CDK4/6 as a Therapeutic Target in HR+/HER2− Breast Cancer Cells—Current Treatment Status
Simple Summary
Abstract
1. Introduction
2. Classification
3. Breast Cancer Cell Metabolism
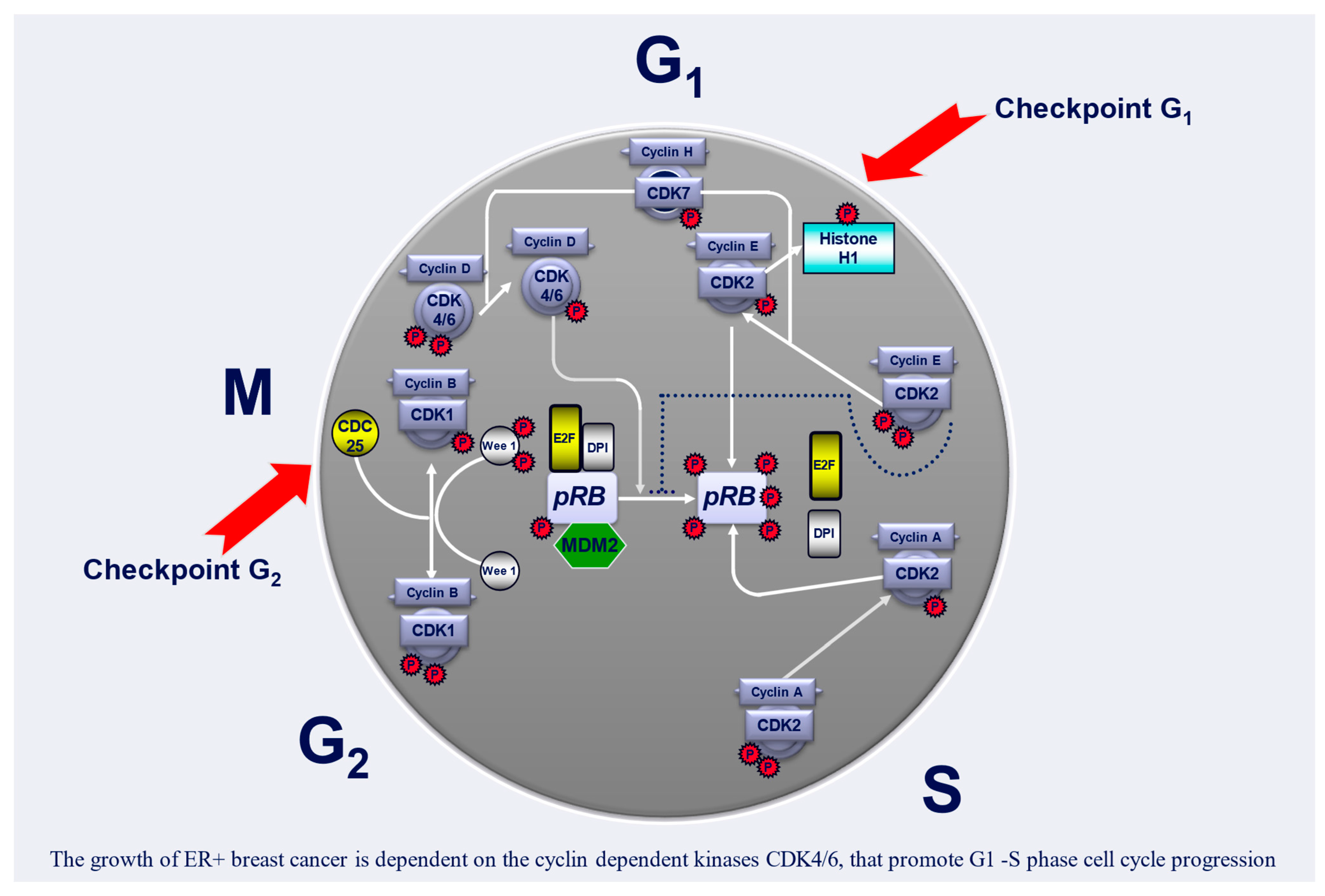
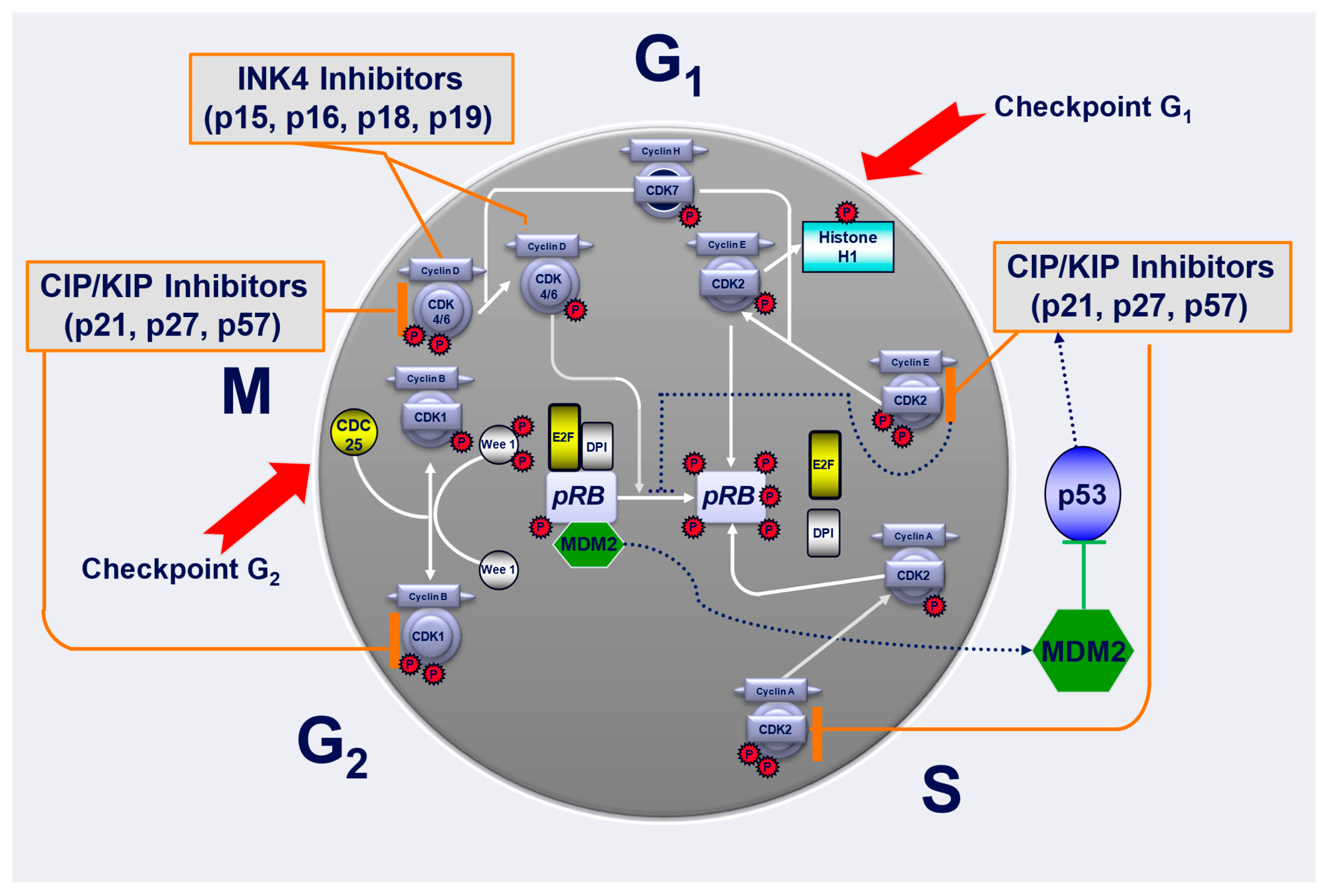
4. Cell Cycle and Its Regulation
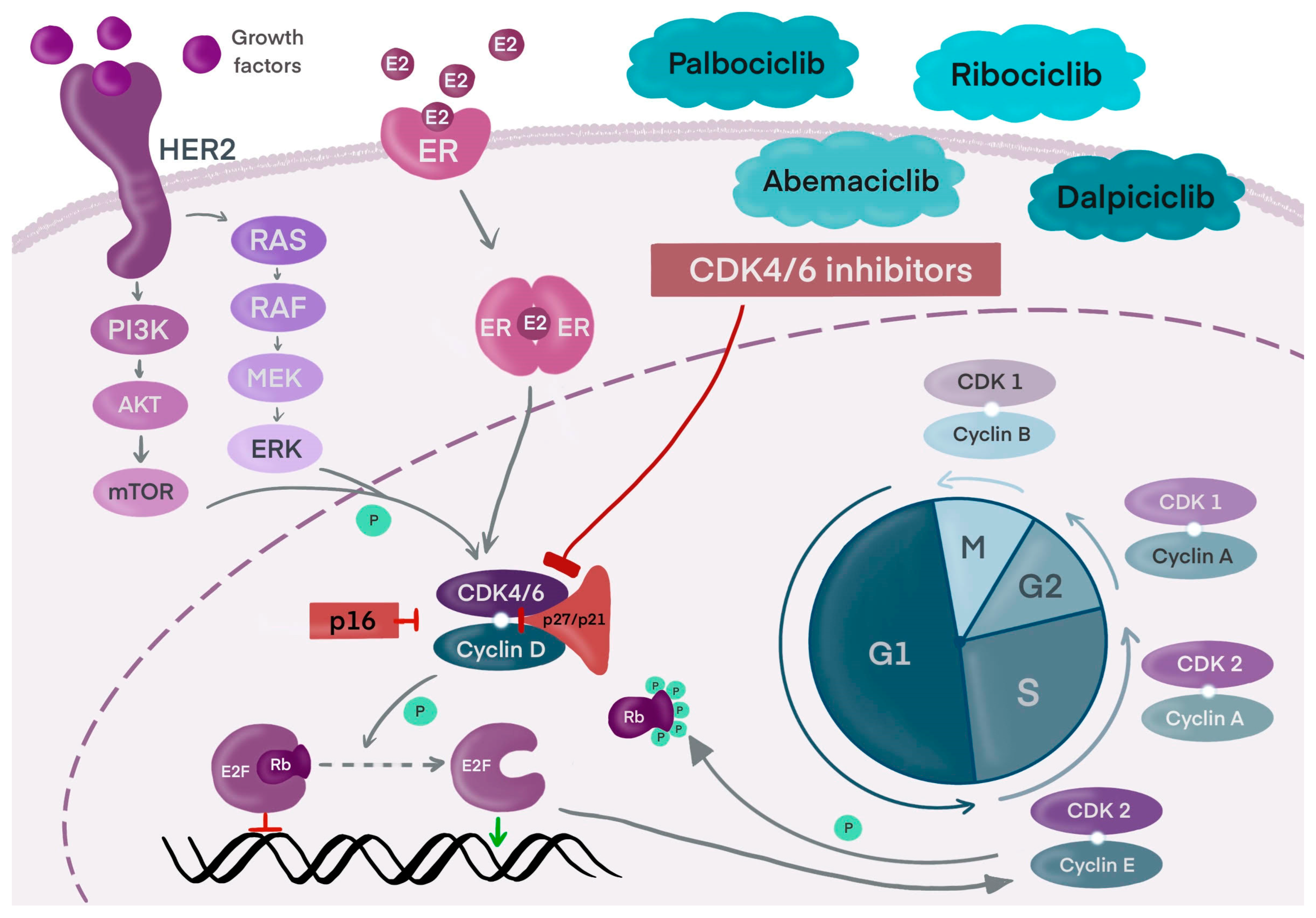
5. Cyclin-Dependent Kinase 4 and 6 (CDK4/6) Inhibitors
6. Common Terminology Criteria for Adverse Events (AEs)
7. Palbociclib
8. Ribociclib
9. Abemaciclib
10. Dalpiciclib
11. Continuation of CDK4/6 Inhibitors in Second Line After Prior Exposure
12. Recommendations for CDK4/6 Inhibitor Treatments
13. CDK4/6 Inhibitors in HER-Positive Tumors
14. Raising Concerns and Cost-Effectiveness of CDK4/6 Inhibitors
15. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990–2016: Evidence from Global Burden of Disease Study 2016. Breast Cancer 2019, 26, 428–445. [Google Scholar] [CrossRef]
- Female Breast Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 14 December 2024).
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Gąsior, Z.; Ekiert, M.; Gisterek, I.; Ignatowicz-Pacyna, A.; Jeleń, M.; Łacko, A.; Matkowski, R.; Nienartowicz, E.; Soter, K.; Szewczyk, K.; et al. Rak Piersi; Centrum Medyczne Kształcenia Podyplomowego: Warszawa, Poland, 2011. [Google Scholar]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary Patterns and Breast Cancer Risk: A Systematic Review. Anticancer Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- Krishnamurti, U.; Silverman, J.F. HER2 in breast cancer: A review and update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; pp. 31–42. [Google Scholar]
- Jerzak, K.J.; Bouganim, N.; Brezden-Masley, C.; Edwards, S.; Gelmon, K.; Henning, J.W.; Hilton, J.F.; Sehdev, S. HR+/HER2− Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review. Curr. Oncol. 2023, 30, 5425–5447. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Q.; Sun, H.; Xie, Y.; Patel, H.; Bo, L.; Lin, H.; Chen, Z.S. Therapeutic evolution in HR+/HER2− breast cancer: From targeted therapy to endocrine therapy. Front. Pharmacol. 2024, 15, 1340764. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wang, X. The Metabolic Mechanisms of Breast Cancer Metastasis. Front. Oncol. 2020, 10, 602416. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Pelicano, H.; Zhang, W.; Liu, J.; Hammoudi, N.; Dai, J.; Xu, R.H.; Pusztai, L.; Huang, P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: Role of mTOR pathway and therapeutic potential. Breast Cancer Res. 2014, 16, 434. [Google Scholar] [CrossRef]
