Predicting Pathogenic Variants of Breast Cancer Using Ultrasound-Derived Machine Learning Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Patient Selection
2.3. Ultrasound Imaging and Data Acquisition
2.4. Genetics Analysis
2.5. Radiomics Feature Extraction
2.6. Feature Selection, Statistical Analysis, and Model Development
3. Results
3.1. Population Characteristics
3.2. Building the Prediction Model—Training Set
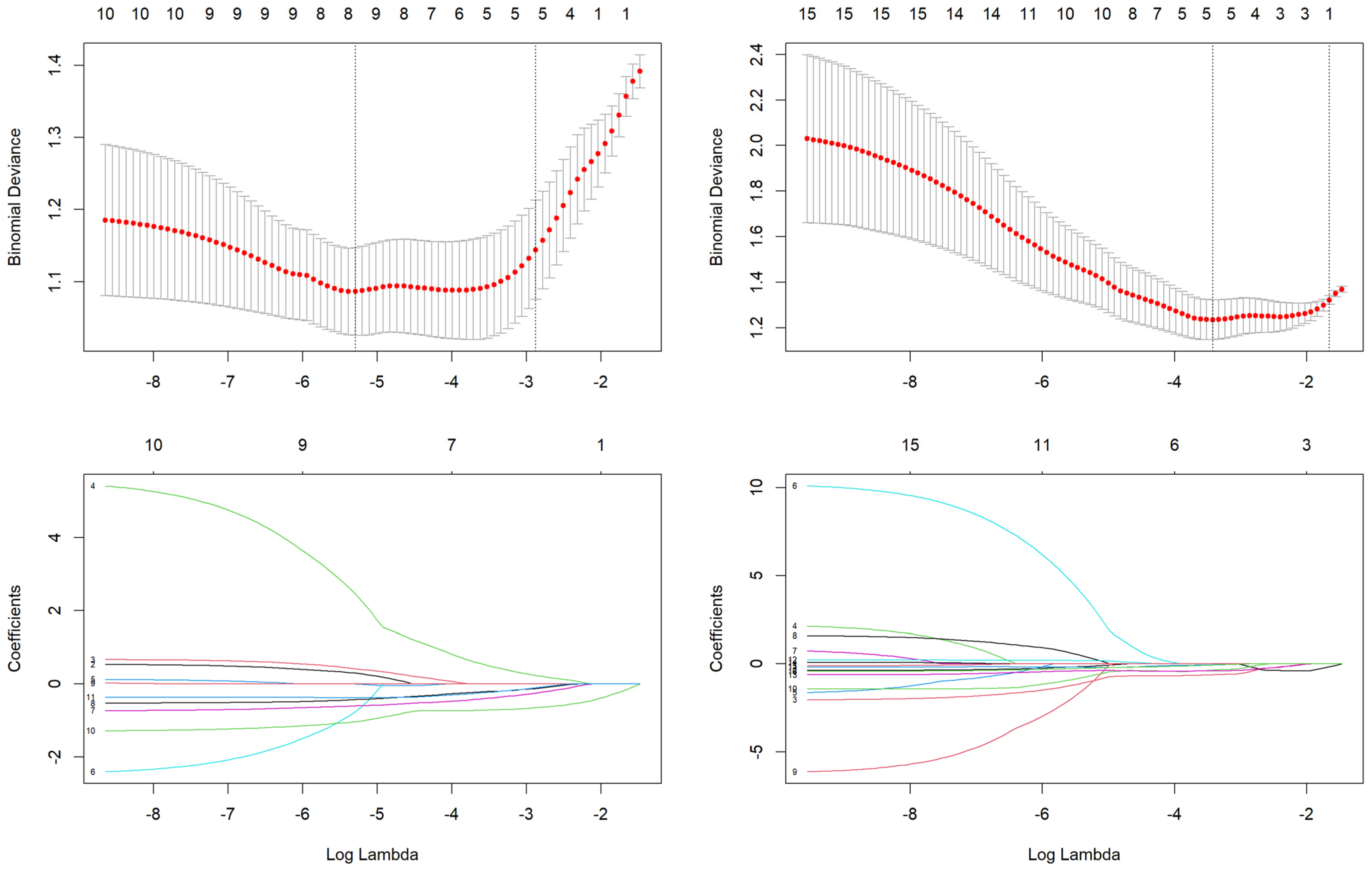
3.2.1. Feature Selection
3.2.2. Model Construction and Radiomic Score Calculation
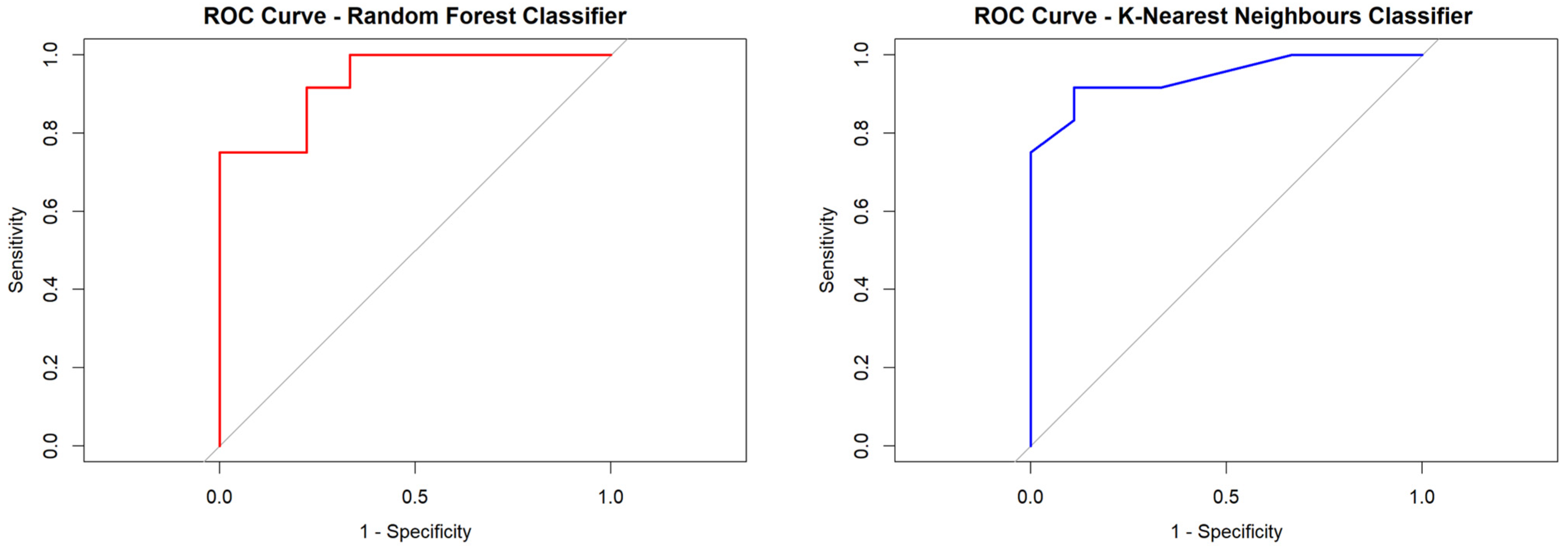
3.2.3. Performance of the Ki67% Proliferation Index and Radiomic Score Derived from Tumor-Only Data (Rad-Score 1)
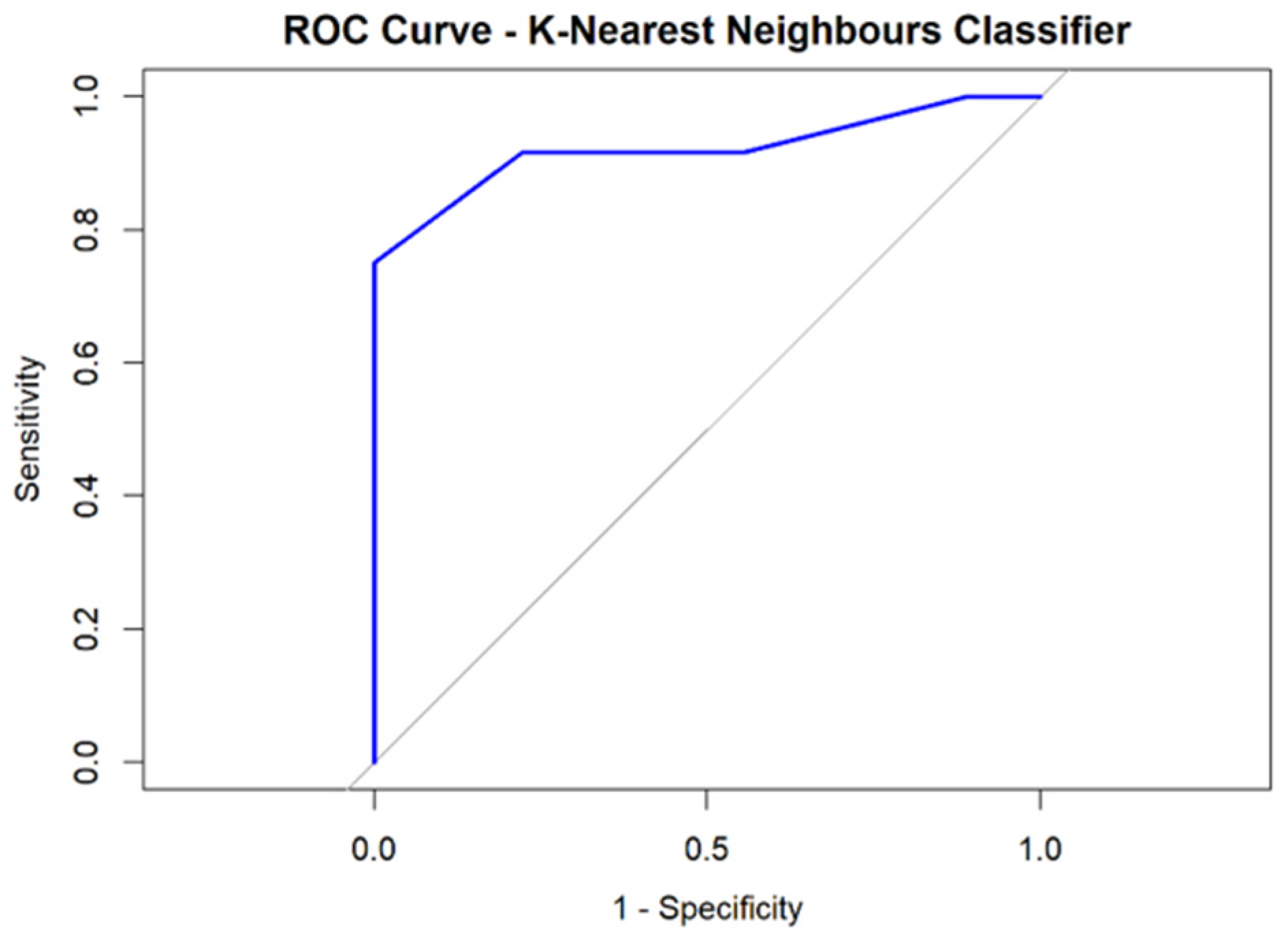
3.2.4. Performance of the Ki67% Proliferation Index and Radiomic Score Derived from Tumor and Peritumoral Data (Rad-Score 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. European