Induction Chemotherapy Followed by Chemoradiotherapy or Chemotherapy Alone for Nonresectable Locally Advanced Pancreatic Cancer: First Results of the CONKO-007 Trial. J. Clin. Oncol. 2022, 40, 4008. [Google Scholar]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs. Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine with or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [PubMed]
- Kobayashi, M.; Mizuno, S.; Murata, Y.; Kishiwada, M.; Usui, M.; Sakurai, H.; Tabata, M.; Ii, N.; Yamakado, K.; Inoue, H.; et al. Gemcitabine-Based Chemoradiotherapy Followed by Surgery for Borderline Resectable and Locally Unresectable Pancreatic Ductal Adenocarcinoma: Significance of the CA19-9 Reduction Rate and Intratumoral Human Equilibrative Nucleoside Transporter 1 Expression. Pancreas 2014, 43, 350–360. [Google Scholar]
- Nakai, Y.; Isayama, H.; Sasaki, T.; Sasahira, N.; Tsujino, T.; Toda, N.; Kogure, H.; Matsubara, S.; Ito, Y.; Togawa, O.; et al. A Multicentre Randomised Phase II Trial of Gemcitabine Alone vs. Gemcitabine and S-1 Combination Therapy in Advanced Pancreatic Cancer: GEMSAP Study. Br. J. Cancer 2012, 106, 1934–1939. [Google Scholar]
- Ozaka, M.; Matsumura, Y.; Ishii, H.; Omuro, Y.; Itoi, T.; Mouri, H.; Hanada, K.; Kimura, Y.; Maetani, I.; Okabe, Y.; et al. Randomized Phase II Study of Gemcitabine and S-1 Combination Versus Gemcitabine Alone in the Treatment of Unresectable Advanced Pancreatic Cancer (Japan Clinical Cancer Research Organization PC-01 Study). Cancer Chemother. Pharmacol. 2012, 69, 1197–1204. [Google Scholar]
- Takeuchi, T.; Mizuno, S.; Murata, Y.; Hayasaki, A.; Kishiwada, M.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Kuriyama, N.; et al. Comparative Study Between Gemcitabine-Based and Gemcitabine Plus S-1-Based Preoperative Chemoradiotherapy for Localized Pancreatic Ductal Adenocarcinoma, with Special Attention to Initially Locally Advanced Unresectable Tumor. Pancreas 2019, 48, 281–291. [Google Scholar]
- Kato, H.; Kishiwada, M.; Hayasaki, A.; Chipaila, J.; Maeda, K.; Noguchi, D.; Gyoten, K.; Fujii, T.; Iizawa, Y.; Tanemura, A.; et al. Role of Serum Carcinoma Embryonic Antigen (CEA) Level in Localized Pancreatic Adenocarcinoma: CEA Level Before Operation is a Significant Prognostic Indicator in Patients with Locally Advanced Pancreatic Cancer Treated with Neoadjuvant Therapy Followed by Surgical Resection: A Retrospective Analysis. Ann. Surg. 2022, 275, e698–e707. [Google Scholar]
- Murata, Y.; Mizuno, S.; Kishiwada, M.; Uchida, K.; Noguchi, D.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Tanemura, A.; et al. Clinical Significance and Predictors of Complete or Near-Complete Histological Response to Preoperative Chemoradiotherapy in Patients with Localized Pancreatic Ductal Adenocarcinoma. Pancreatology 2021, 21, 1482–1490. [Google Scholar] [PubMed]
- Ichikawa, K.; Mizuno, S.; Hayasaki, A.; Kishiwada, M.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Murata, Y.; Azumi, Y.; et al. Prognostic Nutritional Index After Chemoradiotherapy Was the Strongest Prognostic Predictor Among Biological and Conditional Factors in Localized Pancreatic Ductal Adenocarcinoma Patients. Cancers 2019, 11, 514. [Google Scholar] [CrossRef]