- Lei, P.; Wang, W.; Sheldon, M.; Sun, Y.; Yao, F.; Ma, L. Role of Glucose Metabolic Reprogramming in Breast Cancer Progression and Drug Resistance. Cancers 2023, 15, 3390. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Escoté, X.; Fajas, L. Metabolic adaptation to cancer growth: From the cell to the organism. Cancer Lett. 2015, 356, 171–175. [Google Scholar] [CrossRef]
- Łukasik, P.; Baranowska-Bosiacka, I.; Kulczycka, K.; Gutowska, I. Inhibitors of Cyclin-Dependent Kinases: Types and Their Mechanism of Action. Int. J. Mol. Sci. 2021, 22, 2806. [Google Scholar] [CrossRef]
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of cdks, their cyclin activators, and cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Rollins, B.J. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 2004, 117, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Valius, M. The CDK inhibitors in cancer research and therapy. J. Cancer Res. Clin. Oncol. 2011, 137, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770.e754. [Google Scholar] [CrossRef] [PubMed]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, eabc1495. [Google Scholar] [CrossRef]
- Watt, A.C.; Goel, S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022, 24, 17. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK Inhibitors: Cell Cycle Regulators and Beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef]
- Thu, K.L.; Soria-Bretones, I.; Mak, T.W.; Cescon, D.W. Targeting the cell cycle in breast cancer: Towards the next phase. Cell Cycle 2018, 17, 1871–1885. [Google Scholar] [CrossRef]
- Ishida, T.; Ciulli, A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discov. 2021, 26, 484–502. [Google Scholar] [CrossRef]
- Weinberg, R.A. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Brigham; Women’s Hospital. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; Wang, L.; Liu, Y.; Knapp, S.; Liu, Q.; Gray, N.S. Exploration of type II binding mode: A privileged approach for kinase inhibitor focused drug discovery? ACS Chem. Biol. 2014, 9, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, I.M.; Parosanu, A.I.; Nitipir, C. An Overview of the Safety Profile and Clinical Impact of CDK4/6 Inhibitors in Breast Cancer-A Systematic Review of Randomized Phase II and III Clinical Trials. Biomolecules 2023, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.G.; Hillman, S.; Storrick, E.; Mahoney, M.R.; Thall, P.F.; Jatoi, A.; Mandrekar, S.J. Adverse Event Burden Score-A Versatile Summary Measure for Cancer Clinical Trials. Cancers 2020, 12, 3251. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 14 December 2024).