Cancer Information System Breast Cancer in the E.U.; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- The International Agency for Research on Cancer (IARC). Global Cancer Observatory. 2022. Available online: https://gco.iarc.fr/en (accessed on 25 May 2024).
- Filippini, S.E.; Vega, A. Breast cancer genes: Beyond BRCA1 and BRCA2. Front. Biosci. (Landmark Ed.) 2013, 18, 1358–1372. [Google Scholar] [CrossRef] [PubMed]
- Turchiano, A.; Piglionica, M.; Martino, S.; Bagnulo, R.; Garganese, A.; De Luisi, A.; Chirulli, S.; Iacoviello, M.; Stasi, M.; Tabaku, O.; et al. Impact of High-to-Moderate Penetrance Genes on Genetic Testing: Looking over Breast Cancer. Genes 2023, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, J.N.; Neuhausen, S.L.; Adamson, A.; Tao, S.; Ricker, C.; Maoz, A.; Rosenblatt, M.; Nehoray, B.; Sand, S.; Steele, L.; et al. Pathogenic and likely pathogenic variants in PALB2, CHEK2, and other known breast cancer susceptibility genes among 1054 BRCA-negative Hispanics with breast cancer. Cancer 2019, 125, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Han, S.A.; Kim, S.W. BRCA and Breast Cancer-Related High-Penetrance Genes. Adv. Exp. Med. Biol. 2021, 1187, 473–490. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; Schrader, K.A.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015, 1, 23–32, Erratum in JAMA Oncol. 2015, 1, 110. https://doi.org/10.1001/jamaoncol.2015.0410. [Google Scholar] [CrossRef]
- Offit, K.; Couch, F.J.; Nathanson, K.L. Evaluation of ACMG-Guideline-Based Variant Classification of Cancer Susceptibility and Non-Cancer-Associated Genes in Families Affected by Breast Cancer. Am. J. Hum. Genet. 2016, 98, 801–817. [Google Scholar] [CrossRef]
- NCCN. Guidelines for Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (accessed on 1 October 2024).