- Hayasaki, A.; Isaji, S.; Kishiwada, M.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Murata, Y.; Azumi, Y.; Kuriyama, N.; et al. Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer. Cancers 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mizuno, S.; Kishiwada, M.; Hamada, T.; Usui, M.; Sakurai, H.; Tabata, M.; Inoue, H.; Shiraishi, T.; Isaji, S. Impact of Histological Response After Neoadjuvant Chemoradiotherapy on Recurrence-Free Survival in UICC-T3 Pancreatic Adenocarcinoma but Not in UICC-T4. Pancreas 2012, 41, 130–136. [Google Scholar]
- Satoi, S.; Yamaue, H.; Kato, K.; Takahashi, S.; Hirono, S.; Takeda, S.; Eguchi, H.; Sho, M.; Wada, K.; Shinchi, H.; et al. Role of Adjuvant Surgery for Patients with Initially Unresectable Pancreatic Cancer with a Long-Term Favorable Response to Nonsurgical Anticancer Treatments: Results of a Project Study for Pancreatic Surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2013, 20, 590–600. [Google Scholar] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibas, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Győrffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar]
- Sugiura, T.; Toyama, H.; Fukutomi, A.; Asakura, H.; Takeda, Y.; Yamamoto, K.; Hirano, S.; Satoi, S.; Matsumoto, I.; Takahashi, S.; et al. Randomized Phase II Trial of Chemoradiotherapy with S-1 Versus Combination Chemotherapy with Gemcitabine and S-1 as Neoadjuvant Treatment for Resectable Pancreatic Cancer (JASPAC 04). J. Hepatobiliary Pancreat. Sci. 2023, 30, 1249–1260. [Google Scholar]
- Takahashi, S.; Ohno, I.; Ikeda, M.; Konishi, M.; Kobayashi, T.; Akimoto, T.; Kojima, M.; Morinaga, S.; Toyama, H.; Shimizu, Y.; et al. Neoadjuvant S-1 with Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-Label Multicenter Prospective Trial (JASPAC05). Ann. Surg. 2022, 276, e510–e517. [Google Scholar]
- Kim, R.Y.; Christians, K.K.; Aldakkak, M.; Clarke, C.N.; George, B.; Kamgar, M.; Khan, A.H.; Kulkarni, N.; Hall, W.A.; Erickson, B.A.; et al. Total Neoadjuvant Therapy for Operable Pancreatic Cancer. Ann. Surg. Oncol. 2021, 28, 2246–2256. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C.; UICC. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2017; pp. 1–272. [Google Scholar]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017; pp. 1–1024. [Google Scholar]
- Tas, F.; Sen, F.; Odabas, H.; Kılıc, L.; Keskin, S.; Yıldız, I. Performance Status of Patients is the Major Prognostic Factor at All Stages of Pancreatic Cancer. Int. J. Clin. Oncol. 2013, 18, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yang, L.; Lu, Y.; Lu, G. Prognostic Value of Hemoglobin to Red Blood Cell Distribution Width Ratio in Pancreatic Ductal Adenocarcinoma: A Retrospective Study. BMC Gastroenterol. 2024, 24, 288. [Google Scholar] [CrossRef] [PubMed]
- İlhan, A.; Gurler, F.; Yilmaz, F.; Eraslan, E.; Dogan, M. The Relationship Between Hemoglobin-RDW Ratio and Clinical Outcomes in Patients with Advanced Pancreas Cancer. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2060–2067. [Google Scholar] [PubMed]
- Schouten, T.J.; van Goor, I.W.J.M.; Dorland, G.A.; Besselink, M.G.; Bonsing, B.A.; Bosscha, K.; Brosens, L.A.A.; Busch, O.R.; Cirkel, G.A.; van Dam, R.M.; et al. The Value of Biological and Conditional Factors for Staging of Patients with Resectable Pancreatic Cancer Undergoing Upfront Resection: A Nationwide Analysis. Ann. Surg. Oncol. 2024, 31, 4956–4965. [Google Scholar] [CrossRef]