- Palbociclib (IBRANCE). Available online: https://www.ibrance.com/about-ibrance#trial-results (accessed on 14 December 2024).
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Study of Letrozole with or Without Palbociclib (PD-0332991) for the First-Line Treatment of Hormone-Receptor Positive Advanced Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT00721409?cond=NCT00721409&rank=1 (accessed on 14 December 2024).
- Das Majumdar, S.K.; Barik, S.K.; Pattanaik, A.; Das, D.K.; Parida, D.K. Role of Cyclin-Dependent Kinase 4/6 in Metastatic Breast Cancer: Real-World Data From a Tertiary Care Institute in Eastern India. Cureus 2024, 16, e52172. [Google Scholar] [CrossRef]
- A Study of Palbociclib (PD-0332991) + Letrozole vs. Letrozole for 1st Line Treatment of Postmenopausal Women with ER+/HER2− Advanced Breast Cancer (PALOMA-2). Available online: https://clinicaltrials.gov/study/NCT01740427?cond=NCT01740427&rank=1 (accessed on 14 December 2024).
- Finn, R.S.; Boer, K.; Bondarenko, I.; Patel, R.; Pinter, T.; Schmidt, M.; Shparyk, Y.V.; Thummala, A.; Voitko, N.; Bananis, E.; et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 2020, 183, 419–428. [Google Scholar] [CrossRef]
- Slamon, D.J.; Diéras, V.; Rugo, H.S.; Harbeck, N.; Im, S.A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival with Palbociclib Plus Letrozole in Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 994–1000. [Google Scholar] [CrossRef]
- Finn Richard, S.; Martin, M.; Rugo Hope, S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov Oleg, N.; Walshe Janice, M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Palbociclib (PD-0332991) Combined with Fulvestrant in Hormone Receptor+ HER2-Negative Metastatic Breast Cancer After Endocrine Failure (PALOMA-3). Available online: https://clinicaltrials.gov/study/NCT01942135 (accessed on 14 December 2024).
- Anonymous. Palbociclib Plus Fulvestrant Maintains Long-Term Overall Survival Benefit in HR+/HER2− Advanced Breast Cancer. Oncologist 2021, 26 (Suppl. S3), S5–S6. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Rugo, H.S.; Im, S.A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2− ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Leone, J.P. Male Breast Cancer: An Updated Review of Epidemiology, Clinicopathology, and Treatment. J. Oncol. 2022, 2022, 1734049. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.L.; DiCristo, C.; Gordon, D.; Karuturi, M.S.; Oubre, D.; Jepsen, E.; Cuevas, J.; Lakhanpal, S.; Montelongo, M.Z.; Zhang, Z.; et al. Outcomes of male patients with HR+/HER2− advanced breast cancer receiving palbociclib in the real-world POLARIS study. Breast Cancer Res. Treat. 2024, 203, 463–475. [Google Scholar] [CrossRef] [PubMed]
- PALbociclib CoLlaborative Adjuvant Study (PALLAS). Available online: https://clinicaltrials.gov/study/NCT02513394?cond=NCT02513394&rank=1 (accessed on 14 December 2024).
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef]
- Albanell, J.; Martínez, M.T.; Ramos, M.; O’Connor, M.; de la Cruz-Merino, L.; Santaballa, A.; Martínez-Jañez, N.; Moreno, F.; Fernández, I.; Alarcón, J.; et al. Randomized phase II study of fulvestrant plus palbociclib or placebo in endocrine-sensitive, hormone receptor-positive/HER2-advanced breast cancer: GEICAM/2014-12 (FLIPPER). Eur. J. Cancer 2022, 161, 26–37. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil-Gil, M.; Ruíz-Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Fulvestrant-Palbociclib vs Letrozole-Palbociclib as Initial Therapy for Endocrine-Sensitive, Hormone Receptor-Positive, ERBB2-Negative Advanced Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1791–1799. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil Gil, M.J.; Ruiz Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Palbociclib with Fulvestrant or Letrozole in Endocrine-Sensitive Patients with HR-Positive/HER2-Negative Advanced Breast Cancer: A Detailed Safety Analysis of the Randomized PARSIFAL Trial. Oncologist 2023, 28, 23–32. [Google Scholar] [CrossRef]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.B.; Bear, H.; McCarthy, N.; Melé Olivé, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- A Study of Palbociclib in Addition to Standard Endocrine Treatment in Hormone Receptor Positive Her2 Normal Patients with Residual Disease After Neoadjuvant Chemotherapy and Surgery (PENELOPE-B). Available online: https://clinicaltrials.gov/study/NCT01864746 (accessed on 14 December 2024).
- Marmé, F.; Martin, M.; Untch, M.; Thode, C.; Bonnefoi, H.; Kim, S.B.; Bear, H.; Mc Carthy, N.; Gelmon, K.; García-Sáenz, J.A.; et al. Palbociclib combined with endocrine treatment in hormone receptor-positive, HER2-negative breast cancer patients with high relapse risk after neoadjuvant chemotherapy: Subgroup analyses of premenopausal patients in PENELOPE-B. ESMO Open 2024, 9, 103466. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.K.; van Breeschoten, J.; Wouters, M.; van Dartel, M.; van der Flier, S.; Reyners, A.K.L.; de Graeff, P.; Pasmooij, A.M.G.; de Boer, A.; Broekman, K.E.; et al. Palbociclib dose reductions and the effect on clinical outcomes in patients with advanced breast cancer. Breast 2021, 60, 263–271. [Google Scholar] [CrossRef]
- Phase III Palbociclib with Endocrine Therapy vs. Capecitabine in HR+/HER2− MBC with Resistance to Aromatase Inhibitors (PEARL). Available online: https://clinicaltrials.gov/study/NCT02028507 (accessed on 14 December 2024).
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial—PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margelí, M.; Csöszi, T.; Antón, A.; et al. Overall survival with palbociclib plus endocrine therapy versus capecitabine in postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer in the PEARL study. Eur. J. Cancer 2022, 168, 12–24. [Google Scholar] [CrossRef]
- Sobhani, N.; D’Angelo, A.; Pittacolo, M.; Roviello, G.; Miccoli, A.; Corona, S.P.; Bernocchi, O.; Generali, D.; Otto, T. Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer. Cells 2019, 8, 321. [Google Scholar] [CrossRef]
- Study of AZD2014 and Palbociclib in Patients with Estrogen Receptor Positive (ER+) Metastatic Breast Cancer (PASTOR). Available online: https://clinicaltrials.gov/study/NCT02599714 (accessed on 14 December 2024).
- KISQALI (Ribociclib) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209092Orig1s000TOC.cfm (accessed on 14 December 2024).
