Emerging Potential of Metabolomics in Thyroid Cancer—A Comprehensive Review
Simple Summary
Abstract
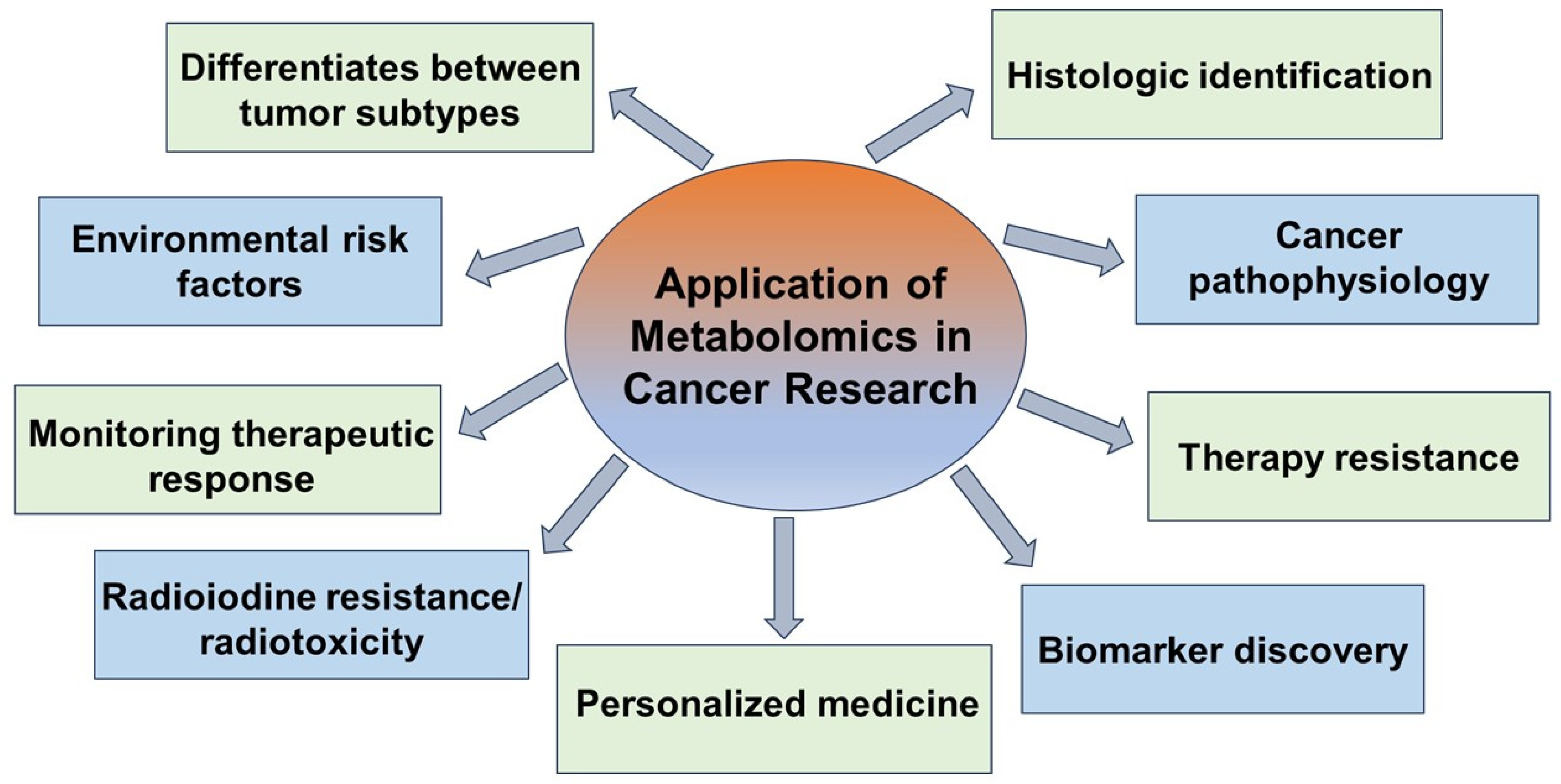
1. Introduction