- Hartlapp, I.; Valta-Seufzer, D.; Siveke, J.T.; Algül, H.; Goekkurt, E.; Siegler, G.; Martens, U.M.; Waldschmidt, D.; Pelzer, U.; Fuchs, M.; et al. Prognostic and Predictive Value of CA 19-9 in Locally Advanced Pancreatic Cancer Treated with Multiagent Induction Chemotherapy: Results from a Prospective, Multicenter Phase II Trial (NEOLAP-AIO-PAK-0113). ESMO Open 2022, 7, 100552. [Google Scholar] [CrossRef]
- Heger, U.; Sun, H.; Hinz, U.; Klaiber, U.; Tanaka, M.; Liu, B.; Sachsenmaier, M.; Springfeld, C.; Michalski, C.W.; Büchler, M.W.; et al. Induction Chemotherapy in Pancreatic Cancer: CA 19-9 May Predict Resectability and Survival. HPB 2020, 22, 224–232. [Google Scholar] [CrossRef]
- Boone, B.A.; Steve, J.; Zenati, M.S.; Hogg, M.E.; Singhi, A.D.; Bartlett, D.L.; Zureikat, A.H.; Bahary, N.; Zeh, H.J., III. Serum CA 19-9 Response to Neoadjuvant Therapy is Associated with Outcome in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 4351–4358. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamada, D.; Asukai, K.; Wada, H.; Hasegawa, S.; Hara, H.; Shinno, N.; Ushigome, H.; Haraguchi, N.; Sugimura, K.; et al. Clinical Implications of the Serum CA19-9 Level in ‘Biological Borderline Resectability’ and ‘Biological Downstaging’ in the Setting of Preoperative Chemoradiation Therapy for Pancreatic Cancer. Pancreatology 2020, 20, 919–928. [Google Scholar]
- White, R.R.; Xie, H.B.; Gottfried, M.R.; Czito, B.G.; Hurwitz, H.I.; Morse, M.A.; Blobe, G.C.; Paulson, E.K.; Baillie, J.; Branch, M.S.; et al. Significance of Histological Response to Preoperative Chemoradiotherapy for Pancreatic Cancer. Ann. Surg. Oncol. 2005, 12, 214–221. [Google Scholar]
- Gu, A.; Li, J.; Li, M.Y.; Liu, Y. Patient-Derived Xenograft Model in Cancer: Establishment and Applications. MedComm 2025, 6, e70059. [Google Scholar]
- Wang, J.; Liao, Z.-X. Research Progress of Microrobots in Tumor Drug Delivery. Food Med. Homol. 2024, 1, 9420025. [Google Scholar] [CrossRef]
- Lee, H.; Park, S. Magnetically Actuated Helical Microrobot with Magnetic Nanoparticle Retrieval and Sequential Dual-Drug Release Abilities. ACS Appl. Mater. Interfaces 2023, 15, 27471–27485. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Oba, A.; Wu, Y.H.A.; Beaty, L.E.; Colborn, K.L.; Janssen, B.V.; Al-Musawi, M.H.; Franco, S.R.; Sugawara, T.; Franklin, O.; et al. Pathological Complete Response in Patients with Resected Pancreatic Adenocarcinoma After Preoperative Chemotherapy. JAMA Netw. Open 2024, 7, e2417625. [Google Scholar] [CrossRef]
- Emanuel, A.; Krampitz, J.; Rosenberger, F.; Kind, S.; Rozer, I. Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers 2022, 14, 2212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wu, Z.; Wang, Z.; Wu, C.; Huang, X.; Tian, B. Prognostic Role of the Prognostic Nutritional Index in Patients with Pancreatic Cancer Who Underwent Curative Resection Without Preoperative Neoadjuvant Treatment: A Systematic Review and Meta-Analysis. Front. Surg. 2022, 9, 992641. [Google Scholar] [CrossRef]