- A Pharmacodynamics Pre-Surgical Study of LEE011 in Early Breast Cancer Patients (MONALEESA-1). Available online: https://clinicaltrials.gov/study/NCT01919229?cond=NCT01919229&rank=1 (accessed on 14 December 2024).
- Study of Efficacy and Safety of LEE011 in Postmenopausal Women with Advanced Breast Cancer. (MONALEESA-2). Available online: https://clinicaltrials.gov/study/NCT01958021?cond=NCT01958021&rank=1 (accessed on 14 December 2024).
- Study of Efficacy and Safety of LEE011 in Men and Postmenopausal Women with Advanced Breast Cancer. (MONALEESA-3). Available online: https://clinicaltrials.gov/study/NCT02422615?cond=NCT02422615&rank=1 (accessed on 14 December 2024).
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martín, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Eggersmann, T.K.; Degenhardt, T.; Gluz, O.; Wuerstlein, R.; Harbeck, N. CDK4/6 Inhibitors Expand the Therapeutic Options in Breast Cancer: Palbociclib, Ribociclib and Abemaciclib. BioDrugs 2019, 33, 125–135. [Google Scholar] [CrossRef]
- Spring, L.M.; Wander, S.A.; Andre, F.; Moy, B.; Turner, N.C.; Bardia, A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future. Lancet 2020, 395, 817–827. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Study of Efficacy and Safety in Premenopausal Women with Hormone Receptor Positive, HER2-Negative Advanced Breast Cancer (MONALEESA-7). Available online: https://clinicaltrials.gov/study/NCT02278120 (accessed on 14 December 2024).
- Lu, Y.S.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre- and Perimenopausal Patients with HR+/HER2− Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial. Clin. Cancer Res. 2022, 28, 851–859. [Google Scholar] [CrossRef]
- Yardley, D.A. MONALEESA clinical program: A review of ribociclib use in different clinical settings. Future Oncol. 2019, 15, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Chan, A.; Bardia, A.; Thaddeus Beck, J.; Sohn, J.; Neven, P.; Tripathy, D.; Im, S.A.; Chia, S.; Esteva, F.J.; et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br. J. Cancer 2021, 125, 679–686. [Google Scholar] [CrossRef]
- Study to Assess the Safety and Efficacy of Ribociclib (LEE011) in Combination with Letrozole for the Treatment of Men and Pre/Postmenopausal Women with HR+ HER2− aBC (COMPLEEMENT-1). Available online: https://clinicaltrials.gov/study/NCT02941926?cond=NCT02941926&rank=1 (accessed on 14 December 2024).
- De Laurentiis, M.; Borstnar, S.; Campone, M.; Warner, E.; Bofill, J.S.; Jacot, W.; Dent, S.; Martin, M.; Ring, A.; Cottu, P.; et al. Full population results from the core phase of CompLEEment-1, a phase 3b study of ribociclib plus letrozole as first-line therapy for advanced breast cancer in an expanded population. Breast Cancer Res. Treat. 2021, 189, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; De Laurentiis, M.; Zamagni, C.; Kudryavcev, I.; Agterof, M.; Brown-Glaberman, U.; Palácová, M.; Chatterjee, S.; Menon-Singh, L.; Wu, J.; et al. Ribociclib plus letrozole in male patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Subgroup analysis of the phase IIIb CompLEEment-1 trial. Breast Cancer Res. Treat. 2022, 193, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Adjuvant Ribociclib with Endocrine Therapy in Hormone Receptor+/HER2− High Risk Early Breast Cancer (EarLEE-1). Available online: https://clinicaltrials.gov/study/NCT03078751?cond=NCT03078751&rank=1 (accessed on 14 December 2024).
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.-S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N. Engl. J. Med. 2024, 390, 1080–1091. [Google Scholar] [CrossRef]
- Untch, M.; Yardley, D.; Im, S.A.; Pluard, T.; Hart, L.; Crown, J.P.; Freyer, G.; Zamagni, C.; López-Barajas, I.B.; Parnizari, F.; et al. 240P Efficacy and safety of ribociclib (RIB) + nonsteroidal aromatase inhibitor (NSAI) in older patients (pts) with HR+/HER2− early breast cancer (EBC) in NATALEE. Ann. Oncol. 2024, 35, S313–S314. [Google Scholar] [CrossRef]
- George, M.A.; Qureshi, S.; Omene, C.; Toppmeyer, D.L.; Ganesan, S. Clinical and Pharmacologic Differences of CDK4/6 Inhibitors in Breast Cancer. Front. Oncol. 2021, 11, 693104. [Google Scholar] [CrossRef]
- Tripathy, D.; Bardia, A.; Sellers, W.R. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin. Cancer Res. 2017, 23, 3251–3262. [Google Scholar] [CrossRef]
- Portman, N.; Alexandrou, S.; Carson, E.; Wang, S.; Lim, E.; Caldon, C.E. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr.-Relat. Cancer 2019, 26, R15–R30. [Google Scholar] [CrossRef]
- FDA Approves Abemaciclib for HR-Positive, HER2-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (accessed on 14 December 2024).
- A Study of Abemaciclib (LY2835219) in Participants with Previously Treated Breast Cancer That Has Spread (MONARCH 1). Available online: https://clinicaltrials.gov/study/NCT02102490?cond=MONARCH-1&rank=1 (accessed on 14 December 2024).
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(−) Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- A Neoadjuvant Study of Abemaciclib (LY2835219) in Postmenopausal Women with Hormone Receptor Positive, HER2 Negative Breast Cancer (neoMONARCH). Available online: https://clinicaltrials.gov/study/NCT02441946?cond=neoMONARCH&rank=1 (accessed on 14 December 2024).
- Hurvitz, S.A.; Martin, M.; Press, M.F.; Chan, D.; Fernandez-Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.S.; Barriga, S.; et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR(+)/HER2(−) Breast Cancer. Clin. Cancer Res. 2020, 26, 566–580. [Google Scholar] [CrossRef]
- A Study of Abemaciclib (LY2835219) Combined with Fulvestrant in Women with Hormone Receptor Positive HER2 Negative Breast Cancer (MONARCH 2). Available online: https://clinicaltrials.gov/study/NCT02107703 (accessed on 14 December 2024).
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Trédan, O.; Park, I.H.; Campone, M.; Chen, S.C.; Manso, L.M.; Paluch-Shimon, S.; et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2− advanced breast cancer: Final overall survival results of MONARCH 3. Ann. Oncol. 2024, 35, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- A Study of Nonsteroidal Aromatase Inhibitors Plus Abemaciclib (LY2835219) in Postmenopausal Women with Breast Cancer (MONARCH 3). Available online: https://clinicaltrials.gov/study/NCT02246621 (accessed on 14 December 2024).
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Endocrine Therapy with or Without Abemaciclib (LY2835219) Following Surgery in Participants with Breast Cancer (monarchE). Available online: https://clinicaltrials.gov/study/NCT03155997?cond=NCT03155997&rank=1 (accessed on 14 December 2024).