- ASCO. Guidelines for Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. Available online: https://ascopubs.org/doi/10.1200/JCO.20.00299 (accessed on 1 October 2024).
- ESMO. Guidelines for Risk Reduction and Screening of Cancer in Hereditary Breast-Ovarian Cancer Syndromes: ESMO Clinical Practice Guideline. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/hereditary-syndromes/risk-reduction-screening-hereditary-breast-ovarian-cancer-syndromes (accessed on 1 October 2024).
- Menko, F.H.; Monkhorst, K.; Hogervorst, F.B.L.; Rosenberg, E.H.; Adank, M.A.; Ruijs, M.W.G.; Bleiker, E.M.A.; Sonke, G.S.; Russell, N.S.; Oldenburg, H.S.A.; et al. Challenges in breast cancer genetic testing. A call for novel forms of multidisciplinary care and long-term evaluation. Crit. Rev. Oncol. 2022, 176, 103642. [Google Scholar] [CrossRef]
- Lee, S.; Rajeev, P.; Finning, S.; Oh, C.; Pothuri, B. Factors associated with delayed genetic testing for patients with BRCA-related cancers (428). Gynecol. Oncol. 2022, 166, S215–S216. [Google Scholar] [CrossRef]
- Armstrong, J.; Lynch, K.; Virgo, K.S.; Schwartz, M.D.; Friedman, S.; Dean, M.; Andrews, J.E.; Bourquardez Clark, E.; Clasen, J.; Conaty, J.; et al. Utilization, Timing, and Outcomes of BRCA Genetic Testing Among Women with Newly Diagnosed Breast Cancer from a National Commercially Insured Population: The ABOARD Study. JCO Oncol. Pr. 2021, 17, e226–e235. [Google Scholar] [CrossRef]
- Kocak, B.; Baessler, B.; Cuocolo, R.; Mercaldo, N.; Pinto Dos Santos, D. Trends and statistics of artificial intelligence and radiomics research in Radiology, Nuclear Medicine, and Medical Imaging: Bibliometric analysis. Eur. Radiol. 2023, 33, 7542–7555. [Google Scholar] [CrossRef] [PubMed]
- Pintican, R.; Duma, M.M.; Szep, M.; Feier, D.; Eniu, D.; Goidescu, I.; Chiorean, A. The Role of US in Depicting Axillary Metastasis in High-Risk Breast Cancer Patients. J. Pers. Med. 2021, 16, 1379. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.V.; Katabathina, V.S.; Bowerson, M.L.; Mityul, M.I.; Shetty, A.S.; Elsayes, K.M.; Balachandran, A.; Bhosale, P.R.; McCullough, A.E.; Menias, C.O. BRCA-associated Cancers: Role of Imaging in Screening, Diagnosis, and Management. RadioGraphics 2017, 37, 1005–1023. [Google Scholar] [CrossRef]
- Deng, T.; Liang, J.; Yan, C.; Ni, M.; Xiang, H.; Li, C.; Ou, J.; Lin, Q.; Liu, L.; Tang, G.; et al. Development and validation of ultrasound-based radiomics model to predict germline BRCA mutations in patients with breast cancer. Cancer Imaging 2024, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, G.; Costa, M.J.; Long, C.; Wetzler, I.R.; Hoyer, J.; Kraus, C.; Popp, B.; Emons, J.; Wunderle, M.; Wenkel, E.; et al. Breast MRI texture analysis for prediction of BRCA-associated genetic risk. BMC Med. Imaging 2020, 20, 86. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, A.; Creech, E.W.; Butler, F.P.; Wiegmann, P.G.; Chatfield, B.M.; Meyer, W.L.; Wilcox, A.P. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Zhang, W.; Guo, Y.; Jin, Q. Radiomics and Its Feature Selection: A Review. Symmetry 2023, 15, 1834. [Google Scholar] [CrossRef]
- Joseph, V.R. Optimal ratio for data splitting. Stat. Anal. Data Min. 2022, 15, 531–538. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 11 December 2023).