- Kawahara, S.; Aoyama, T.; Murakawa, M.; Kanemoto, R.; Takahashi, D.; Kamioka, Y.; Hashimoto, I.; Maezawa, Y.; Kobayashi, S.; Ueno, M.; et al. Prognostic Nutritional Index is an Independent Risk Factor for Continuing S-1 Adjuvant Chemotherapy in Patients with Pancreatic Cancer Who Received Neoadjuvant Chemotherapy and Surgical Resection. BMC Cancer 2024, 24, 1469. [Google Scholar] [CrossRef]
- Kubo, H.; Ohgi, K.; Sugiura, T.; Ashida, R.; Yamada, M.; Otsuka, S.; Yamazaki, K.; Todaka, A.; Sasaki, K.; Uesaka, K. The Association Between Neoadjuvant Therapy and Pathological Outcomes in Pancreatic Cancer Patients After Resection: Prognostic Significance of Microscopic Venous Invasion. Ann. Surg. Oncol. 2022, 29, 4992–5002. [Google Scholar] [CrossRef]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S.V.; Windsor, J.A. ‘Artery-First’ Approaches to Pancreatoduodenectomy. Br. J. Surg. 2012, 99, 1027–1035. [Google Scholar] [CrossRef]
- Schneider, M.; Hackert, T.; Strobel, O.; Büchler, M.W. Technical Advances in Surgery for Pancreatic Cancer. Br. J. Surg. 2021, 108, 777–785. [Google Scholar] [CrossRef]
- Cai, B.; Lu, Z.; Neoptolemos, J.P.; Diener, M.K.; Li, M.; Yin, L.; Gao, Y.; Wei, J.; Chen, J.; Guo, F.; et al. Sub-Adventitial Divestment Technique for Resecting Artery-Involved Pancreatic Cancer: A Retrospective Cohort Study. Langenbeck’s Arch. Surg. 2021, 406, 691–701. [Google Scholar] [CrossRef]
- Hackert, T.; Strobel, O.; Michalski, C.W.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.; Berchtold, C.; Ulrich, A.; Büchler, M.W. The TRIANGLE Operation—Radical Surgery After Neoadjuvant Treatment for Advanced Pancreatic Cancer: A Single-Arm Observational Study. HPB 2017, 19, 1001–1007. [Google Scholar] [PubMed]
- Mizuno, S.; Isaji, S.; Tanemura, A.; Kishiwada, M.; Murata, Y.; Azumi, Y.; Kuriyama, N.; Usui, M.; Sakurai, H.; Tabata, M. Anterior Approach to the Superior Mesenteric Artery by Using Nerve Plexus Hanging Maneuver for Borderline Resectable Pancreatic Head Carcinoma. J. Gastrointest. Surg. 2014, 18, 1209–1217. [Google Scholar] [PubMed]
- Strickler, J.H.; Satake, H.; George, T.J.; Yaeger, R.; Hollebecque, A.; Garrido-Laguna, I.; Schuler, M.; Burns, T.F.; Coveler, A.L.; Falchook, G.S.; et al. Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. N. Engl. J. Med. 2023, 388, 33–43. [Google Scholar] [PubMed]
- Carbone, D.; De Franco, M.; Pecoraro, C.; Bassani, D.; Pavan, M.; Cascioferro, S.; Parrino, B.; Cirrincione, G.; Dall’Acqua, S.; Moro, S.; et al. Discovery of the 3-Amino-1,2,4-Triazine-Based Library as Selective PDK1 Inhibitors with Therapeutic Potential in Highly Aggressive Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 3679. [Google Scholar] [CrossRef]
- Du, S.; Yan, J.; Xue, Y.; Zhong, Y.; Dong, Y. Adoptive Cell Therapy for Cancer Treatment. Exploration 2023, 3, 20210058. [Google Scholar]
- Garajov, I.; Peroni, M.; Gelsomino, F.; Leonardi, F. A Simple Overview of Pancreatic Cancer Treatment for Clinical Oncologists. Curr. Oncol. 2023, 30, 9587–9601. [Google Scholar] [CrossRef]

| All (n = 351) | R (n = 83) | BR-PV (n = 40) | BR-A (n = 84) | UR-LA (n = 144) | |
|---|---|---|---|---|---|
| Completion of CRT | 347 (98.9%) | 80 (96.4%) | 39 (97.5%) | 84 (100%) | 144 (100%) |
| Grade 3 or higher adverse events | 181 (51.6%) | 40 (48.2%) | 16 (40.0%) | 42 (50.0%) | 83 (57.6%) |