- Rastogi, P.; O’Shaughnessy, J.; Martin, M.; Boyle, F.; Cortes, J.; Rugo, H.S.; Goetz, M.P.; Hamilton, E.P.; Huang, C.-S.; Senkus, E.; et al. Adjuvant Abemaciclib Plus Endocrine Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative, High-Risk Early Breast Cancer: Results From a Preplanned monarchE Overall Survival Interim Analysis, Including 5-Year Efficacy Outcomes. J. Clin. Oncol. 2024, 42, 987–993. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.S.; Huober, J.; Jaliffe, G.G.; Cicin, I.; et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023, 24, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhong, J.; Wang, J.; Song, G.; Di, L.; Shao, B.; Zhang, R.; Liu, Y.; Zhu, A.; Wang, N.; et al. Abemaciclib plus endocrine therapy versus chemotherapy after progression on prior palbociclib in HR+/HER2− metastatic breast cancer: A single center real-world study in China. Cancer Med. 2024, 13, e7249. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Yuan, J.; Wang, J.; Chen, Z.; Liu, Z.; Li, Z.; Lai, Y.; Gao, J.; Shen, L. CDK4/6 inhibitor-SHR6390 exerts potent antitumor activity in esophageal squamous cell carcinoma by inhibiting phosphorylated Rb and inducing G1 cell cycle arrest. J. Transl. Med. 2017, 15, 127. [Google Scholar] [CrossRef]
- Long, F.; He, Y.; Fu, H.; Li, Y.; Bao, X.; Wang, Q.; Wang, Y.; Xie, C.; Lou, L. Preclinical characterization of SHR6390, a novel CDK 4/6 inhibitor, in vitro and in human tumor xenograft models. Cancer Sci. 2019, 110, 1420–1430. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Satti, S.A. The emerging CDK4/6 inhibitor for breast cancer treatment. Mol. Cell Pharmacol. 2021, 13, 9–12. [Google Scholar]
- Xu, B.; Zhang, Q.; Zhang, P.; Hu, X.; Li, W.; Tong, Z.; Sun, T.; Teng, Y.; Wu, X.; Ouyang, Q.; et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: A randomized, phase 3 trial. Nat. Med. 2021, 27, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Q.; Tong, Z.; Sun, T.; Li, W.; Ouyang, Q.; Hu, X.; Cheng, Y.; Yan, M.; Pan, Y.; et al. Dalpiciclib plus letrozole or anastrozole versus placebo plus letrozole or anastrozole as first-line treatment in patients with hormone receptor-positive, HER2-negative advanced breast cancer (DAWNA-2): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2023, 24, 646–657. [Google Scholar] [CrossRef]
- Giordano, A.; Lin, N.U.; Tolaney, S.M.; Mayer, E.L. Is there a role for continuation of CDK4/6 inhibition after progression on a prior CDK4/6 inhibitor in HR+/HER2− metastatic breast cancer? Ann. Oncol. 2024, 35, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; DeMichele, A.; Clark, A.S.; Zelnak, A.; Yardley, D.A.; Karuturi, M.; Sanft, T.; Blau, S.; Hart, L.; et al. Phase I/II Trial of Exemestane, Ribociclib, and Everolimus in Women with HR(+)/HER2(−) Advanced Breast Cancer after Progression on CDK4/6 Inhibitors (TRINITI-1). Clin. Cancer Res. 2021, 27, 4177–4185. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Hamilton, E.; Schiavon, G.; Grinsted, L.M.; De Bruin, E.C.; Catanese, M.T.; Rugo, H.S. 338TiP CAPItello-292: A phase 1b/3 study of capivasertib, palbociclib and fulvestrant versus placebo, palbociclib and fulvestrant in HR+/HER2− advanced breast cancer. Ann. Oncol. 2021, 32, S514. [Google Scholar] [CrossRef]
- FDA Approves Elacestrant for ER-Positive, HER2-Negative, ESR1-Mutated Advanced or Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer (accessed on 14 December 2024).
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Rugo, H.; Bardia, A.; Cortés, J.; Curigliano, G.; Hamilton, E.; Hurvitz, S.; Loibl, S.; Scartoni, S.; Sahmoud, T.; Grzegorzewski, K.; et al. Abstract OT2-01-03: ELEVATE: A phase 1b/2, open-label, umbrella study evaluating elacestrant in various combinations in women and men with metastatic breast cancer (mBC). Cancer Res. 2023, 83, OT2-01-03. [Google Scholar] [CrossRef]
- Goetz, M.P.; Bagegni, N.A.; Batist, G.; Brufsky, A.; Cristofanilli, M.A.; Damodaran, S.; Daniel, B.R.; Fleming, G.F.; Gradishar, W.J.; Graff, S.L.; et al. Lasofoxifene versus fulvestrant for ER+/HER2− metastatic breast cancer with an ESR1 mutation: Results from the randomized, phase II ELAINE 1 trial. Ann. Oncol. 2023, 34, 1141–1151. [Google Scholar] [CrossRef]
- Damodaran, S.; Moore, H.C.F.; Anderson, I.C.; Cherian, M.A.; O’Sullivan, C.C.; Plourde, P.V.; Portman, D.J.; Goetz, M.P. Lasofoxifene (LAS) plus abemaciclib (Abema) for treating ESR1-mutated ER+/HER2− metastatic breast cancer (mBC) after progression on prior therapies: ELAINE 2 study update. J. Clin. Oncol. 2023, 41, 1057. [Google Scholar] [CrossRef]
- Lainé, M.; Fanning, S.W.; Chang, Y.F.; Green, B.; Greene, M.E.; Komm, B.; Kurleto, J.D.; Phung, L.; Greene, G.L. Lasofoxifene as a potential treatment for therapy-resistant ER-positive metastatic breast cancer. Breast Cancer Res. 2021, 23, 54. [Google Scholar] [CrossRef]
- Goetz, M.P.; Wander, S.A.; Bachelot, T.; Batist, G.; Cortes, J.; Cristofanilli, M.; Curigliano, G.; de Nonneville, A.; Gal-Yam, E.N.; Jhaveri, K.L.; et al. Open-label, randomized, multicenter, phase 3, ELAINE 3 study of the efficacy and safety of lasofoxifene plus abemaciclib for treating ER+/HER2−, locally advanced or metastatic breast cancer with an ESR1 mutation. J. Clin. Oncol. 2024, 42, TPS1127. [Google Scholar] [CrossRef]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Sakach, E.; Sathe, C.; Ahn, H.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; et al. Randomized Phase II Trial of Endocrine Therapy with or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: MAINTAIN Trial. J. Clin. Oncol. 2023, 41, 4004–4013. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Bianchini, G.; Hamilton, E.; Graff, S.L.; Park, K.H.; Jeselsohn, R.; Demirci, U.; Martin, M.; Layman, R.M.; Hurvitz, S.A.; et al. Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial. J. Clin. Oncol. 2024, 43, 9. [Google Scholar] [CrossRef]
- Mayer, E.L.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.; Miller, K.D.; Jolly, T.; et al. Abstract GS3-06: GS3-06 Palbociclib After CDK4/6i and Endocrine Therapy (PACE): A Randomized Phase II Study of Fulvestrant, Palbociclib, and Avelumab for Endocrine Pre-treated ER+/HER2− Metastatic Breast Cancer. Cancer Res. 2023, 83, GS3-06. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Harper-Wynne, C.; Perello, A.; Hennequin, A.; Fernandez, A.; Colleoni, M.; Carañana, V.; Quiroga, V.; Medioni, J.; Iranzo, V.; et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[-]) advanced breast cancer (ABC): PALMIRA trial. J. Clin. Oncol. 2023, 41, 1001. [Google Scholar] [CrossRef]