- Nero, C.; Ciccarone, F.; Boldrini, L.; Lenkowicz, J.; Paris, I.; Capoluongo, E.D.; Testa, A.C.; Fagotti, A.; Valentini, V.; Scambia, G. Germline BRCA 1-2 status prediction through ovarian ultrasound images radiogenomics: A hypothesis generating study (PROBE study). Sci. Rep. 2020, 10, 16511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallivanone, F.; Bertoli, G.; Porro, D. Radiogenomics Breast Cancer Diagnosis and Characterization: Current Status and Future Directions. Methods Protoc. 2022, 5, 78. [Google Scholar] [CrossRef]
- Pintican, R.M.; Chiorean, A.; Duma, M.; Feier, D.; Szep, M.; Eniu, D.; Goidescu, I.; Dudea, S. Are Mutation Carrier Patients Different from Non-Carrier Patients? Genetic, Pathology, and US Features of Patients with Breast Cancer. Cancers 2022, 14, 2759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; Landefeld, C.S.; et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer. JAMA 2019, 322, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Braman, N.; Prasanna, P.; Whitney, J.; Singh, S.; Beig, N.; Etesami, M.; Bates, D.D.B.; Gallagher, K.; Bloch, B.N.; Vulchi, M.; et al. Association of Peritumoral Radiomics with Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)–Positive Breast Cancer. JAMA Netw. Open 2019, 2, e19256120. [Google Scholar] [CrossRef] [PubMed]

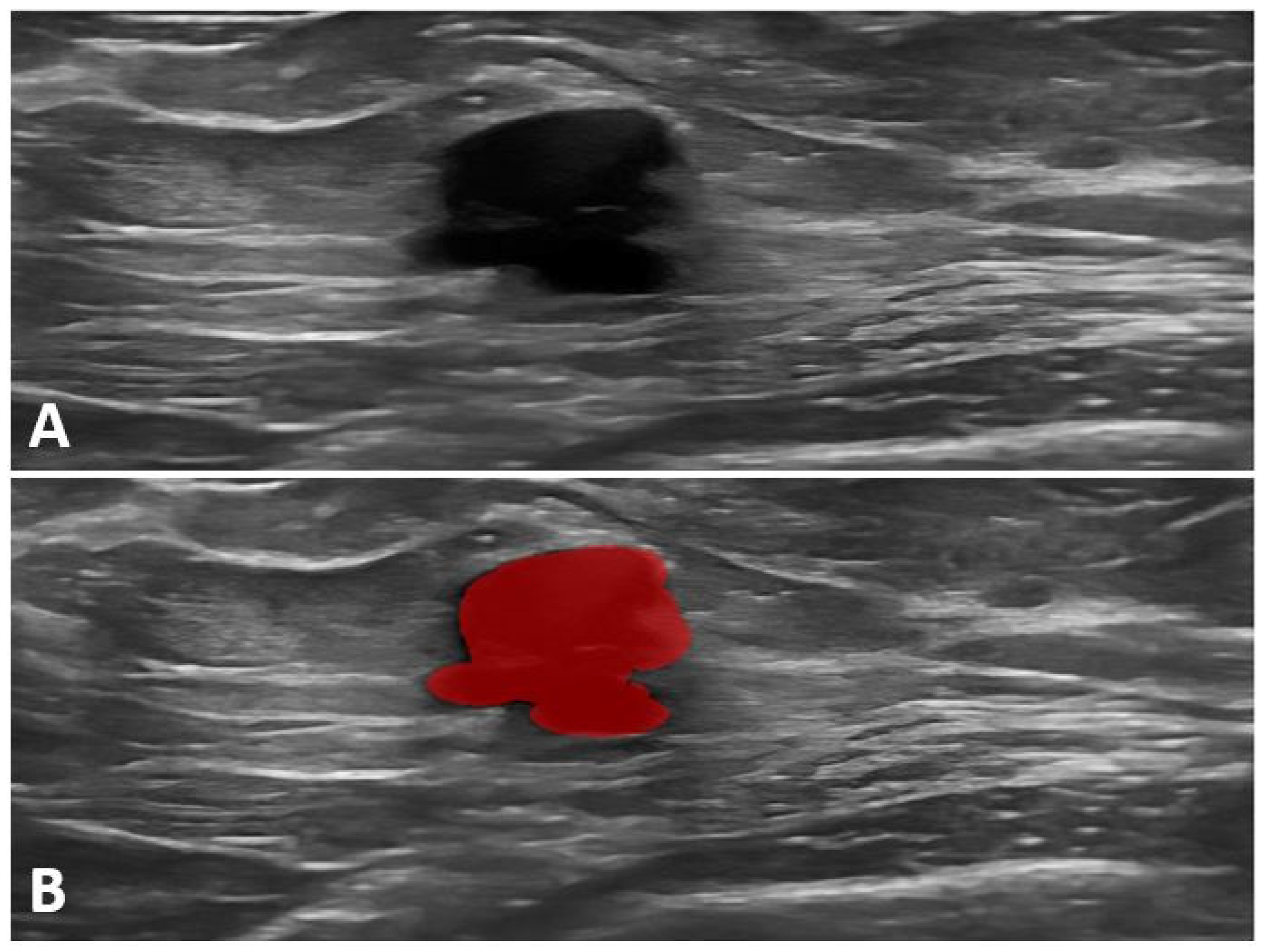
| Gene | Penetrance | Lifetime Breast Cancer Risk % | Prophylactic Mastectomy | Guidelines |
|---|---|---|---|---|
| BRCA1 | High | 60–87 | Recommended | NCCN, ASCO, ESMO, NICE |
| BRCA2 | High | 45–84 | Recommended | NCCN, ASCO, ESMO |
| TP53 | High | 49–85 | Recommended | NCCN, ASCO, ESMO |
| PTEN | High | 25–50 | Recommended | NCCN, ASCO, ESMO |
| CDH1 | High | 39–52 * | Recommended | NCCN, ASCO, ESMO |
| PALB2 | Moderate | 33–58 | Suggested/enhanced Surveillance | NCCN |
| ASCO | ||||
| STK11 | Moderate | 32–54 | Suggested/enhanced Surveillance | NCCN |
| ASCO |
| Patients with Pathogenic Variants (N = 50) | Patients Without Pathogenic Variants (N = 38) | p-Value | |
|---|---|---|---|
| Age (median) | 45 (34–65) | 46 (34–65) | 0.721 |
| Histology 1 | 0.062 | ||
| IDC-NST | 40 | 24 | |
| ILC | 9 | 7 | |
| DCIS | 1 | 6 | |
| Other | 0 | 1 | |
| Nottingham grade | 0.033 | ||
| 0 | 0 | 3 | |
| 1 | 2 | 0 | |
| 2 | 22 | 23 | |
| 3 | 26 | 12 | |
| Ki67% | 0.005 | ||
| <20 | 4 | 12 | |
| >20 | 46 | 26 | |
| ER | 0.714 | ||