| White blood cell decreased | 139 (39.6%) | 30 (36.1%) | 12 (30.0%) | 30 (35.7%) | 67 (46.5%) |
| Neutrophil count decreased | 113 (32.2%) | 24 (28.9%) | 6 (15.0%) | 29 (34.5%) | 54 (37.5%) |
| Febrile neutropenia | 2 (0.6%) | 0 (0%) | 0 (0%) | 1 (1.2%) | 1 (0.7%) |
| Anemia | 18 (5.1%) | 5 (6.0%) | 1 (2.5%) | 3 (3.6%) | 9 (6.3%) |
| Platelet count decreased | 17 (4.8%) | 7 (8.4%) | 0 (0%) | 4 (4.8%) | 6 (4.2%) |
| Anorexia | 13 (3.7%) | 3 (3.6%) | 1 (2.5%) | 5 (6.0%) | 4 (2.8%) |
| Diarrhea | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.7%) |
| Eczema | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Peripheral sensoryneuropathy | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| (A) All Patients | n = 319 |
| Before GS-CRT | |
| Age | 69 (40–87) |
| Sex (male/female) | 193/126 |
| PS (0/1 or 2/3) | 305/14 |
| BMI (Kg/m2) | 21.2 (14.1–37.3) |
| Hb (g/dL) | 13.1 (6.9–17.0) |
| Alb (g/dL) | 3.7 (2.4–4.7) |
| PNI (prognostic nutrition index) | 38.5 (26.2–48.7) |
| White blood cell counts (/mm3) | 5760 (2520–13,320) |
| Neutrophil counts (/mm3) | 3590 (1150–11,920) |
| Lymphocyte counts (/mm3) | 1450 (270–4630) |
| NLR (neutrophils/lymphocytes ratio) | 2.5 (0.6–21.2) |
| CA19-9 level (U/L) | 199.4 (0.1–61,621) |
| CEA level (ng/mL) | 3.7 (0.8–97.3) |
| Portal venous contact or invasion ≥ 180° (yes/no) | 150/169 |
| Celiac axis contact or invasion ≥ 180° (yes/no) | 55/264 |
| Superior mesenteric artery contact or invasion ≥ 180° (yes/no) | 77/242 |
| Common hepatic artery contact or invasion ≥ 180° (yes/no) | 67/252 |
| JPS8th T factor (T1–T3/T4) | 121/198 |
| JPS8th N factor (N0/N1a,N1b) | 264/55 |
| Tumor size on CT (mm) | 32.9 (12.6–90.6) |
| (B) Resected Patients | n = 184 |
| Pre-operative factors | |
| Age | 67 (40–84) |
| Sex (male/female) | 110/74 |
| PS (0/1 or 2/3) | 180/4 |
| BMI (Kg/m2) | 21.3 (13.6–34.3) |
| Hb (g/dL) | 11.5 (7.6–14.2) |
| Alb (g/dL) | 3.7 (1.7–4.3) |
| PNI (prognostic nutrition index) | 41.0 (22.6–53.0) |
| White blood cell counts (/mm3) | 4530 (2300–9930) |
| Neutrophil counts (/mm3) | 2880 (900–7880) |
| Lymphocyte counts (/mm3) | 900 (316–2120) |
| NLR (neutrophils/lymphocytes ratio) | 3.5 (1.0–11.2) |
| CA19-9 level (U/L) | 28.9 (0.1–1034.3) |
| CEA level (ng/mL) | 3.0 (0.7–41.2) |
| Portal venous contact or invasion ≥ 180° (yes/no) | 67/117 |
| Celiac axis contact or invasion ≥ 180° (yes/no) | 17/167 |
| Superior mesenteric artery contact or invasion ≥ 180° (yes/no) | 26/158 |
| Common hepatic artery contact or invasion ≥ 180° (yes/no) | 28/156 |
| JPS8th T factor (T1–T3/T4) | 99/85 |
| JPS8th N factor (N0/N1a,N1b) | 171/13 |
| Tumor size on CT (mm) | 25.7 (10.4–82.0) |
| Duration from initial treatment (day) | 114 (79–434) |
| Intra-operative factors | |
| Operative procedures (PD, TP/DP) | 152/32 |
| Operation time (minutes) | 525 (229–906) |
| Blood loss (ml) | 757 (60–11,937) |
| Combined resection of portal vein (yes/no) | 141/43 |
| Combined resection of common hepatic artery (yes/no) | 18/166 |
| Combined resection of celiac axis (yes/no) | 6/178 |
| Histopathological factors | |
| Degree of pathological differentiation (well/mod-poor/NE) | 88/80/16 |
| Degree of lymphatic invasion (ly0/ly1–3/NE) | 158/22/4 |