- Ramos-Esquivel, A.; Ramírez-Jiménez, I.; Víquez-Jaikel, A. Continuation of CDK4/6 Inhibition and Switching of Hormonal Therapy After Progression on Prior CDK4/6 Inhibitors in HR+/HER2− Breast Cancer: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e73738. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef]
- Teomete, M.; Cabuk, D.; Korkmaz, T.; Seber, S.; Ozturk, O.F.; Aver, B.; Karaalp, A.; Basaran, G. Recommendations for cyclin-dependent kinase 4/6 inhibitor treatments in the context of co-morbidity and drug interactions (Review). Oncol. Lett. 2024, 27, 145. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Del Re, M.; Curigliano, G.; van Schaik, R.H.; Lancellotti, P.; Danesi, R. Drug-drug interactions in breast cancer patients treated with CDK4/6 inhibitors. Cancer Treat. Rev. 2019, 74, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Roncato, R.; Angelini, J.; Pani, A.; Cecchin, E.; Sartore-Bianchi, A.; Siena, S.; De Mattia, E.; Scaglione, F.; Toffoli, G. CDK4/6 Inhibitors in Breast Cancer Treatment: Potential Interactions with Drug, Gene, and Pathophysiological Conditions. Int. J. Mol. Sci. 2020, 21, 6350. [Google Scholar] [CrossRef]
- Battisti, N.M.L.; De Glas, N.; Sedrak, M.S.; Loh, K.P.; Liposits, G.; Soto-Perez-de-Celis, E.; Krok-Schoen, J.L.; Menjak, I.B.; Ring, A. Use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in older patients with ER-positive HER2-negative breast cancer: Young International Society of Geriatric Oncology review paper. Ther. Adv. Med. Oncol. 2018, 10, 1758835918809610. [Google Scholar] [CrossRef]
- Martorana, F.; Sanò, M.V.; Valerio, M.R.; Fogli, S.; Vigneri, P.; Danesi, R.; Gebbia, V. Abemaciclib pharmacology and interactions in the treatment of HR+/HER2− breast cancer: A critical review. Ther. Adv. Drug Saf. 2024, 15, 20420986231224214. [Google Scholar] [CrossRef]
- Kassem, L.; Shohdy, K.S.; Lasheen, S.; Abdel-Rahman, O.; Bachelot, T. Hematological adverse effects in breast cancer patients treated with cyclin-dependent kinase 4 and 6 inhibitors: A systematic review and meta-analysis. Breast Cancer 2018, 25, 17–27. [Google Scholar] [CrossRef]
- Pavlovic, D.; Niciforovic, D.; Papic, D.; Milojevic, K.; Markovic, M. CDK4/6 inhibitors: Basics, pros, and major cons in breast cancer treatment with specific regard to cardiotoxicity—A narrative review. Ther. Adv. Med. Oncol. 2023, 15, 17588359231205848. [Google Scholar] [CrossRef]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 Receptor and Breast Cancer: Ten Years of Targeted Anti–HER-2 Therapy and Personalized Medicine. The Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef]
- Stanowicka-Grada, M.; Senkus, E. Anti-HER2 Drugs for the Treatment of Advanced HER2 Positive Breast Cancer. Curr. Treat. Options Oncol. 2023, 24, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- A Study of Abemaciclib (LY2835219) in Women with HR+, HER2+ Locally Advanced or Metastatic Breast Cancer (monarcHER). Available online: https://clinicaltrials.gov/study/NCT02675231 (accessed on 14 December 2024).
- Tolaney, S.M.; Wardley, A.M.; Zambelli, S.; Hilton, J.F.; Troso-Sandoval, T.A.; Ricci, F.; Im, S.A.; Kim, S.B.; Johnston, S.R.; Chan, A.; et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): A randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Goel, S.; Nadal, J.; Denys, H.; Borrego, M.R.; Litchfield, L.M.; Liu, J.; Appiah, A.K.; Chen, Y.; André, F. Overall Survival and Exploratory Biomarker Analyses of Abemaciclib plus Trastuzumab with or without Fulvestrant versus Trastuzumab plus Chemotherapy in HR+, HER2+ Metastatic Breast Cancer Patients. Clin. Cancer Res. 2024, 30, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.; Villagrasa, P.; Pascual, T.; Oliveira, M.; Pernas, S.; Paré, L.; Escrivá-de-Romaní, S.; Manso, L.; Adamo, B.; Martínez, E.; et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020, 26, 5820–5829. [Google Scholar] [CrossRef]
- Randomized, Open Label, Clinical Study of the Targeted Therapy, Palbociclib, to Treat Metastatic Breast Cancer (PATINA). Available online: https://clinicaltrials.gov/study/NCT02947685 (accessed on 14 December 2024).
- Yan, M.; Niu, L.; Lv, H.; Zhang, M.; Wang, J.; Liu, Z.; Chen, X.; Lu, Z.; Zhang, C.; Zeng, H.; et al. Dalpiciclib and pyrotinib in women with HER2-positive advanced breast cancer: A single-arm phase II trial. Nat. Commun. 2023, 14, 6272. [Google Scholar] [CrossRef]
- Yan, M.; Niu, L.; Lv, H.; Zhang, M.; Liu, Z.; Chen, X.; Lu, Z.; Zhang, C.; Zeng, H.; Zhao, S.; et al. Updated results from a phase 2 study on dalpiciclib and pyrotinib, a dual-oral chemotherapy-free regimen in HER2-positive advanced breast cancer (DAP-HER-01). J. Clin. Oncol. 2023, 41, 1045. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Xin, Q.; Zhang, Y.; Wang, K.; Li, M. Recent progress of CDK4/6 inhibitors’ current practice in breast cancer. Cancer Gene Ther. 2024, 31, 283–1291. [Google Scholar] [CrossRef]
- Haslam, A.; Ranganathan, S.; Prasad, V.; Olivier, T. CDK4/6 inhibitors as adjuvant therapy in early breast cancer? Uncertain benefits, guaranteed harms. Eur. J. Cancer 2024, 207, 114192. [Google Scholar] [CrossRef]
- van Ommen-Nijhof, A.; Konings, I.R.; van Zeijl, C.J.J.; Uyl-de Groot, C.A.; van der Noort, V.; Jager, A.; Sonke, G.S. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer—The SONIA study: Study protocol for a randomized controlled trial. BMC Cancer 2018, 18, 1146. [Google Scholar] [CrossRef]
- Sonke, G.S.; Nijhof, A.V.O.; Wortelboer, N.; Noort, V.v.d.; Swinkels, A.C.P.; Blommestein, H.M.; Beeker, A.; Beelen, K.; Hamming, L.C.; Heijns, J.B.; et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC). J. Clin. Oncol. 2023, 41, LBA1000. [Google Scholar] [CrossRef]
- Masurkar, P.P.; Prajapati, P.; Canedo, J.; Goswami, S.; Earl, S.; Bhattacharya, K. Cost-effectiveness of CDK4/6 inhibitors in HR+/HER2− metastatic breast cancer: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2024, 40, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Wortelboer, N.; Kent, S.; Konings, I.R.; Van Ommen-Nijhof, A.; van der Noort, V.; van den Pol, E.; Páez, C.G.; van Bekkum, M.; Droogendijk, H.J.; Erdkamp, F.; et al. 352P Cost-effectiveness of CDK4/6 inhibitors in first- vs second-line for advanced breast cancer (ABC) in the phase III SONIA trial (BOOG 2017-03). Ann. Oncol. 2024, 35, S364. [Google Scholar] [CrossRef]