| + | 31 | 25 | |
| − | 19 | 13 | |
| PR | 0.842 | ||
| + | 20 | 16 | |
| − | 30 | 22 | |
| HER 2 | 0.465 | ||
| + | 11 | 6 | |
| − | 39 | 32 |
| Tumor-Only Features (Rad-Score 1) | Coefficient (β) | Feature Description |
|---|---|---|
| X.S.0.1.Contrast | 0.11 | Measures the intensity difference between neighboring pixels, indicating texture roughness or heterogeneity |
| X.S.2.2.AngScMom | 0.94 | It quantifies the homogeneity of an image by summing the squared values of the gray-level co-occurrence matrix (GLCM). |
| X.S.5.5.SumVarnc | −0.01 | Represents texture uniformity or energy; higher values indicate more homogenous textures |
| X135dr_GLevNonU | −0.49 | Assesses the distribution of gray levels; lower values indicate more uniform textures |
| Teta2 | −0.29 | Represents an angular-related feature in texture analysis, linked to orientation or directional patterns |
| ZWavEnLL_s6 | −0.73 | Energy in specific wavelet frequency bands, which may correlate with microstructural variations or subtle changes in tissue composition |
| ZWavEnLH_s6 | −0.32 | Energy in specific wavelet frequency bands, which may correlate with microstructural variations or subtle changes in tissue composition |
| Tumoral + Peritumoral Features (Rad-Score 2) | Coefficient (β) | |
| Perc.01. | −0.59 | First percentile of intensity values, representing the lower bound of pixel intensity distribution |
| X.S.5.5.SumEntrp | −0.31 | Measures randomness in the image texture; higher values indicate more complexity and heterogeneity |
| Horzl_RLNonUni | −0.03 | Evaluates the variability of consecutive pixel runs in the horizontal direction; lower values suggest more uniform textures |
| WavEnHL_s.3 | −0.38 | Energy in the high–horizontal and low–vertical frequency wavelet decomposition at scale 3, indicating texture detail at a specific resolution |
| WavEnHH_s.6 | −0.04 | Quantify wavelet energy at specific high–low and high–high frequency bands at scales 3 and 6, which may correlate with fine-to-coarse microstructural tissue variations or changes in composition |
| Classifier | AUC | Specificity | Sensitivity | PPV | NPV | Accuracy (95% CI) |
|---|---|---|---|---|---|---|
| Random Forest | 0.935 | 0.888 | 0.750 | 0.900 | 0.727 | 0.809 (0.580 to 0.945) |
| Boosting Classification | 0.888 | 0.777 | 0.916 | 0.846 | 0.875 | 0.857 (0.636 to 0.969) |