| Degree of perineural invasion (ne0/ne1–3/NE) | 72/108/4 |
| Degree of venous invasion (v0/v1–3/NE) | 147/33/4 |
| Degree of the residual tumor (R0/R1–2) | 168/16 |
| Degree of histological response (grade1–2/grade 3–4) | 116/68 |
| Post-operative factors | |
| Postoperative complications (yes/no) CD > 3 | 53/131 |
| Adjuvant chemotherapy (yes/no) | 155/29 |
| (C) Resected UR-LA Patients | n = 49 |
| Pre-operative factors | |
| Age | 67 (50–76) |
| Sex (male/female) | 29/20 |
| PS (0/1 or 2/3) | 48/1 |
| BMI (Kg/m2) | 21.8 (15.9–34.3) |
| Hb (g/dL) | 11.2 (7.6–14.6) |
| Alb (g/dL) | 3.6 (1.7–4.5) |
| PNI (prognostic nutrition index) | 39.3 (22.6–52.1) |
| White blood cell counts (/mm3) | 4510 (2570–9930) |
| Neutrophil counts (/mm3) | 2850 (1640–7880) |
| Lymphocyte counts (/mm3) | 810 (316–2400) |
| NLR (neutrophils/lymphocytes ratio) | 3.6 (1.5–11.2) |
| CA19-9 level (U/L) | 27.4 (0.7–1034.3) |
| CEA level (ng/mL) | 2.8 (1.4–9.2) |
| Portal venous contact or invasion ≥ 180° (yes/no) | 28/21 |
| Celiac axis contact or invasion ≥ 180° (yes/no) | 15/34 |
| Superior mesenteric artery contact or invasion ≥ 180° (yes/no) | 23/26 |
| Common hepatic artery contact or invasion ≥ 180° (yes/no) | 22/27 |
| JPS8th T factor (T1–T3/T4) | 2/47 |
| JPS8th N factor (N0/N1a,N1b) | 43/6 |
| Tumor size on CT (mm) | 29.7 (14.4–82.0) |
| Duration from initial treatment (day) | 152 (78–489) |
| Intra-operative factors | |
| Operative procedures (PD, TP/DP) | 36/13 |
| Operation time (minutes) | 600 (333–801) |
| Blood loss (mL) | 1155 (318–11,937) |
| Combined resection of portal vein (yes/no) | 39/10 |
| Combined resection of common hepatic artery (yes/no) | 10/39 |
| Combined resection of celiac axis (yes/no) | 5/44 |
| Histopathological factors | |
| Degree of pathological differentiation (well/mod-poor/NE) | 22/23/4 |
| Degree of lymphatic invasion (ly0/ly1–3) | 43/6 |
| Degree of perineural invasion (ne0/ne1–3) | 11/38 |
| Degree of venous invasion (v0/v1–3) | 42/7 |
| Degree of the residual tumor (R0/R1) | 39/10 |
| Degree of histological response (grade1–2/grade 3–4) | 36/13 |
| Post-operative factors | |
| Postoperative complications CD > 3 (yes/no) | 13/36 |
| Adjuvant chemotherapy (yes/no) | 39/10 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables Before GS-CRT | HR | 95%CI | p-Value | HR | 95%CI | p-Value |
| Age | 0.072 | 0.385 | ||||
| Sex (male/female) | 0.606 | |||||
| PS (0/1 or 2/3) | 0.069 | 1.9 | 1.031–3.504 | 0.04 | ||
| BMI (Kg/m2) | 0.423 | |||||
| Hb (g/bL) | 0.91 | 0.844–0.973 | 0.007 | 0.9 | 0.841–0.971 | 0.006 |
| Alb (g/dL) | 0.316 | |||||
| PNI (prognostic nutrition index) | 0.248 | |||||
| White blood cell counts (/mm3) | 0.794 | |||||
| Neutrophil counts (/mm3) | 0.802 | |||||
| Lymphocyte counts (/mm3) | 0.632 | |||||
| NLR (neutrophils/lymphocytes ratio) | 0.533 | |||||
| CA19-9 level (U/L) | 0.339 | |||||
| CEA level (ng/mL) | 0.095 | 0.065 | ||||
| Portal venous contact or invasion ≥ 180° (yes/no) | 1.327 | 1.027–1.715 | 0.03 | 0.291 | ||
| Celiac axis contact or invasion ≥ 180° (yes/no) | 1.79 | 1.305–2.451 | <0.001 | 1.45 | 1.039–2.035 | 0.029 |
| Superior mesenteric artery contact or invasion ≥ 180° (yes/no) | 1.786 | 1.337–2.384 | <0.001 | 0.09 | ||