- Elazzazy, S.; Al-Ziftawi, N.H.; Mohamed Ibrahim, M.I.; Bujassoum, S.; Hamad, A. Comparative cost-effectiveness analysis of CDK4/6 inhibitors in the first-line treatment of HR-positive and HER2-negative advanced breast cancer: A Markov’s model-based evaluation. Front. Oncol. 2024, 14, 1413676. [Google Scholar] [CrossRef]
- Le, V.; Zhong, L.; Narsipur, N.; Hays, E.; Tran, D.K.; Rosario, K.; Wilson, L. Cost-effectiveness of ribociclib plus endocrine therapy versus placebo plus endocrine therapy in HR-positive, HER2-negative breast cancer. J. Manag. Care Spec. Pharm. 2021, 27, 327–338. [Google Scholar] [CrossRef]
- Zeng, N.; Han, J.; Liu, Z.; He, J.; Tian, K.; Chen, N. CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2− Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis. Cancers 2023, 15, 3386. [Google Scholar] [CrossRef]
- Reddy, S.; Barkhane, Z.; Elmadi, J.; Satish Kumar, L.; Pugalenthi, L.S.; Ahmad, M. Cyclin-Dependent Kinase 4 and 6 Inhibitors: A Quantum Leap in the Treatment of Advanced Breast Cancers. Cureus 2022, 14, e23901. [Google Scholar] [CrossRef]
| Subtype | Luminal A | Luminal B | HER2-Enriched | Basal-like/TNBC |
|---|---|---|---|---|
| IHC Phenotype | ER+ HER2− | ER+ HER2− | ER- HER2+ | ER- HER2− |
| PR ≥ 20% Ki67 < 20% | And/or PR < 20% and/or Ki67 ≥ 20% | PR- | PR- | |
| DNA mutations | TP53 (12%) PIK3CA (49%) | TP53 (32%) PIK3CA (32%) | TP53 (84%) PIK3CA (7%) | TP53 (75%) PIK3CA (42%) |
| Glucose metabolism | Lower than HER-enriched Mainly TCA cycle, reverse Warburg effect | Lower than HER-enriched Mainly TCA cycle, reverse Warburg effect | Higher than luminal A and B Mixed metabolism—glycolysis and TCA | The highest glucose metabolism activity Mainly glycolysis, Warburg effect The highest level of GLUT1 expression |
| Amino acid metabolism | Lower than in HER-enriched | Lower than in HER-enriched | The highest | The lowest |
| Lipid metabolism | Lower than in HER-enriched | Lower than in HER-enriched | The highest | The lowest |
| Glutamine metabolism | Lower than in luminal B | Higher than in luminal A | The highest | - |
| CTCAE Term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Diarrhea | <4 stools per day, Mild increase in ostomy output | 4–6 stools per day, Moderate increase in ostomy output, limiting instrumental ADL | ≥7 stools per day, Severe increase in ostomy output, limiting self-care ADL | Life-threatening consequences, urgent intervention indicated | Death |
| Neutropenia | <LLN—1500/mm3; <LLN—1.5 × 10−9/L | <1500–1000/mm3; <1.5–1.0 × 10−9/L | <1000–500/mm3; <1.0–0.5 × 10−9/L | <500/mm3; <0.5 × 10−9/L | - |
| Leukopenia | <LLN—3000/mm3; <LLN—3.0 × 10−9/L | <3000–2000/mm3; <3.0–2.0 × 10−9/L | <2000–1000 /mm3; <2.0–1.0 × 10−9 /L | <1000/mm3; <1.0 × 10−9/L | - |
| Thrombocytopenia | <LLN—75,000/mm3; <LLN—75.0 × 10−9/L | <75,000–50,000/mm3; <75.0–50.0 × 10−9/L | <50,000–25,000 /mm3; <50.0–25.0 × 10−9 /L | <25,000/mm3; <25.0 × 10−9/L | - |
| Anemia | Hgb <LLN—10.0 g/dL; <LLN—6.2 mmol/L; <LLN—100 g/L | Hgb <10.0–8.0 g/dL; <6.2–4.9 mmol/L; <100–80 g/L | Hgb <8.0 g/dL; <4.9 mmol/L; <80 g/L; transfusion indicated | Life-threatening consequences, urgent intervention indicated | Death |
| Stage of Breast Cancer, Type of Therapy (Adjuvant/Neoadjuvant) | Drug | Study | Phase | Population and Menopausal Status | Number of Participants | Primary or Secondary Endpoints 1. With Inhibitor 2. Without Inhibitor | Most Common AEs |
|---|---|---|---|---|---|---|---|
| EBC Adjuvant | PALBOCICLIB | PALLAS (NCT02513394) | III | ER+/HER2− EBC | 5796 | iDFS (4 years) 1. 84.2% 2. 84.5% | Neutropenia, leukopenia, fatigue, anemia, alopecia, upper respiratory tract infection |
| EBC Adjuvant | PALBOCICLIB | PENELOPE-B (NCT01864746) | III | HR +/HER2− normal primary BC but high relapse risk after neoadjuvant chemotherapy | 1250 | iDFS (3 years) 1. 81.2% 2. 77.7% | Leukopenia, neutropenia, anemia, thrombocytopenia, febrile neutropenia, fatigue, nausea, stomatitis |
| EBC | RIBOCICLIB | MONALEESA-1 (NCT01919229) | II | HR+/HER2− EBC postmenopausal | 14 | Mean decrease in Ki67-expressing cells, 1. 96% or 92%, 2. 69% | Nausea, decreased appetite, diarrhea, abdominal pain, fatigue, asthenia |
| EBC Adjuvant | RIBOCICLIB | EarLEE-1 (NCT03078751) | II | HR+/HER2− high risk EBC | 54 | % of AEs and SAEs 1. Aes—96.2%, SAEs—15.4% 2. AEs—87.5%, SAEs—8.3% | Neutropenia, anemia, thrombocytopenia, diarrhea, nausea, fatigue, increased ALT, increased AST SAEs: disseminated intravascular coagulation, AML, pulmonary embolism, cellulitis, cardiac failure congestive |
| EBC Adjuvant | RIBOCICLIB | NATALEE (NCT03701334) Data cutoff 11 January 2023 | III | HR+/HER2− EBC | 5101 | 3-y iDFS 1. 90.4% 2. 87.1% | Neutropenia, arthralgia, fatigue, nausea |
| EBC Adjuvant | ABEMACICLIB | MonarchE (NCT03155997) | III | HR+/HER2− High-risk, node-positive, early stage surgery of the primary | 5637 | 4-y iDFS 1. 85.8% 2. 79.4% | Leukopenia, neutropenia, anemia, thrombocytopenia, fatigue, nausea, abdominal pain |
| EBC Neoadjuvant | ABEMACICLIB | NeoMONARCH (NCT02441946) | II | HR+/HER2− EBC postmenopausal | 224 | Percent change in Ki67 expression (from baseline to 2 weeks) 1. Ab + An (−93%) 2. Ab (−91%) 3. An (−63%) | Diarrhea, nausea, fatigue, constipation |
| ABC/MBC | PALBOCICLIB | PALOMA-1/TRIO-18 (NCT00721409) | II | ER+/HER2− ABC postmenopausal | 177 | PFS 1. 20.2 months 2. 10.2 months | Neutropenia, leukopenia, fatigue anemia, nausea, backpain |
| ABC/MBC | PALBOCICLIB | PALOMA-2 (NCT01740427) | III | ER+/HER2− ABC postmenopausal | 666 | PFS 1. 19.3 months 2. 12.9 months | Neutropenia, leukopenia, nausea, fatigue, arthralgia, alopecia |
| ABC/MBC | PALBOCICLIB | PASTOR (NCT02599714) | I | ER+/HER2− locally advanced or MBC prior hormonal therapy postmenopausal | 54 | Number of AEs—parts A and B PFS—part B | No results posted |
| ABC/MBC | PALBOCICLIB | PARFISAL (NCT02491983) | II | ER+/HER2− locally advanced or MBC | 486 | PFS (median follow-up of 32 months) 1. palbociclib + letrozole—32.8 months 2. palbociclib + fulvestrant—27.9 months | Neutropenia, leukopenia, anemia, asthenia, arthralgia, fatigue, diarrhea |