| K-Nearest Neighbors | 0.939 | 0.888 | 0.750 | 0.900 | 0.727 | 0.809 (0.580 to 0.945 |
| Support Vector Machine | 0.851 | 0.777 | 0.750 | 0.818 | 0.700 | 0.761 (0.528 to 0.917) |
| Feature Importance (1-AUC) | Rad-score 1 | Ki67% | ||||
| Random Forest | 0.133 | 0.297 | ||||
| Boosting Classification | 0.375 | 0.281 | ||||
| K-Nearest Neighbors | 0.403 | 0.142 | ||||
| Support Vector Machine | 0.260 | 0.195 |
| Classifier | AUC | Specificity | Sensitivity | PPV | NPV | Accuracy (95% CI) |
|---|---|---|---|---|---|---|
| Random Forest | 0.824 | 0.666 | 0.666 | 0.727 | 0.600 | 0.666 (0.430 to 0.854) |
| Boosting Classification | 0.851 | 0.555 | 0.916 | 0.733 | 0.833 | 0.761 (0.528 to 0.917) |
| K-Nearest Neighbors | 0.930 | 0.666 | 0.916 | 0.785 | 0.857 | 0.809 (0.580 to 0.945) |
| Support Vector Machine | 0.907 | 0.777 | 0.916 | 0.846 | 0.875 | 0.857 (0.636 to 0.969) |
| Feature Importance (1-AUC) | Rad-score 2 | Ki67% | ||||
| Random Forest | 0.292 | 0.022 | ||||
| Boosting Classification | 0.282 | 0.152 | ||||
| K-Nearest Neighbors | 0.388 | 0.164 | ||||
| Support Vector Machine | 0.224 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antone, N.Z.; Pintican, R.; Manole, S.; Fodor, L.-A.; Lucaciu, C.; Roman, A.; Trifa, A.; Catana, A.; Lisencu, C.; Buiga, R.; et al. Predicting Pathogenic Variants of Breast Cancer Using Ultrasound-Derived Machine Learning Models. Cancers 2025, 17, 1019. https://doi.org/10.3390/cancers17061019
Antone NZ, Pintican R, Manole S, Fodor L-A, Lucaciu C, Roman A, Trifa A, Catana A, Lisencu C, Buiga R, et al. Predicting Pathogenic Variants of Breast Cancer Using Ultrasound-Derived Machine Learning Models. Cancers. 2025; 17(6):1019. https://doi.org/10.3390/cancers17061019
Chicago/Turabian StyleAntone, Nicoleta Zenovia, Roxana Pintican, Simona Manole, Liviu-Andrei Fodor, Carina Lucaciu, Andrei Roman, Adrian Trifa, Andreea Catana, Carmen Lisencu, Rares Buiga, and et al. 2025. "Predicting Pathogenic Variants of Breast Cancer Using Ultrasound-Derived Machine Learning Models" Cancers 17, no. 6: 1019. https://doi.org/10.3390/cancers17061019
APA StyleAntone, N. Z., Pintican, R., Manole, S., Fodor, L.-A., Lucaciu, C., Roman, A., Trifa, A., Catana, A., Lisencu, C., Buiga, R., Vlad, C., & Achimas Cadariu, P. (2025). Predicting Pathogenic Variants of Breast Cancer Using Ultrasound-Derived Machine Learning Models. Cancers, 17(6), 1019. https://doi.org/10.3390/cancers17061019