| Common hepatic artery contact or invasion ≥ 180° (yes/no) | 1.435 | 1.063–1.939 | 0.018 | 0.28 | ||
| JPS8th T factor (T1–T3/T4) | 1.94 | 1.468–2.567 | <0.001 | 1.8 | 1.334–2.416 | <0.001 |
| JPS8th N factor (N0/N1a,N1b) | 0.804 | |||||
| Tumor size on CT (mm) | 1.012 | 1.002–1.023 | 0.015 | 0.459 | ||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Pre-operative factors | ||||||
| Age | 0.697 | |||||
| Sex (male/female) | 0.373 | |||||
| PS (0/1 or 2/3) | 5.34 | 1.645–17.359 | 0.005 | 5.34 | 1.612–17.672 | 0.006 |
| BMI (Kg/m2) | 0.218 | |||||
| Hb (g/bL) | 0.87 | 0.769–0.986 | 0.029 | 0.346 | ||
| Alb (g/dL) | 0.1 | |||||
| PNI (prognostic nutrition index) | 0.305 | |||||
| White blood cell counts (/mm3) | 0.85 | |||||
| Neutrophil counts (/mm3) | 0.855 | |||||
| Lymphocyte counts (/mm3) | 0.6 | |||||
| NLR (neutrophils/lymphocytes ratio) | 0.308 | |||||
| CA19-9 level (U/L) | 1 | 1.001–1.004 | <0.001 | 1 | 1.001–1.003 | <0.001 |
| CEA level (ng/mL) | 0.733 | |||||
| Portal venous contact or invasion ≥180° (yes/no) | 1.47 | 1.000–2.171 | 0.05 | 0.174 | ||
| Celiac axis contact or invasion ≥ 180° (yes/no) | 0.052 | 0.406 | ||||
| Superior mesenteric artery contact or invasion ≥ 180° (yes/no) | 1.91 | 1.157–3.136 | 0.011 | 0.336 | ||
| Common hepatic artery contact or invasion ≥ 180° (yes/no) | 0.848 | |||||
| JPS8th T factor (T1–T3/T4) | 1.75 | 1.194–2.567 | 0.004 | 1.89 | 1.275–2.813 | 0.002 |
| JPS8th N factor (N0/N1a,N1b) | 0.907 | |||||
| Tumor size on CT (mm) | 1.02 | 1.001–1.033 | 0.033 | 0.833 | ||
| Duration from initial treatment (day) | 0.292 | |||||
| Intra-operative factors | ||||||
| Operative procedures (PD, TP/DP) | 0.342 | |||||
| Operation time (minutes) | 0.342 | |||||
| Blood loss (ml) | 1 | 1.000–1.000 | 0.018 | 0.641 | ||
| Combined resection of portal vein (yes/no) | 0.243 | |||||
| Combined resection of common hepatic artery (yes/no) | 0.701 | |||||
| Combined resection of celiac axis (yes/no) | 0.122 | |||||
| Histopathological factors | ||||||
| Degree of pathological differentiation (well/mod-poor/NE) | 0.027 | 0.107 | ||||
| Degree of lymphatic invasion (ly0/ly1–3) | 0.145 | |||||
| Degree of perineural invasion (ne0/ne1–3) | 1.8 | 1.197–2.696 | 0.005 | 0.073 | ||
| Degree of venous invasion (v0/v1–3) | 0.131 | |||||
| Degree of the residual tumor (R0/R1) | 2.13 | 1.183–3.835 | 0.012 | 0.599 | ||
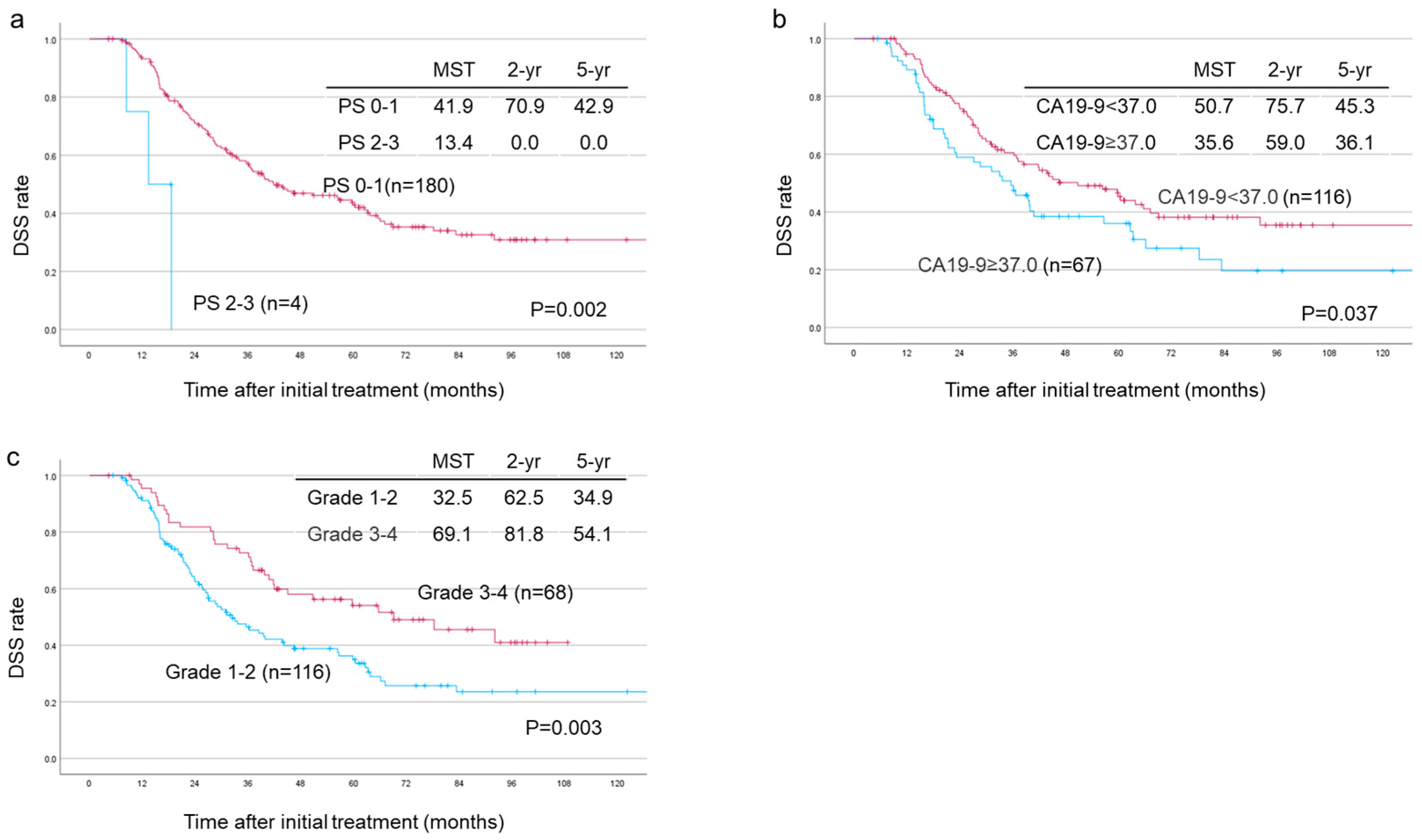
| Degree of histological response (grade1–2/grade 3–4) | 1.84 | 1.218–2.779 | 0.004 | 1.7 | 1.084–2.69 | 0.021 |
| Post-operative factors | ||||||
| Postoperative complications CD > 3 (yes/no) | 0.261 | |||||
| Adjuvant chemotherapy (yes/no) | 0.15 | 2.25 | 1.204–4.217 | 0.011 | ||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Pre-operative factors | ||||||
| Age | 0.925 | |||||
| Sex (male/female) | 0.31 | |||||
| PS (0/1 or 2/3) | 0.637 | |||||
| BMI (Kg/m2) | 0.305 | |||||
| Hb (g/bL) | 0.061 | 0.754 | ||||
| Alb (g/dL) | 0.147 | |||||
| PNI (prognostic nutrition index) | 0.94 | 0.892–0.996 | 0.036 | 0.93 | 0.866–0.988 | 0.02 |
| White blood cell counts (/mm3) | 0.216 | |||||
| Neutrophil counts (/mm3) | 0.86 | |||||
| Lymphocyte counts (/mm3) | 0.999 | 0.998–1.000 | 0.045 | 0.161 | ||
| NLR (neutrophils/lymphocytes ratio) | 0.258 | |||||