| ABC/MBC | RIBOCICLIB | MONALEESA-2 (NCT01958021) | III | HR+/HER2− MBC without prior therapy postmenopausal | 668 | PFS 1. 19.3 months 2. 14.7 months | Neutropenia, leukopenia, nausea, fatigue, diarrhea, alopecia, increased ALT, increased AST |
| ABC/MBC | RIBOCICLIB | MONALEESA-3 (NCT02422615) | III | HR+/HER2− ABC without or one line prior endocrine therapy postmenopausal | 726 | PFS 1. 20.5 months 2. 12.8 months OS (data cut-off—30 October 2020) 1. 53.7 months 2. 41.5 months | Neutropenia, leukopenia, nausea, fatigue, diarrhea, alopecia, rash, arthralgia, anemia, increased ALT, increased AST |
| ABC/MBC | RIBOCICLIB | MONALEESA-7 (NCT02278120) | III | ER+/HER2− advanced MBC premenopausal or perimenopausal | 672 | PFS 1. 23.8 months 2. 13.0 months | Neutropenia, leukopenia, increased ALT, increased AST, anemia, nausea, diarrhea, hypertension |
| ABC/MBC | ABEMACICLIB | MONARCH-1 (NCT02102490) | II | HR+/HER2− MBC or ABC prior treatment with at least two chemotherapy regimens | 132 | PFS 6.0 months (95% confidence interval (CI) 4.2 to 7.5) | Diarrhea, fatigue, nausea, decreased appetite, abdominal pain, thrombocytopenia, neutropenia, |
| ABC/MBC | ABEMACICLIB | MONARCH-3 (NCT02246621) | III | HR+/HER2− MBC, recurrent or locoregionally postmenopausal | 493 | PFS 1. 29.0 months 2. 14.8 months Final mOS 1. 66.8 months 2. 53.7 months | Neutropenia, diarrhea, nausea, fatigue, infections |
| ABC/MBC No prior systemic therapy | DALPICICLIB | DAWNA-2 (NCT03966898) Data cutoff 1 June 2022 | III | HR+/HER2− MBC or recurrent BC | 426 | PFS 1. 30.6 months 2. 18.2 months | Neutropenia, leukopenia |
| ABC/MBC prior endocrine therapy | PALBOCICLIB | PALOMA-3 (NCT01942135) | III | HR+/HER2− MBC prior endocrine therapy | 521 | PFS 1. 9.2 months 2. 3.8 months OS 1. 34.8 months 2. 28.0 months | Neutropenia, leukopenia, fatigue, nausea, headache, alopecia |
| ABC/MBC prior endocrine therapy | ABEMACICLIB | MONARCH-2 (NCT02107703) | III | HR+/HER2− MBC or local ABC prior endocrine therapy | 669 | PFS 1. 16.4 months 2. 9.3 months | Neutropenia, diarrhea, nausea, fatigue, abdominal pain |
| ABC/MBC prior endocrine therapy | DALPICICLIB | DAWNA-1 (NCT03927456) | III | HR+/HER2− MBC or recurrent BC prior endocrine therapy | 361 | PFS 1. 15.7 months 2. 7.2 months | Neutropenia, leukopenia, thrombocytopenia, prolonged OT, liver enzyme abnormalities |
| ABC/MBC CDK4/6 inhibitor resistance | RIBOCICLIB | TRINITI-1 (NCT02732119) | I/II | HR+/HER2− locally advanced or MBC Progression on a CDK4/6 inhibitor Group 1: 300 mg ribociclib + 2.5 mg everolimus +25 mg exemestane Group 2: 200 mg ribociclib + 5 mg everolimus + 25 mg exemestane | 104 | CBR—phase II 1. 65.2% 2. 59.4% | Neutropenia, anemia, thrombocytopenia, stomatitis, diarrhea |
| Special populations | PALBOCICLIB | PEARL (NCT02028507) | III | HR+/HER2 MBC resistance to aromatase inhibitors postmenopausal | 693 | PFS 1. 7.4 months 2. 9.4 months OS 1. 32.6 months 2. 30.9 months | Neutropenia, leukopenia, hand/foot syndrome, diarrhea, fatigue |
| Special populations Neoadjuvant | PALBOCICLIB | NeoPalAna (NCT01723774) | II | ER+/HER2− stage 2 or 3 Arm 1—PIK3CA wild type Arm 2—PIK3CA mutant type Arm 3—endocrine resistant | 50 | Ki67 ≤ 2.7% (complete cell arrest) following 2 weeks 1. 79.3% 2. 100% 3. 57.6% | Neutropenia, leukopenia, thrombocytopenia, anemia, nausea, headache, arthralgia, diarrhea |
| Special populations | PALBOCICLIB | FLIPPER (NCT02690480) | II | HR+/HER2− MBC Postmenopausal de novo metastatic disease or prior ET ≥ 5 years and remained disease free for >12 months | 189 | PFS 1. 31.8 months 2. 22.0 months 1-y PFS 1. 83.5% 2. 71.9% | Neutropenia, anemia, leukopenia, thrombocytopenia, fatigue, diarrhea |
| Special populations | RIBOCICLIB | PATINA (NCT02947685) | III | HR+/HER2+ MBC | 496 | PFS | No results posted |
| Palbociclib | Ribociclib | Abemaciclib | |
|---|---|---|---|
| Hematologic toxicity, especially neutropenia | +++ | ++ | + |
| Gastrointestinal toxicity, including diarrhea | + | + | +++ |
| Cardiotoxicity, including QT prolongation | − | ++ | − |
| Trial | Phase | Patients | Number of Patients | Treatment | Results | Adverse Events |
|---|---|---|---|---|---|---|
| DAP-HER-01 (NCT04293276) | II | HER2+ ABC ≤1 prior endocrine therapy | 41 | Dalpiciclib + pyrotinib | mPFS in evaluable patients: 11.0 months | Leukopenia, neutropenia, diarrhea |
| DAP-HER-02 (NCT05328440) | II | HR+/HER2+ ABC | 120 | Dalpiciclib + pyrotinib + fulvestrant/inetetamab | PFS | No results posted |
| MonarcHER (NCT02675231) | II | HR+/HER2+ locally advanced or MBC ≥2 prior HER2-directed therapies | 237 | Arm A abemaciclib + trastuzumab + fulvestrant Arm B abemaciclib + trastuzumab Arm C trastuzumab + SOC therapy | PFS 1. 8.3 months 2. 5.7 months 3. 5.7 months | Neutropenia, diarrhea, anemia, nausea, fatigue, abdominal pain |
| PATINA (NCT02947685) | III | HR+/HER2+ MBC | 496 | Palbociclib + trastuzumab/pertuzumab + endocrine therapy | PFS | No results posted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, K.; Liszcz-Tymoszuk, A.; Czerw, N.; Czerw, A.; Sygit, K.; Kozłowski, R.; Deptała, A.; Badowska-Kozakiewicz, A. CDK4/6 as a Therapeutic Target in HR+/HER2− Breast Cancer Cells—Current Treatment Status. Cancers 2025, 17, 1039. https://doi.org/10.3390/cancers17061039
Krupa K, Liszcz-Tymoszuk A, Czerw N, Czerw A, Sygit K, Kozłowski R, Deptała A, Badowska-Kozakiewicz A. CDK4/6 as a Therapeutic Target in HR+/HER2− Breast Cancer Cells—Current Treatment Status. Cancers. 2025; 17(6):1039. https://doi.org/10.3390/cancers17061039
Chicago/Turabian StyleKrupa, Kamila, Anna Liszcz-Tymoszuk, Natalia Czerw, Aleksandra Czerw, Katarzyna Sygit, Remigiusz Kozłowski, Andrzej Deptała, and Anna Badowska-Kozakiewicz. 2025. "CDK4/6 as a Therapeutic Target in HR+/HER2− Breast Cancer Cells—Current Treatment Status" Cancers 17, no. 6: 1039. https://doi.org/10.3390/cancers17061039
APA StyleKrupa, K., Liszcz-Tymoszuk, A., Czerw, N., Czerw, A., Sygit, K., Kozłowski, R., Deptała, A., & Badowska-Kozakiewicz, A. (2025). CDK4/6 as a Therapeutic Target in HR+/HER2− Breast Cancer Cells—Current Treatment Status. Cancers, 17(6), 1039. https://doi.org/10.3390/cancers17061039