| CA19-9 level (U/L) | 1.003 | 1.001–1.006 | 0.012 | 0.125 | ||
| CEA level (ng/mL) | 1.172 | 1.008–1.363 | 0.039 | 0.261 | ||
| Portal venous contact or invasion ≥ 180° (yes/no) | 0.786 | |||||
| Celiac axis contact or invasion ≥ 180° (yes/no) | 0.094 | |||||
| Superior mesenteric artery contact or invasion ≥ 180° (yes/no) | 0.309 | |||||
| Common hepatic artery contact or invasion ≥ 180° (yes/no) | 0.26 | |||||
| JPS8th T factor (T1–T3/T4) | 0.104 | 0.895 | ||||
| JPS8th N factor (N0/N1a,N1b) | 0.981 | |||||
| Tumor size on CT (mm) | 0.476 | |||||
| Duration from initial treatment (day) | 0.701 | |||||
| Intra-operative factors | ||||||
| Operative procedures (PD, TP/DP) | 0.355 | |||||
| Operation time (minutes) | 0.373 | |||||
| Blood loss (ml) | 0.1 | 0.297 | ||||
| Combined resection of portal vein (yes/no) | 0.777 | |||||
| Combined resection of common hepatic artery (yes/no) | 0.472 | |||||
| Combined resection of celiac axis (yes/no) | 0.471 | |||||
| Histopathological factors | ||||||
| Degree of pathological differentiation (well/mod-por/NE) | 0.22 | |||||
| Degree of lymphatic invasion (ly0/ly1–3) | 0.527 | |||||
| Degree of perineural invasion (ne0/ne1–3) | 0.168 | |||||
| Degree of venous invasion (v0/v1–3) | 7.84 | 2.209–27.808 | 0.001 | 8.13 | 2.102–31.408 | 0.002 |
| Degree of the residual tumor (R0/R1) | 0.297 | |||||
| Degree of histological response(grade1–2/grade 3–4) | 0.07 | 0.239 | ||||
| Post-operative factors | ||||||
| Postoperative complications CD > 3 (yes/no) | 0.752 | |||||
| Adjuvant chemotherapy (yes/no) | 3.43 | 1.349–8.742 | 0.01 | 3.04 | 1.051–8.773 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishiwada, M.; Mizuno, S.; Hayasaki, A.; Kaluba, B.; Fujii, T.; Noguchi, D.; Ito, T.; Iizawa, Y.; Tanemura, A.; Murata, Y.; et al. Impact of Surgical Resection After Induction Gemcitabine Plus S-1-Based Chemoradiotherapy in Patients with Locally Advanced Pancreatic Ductal Adenocarcinoma: A Focus on UR-LA Cases. Cancers 2025, 17, 1048. https://doi.org/10.3390/cancers17061048
Kishiwada M, Mizuno S, Hayasaki A, Kaluba B, Fujii T, Noguchi D, Ito T, Iizawa Y, Tanemura A, Murata Y, et al. Impact of Surgical Resection After Induction Gemcitabine Plus S-1-Based Chemoradiotherapy in Patients with Locally Advanced Pancreatic Ductal Adenocarcinoma: A Focus on UR-LA Cases. Cancers. 2025; 17(6):1048. https://doi.org/10.3390/cancers17061048
Chicago/Turabian StyleKishiwada, Masashi, Shugo Mizuno, Aoi Hayasaki, Benson Kaluba, Takehiro Fujii, Daisuke Noguchi, Takahiro Ito, Yusuke Iizawa, Akihiro Tanemura, Yasuhiro Murata, and et al. 2025. "Impact of Surgical Resection After Induction Gemcitabine Plus S-1-Based Chemoradiotherapy in Patients with Locally Advanced Pancreatic Ductal Adenocarcinoma: A Focus on UR-LA Cases" Cancers 17, no. 6: 1048. https://doi.org/10.3390/cancers17061048
APA StyleKishiwada, M., Mizuno, S., Hayasaki, A., Kaluba, B., Fujii, T., Noguchi, D., Ito, T., Iizawa, Y., Tanemura, A., Murata, Y., & Kuriyama, N. (2025). Impact of Surgical Resection After Induction Gemcitabine Plus S-1-Based Chemoradiotherapy in Patients with Locally Advanced Pancreatic Ductal Adenocarcinoma: A Focus on UR-LA Cases. Cancers, 17(6), 1048. https://doi.org/10.3390/cancers17061048