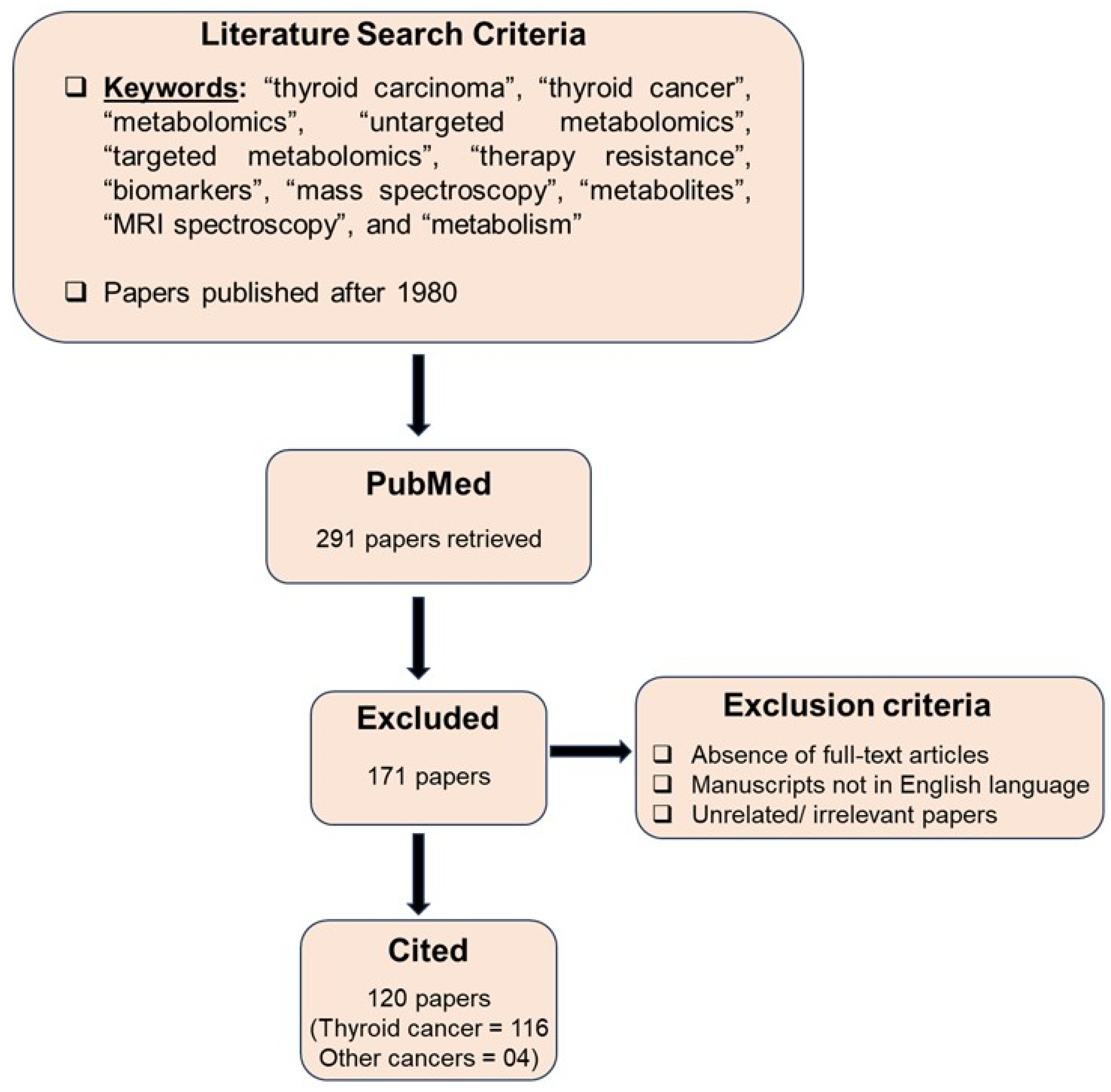
2. Search Strategy
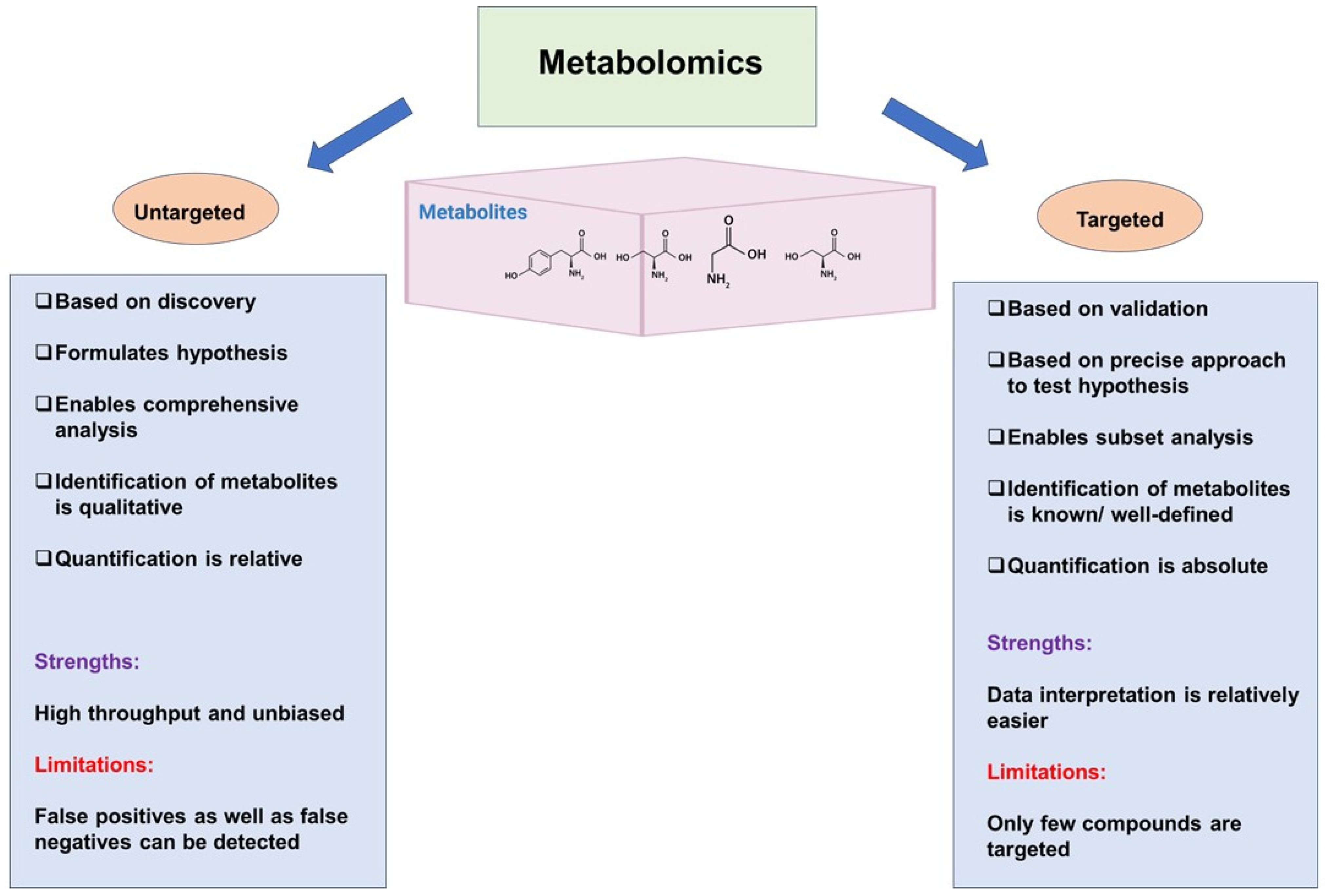
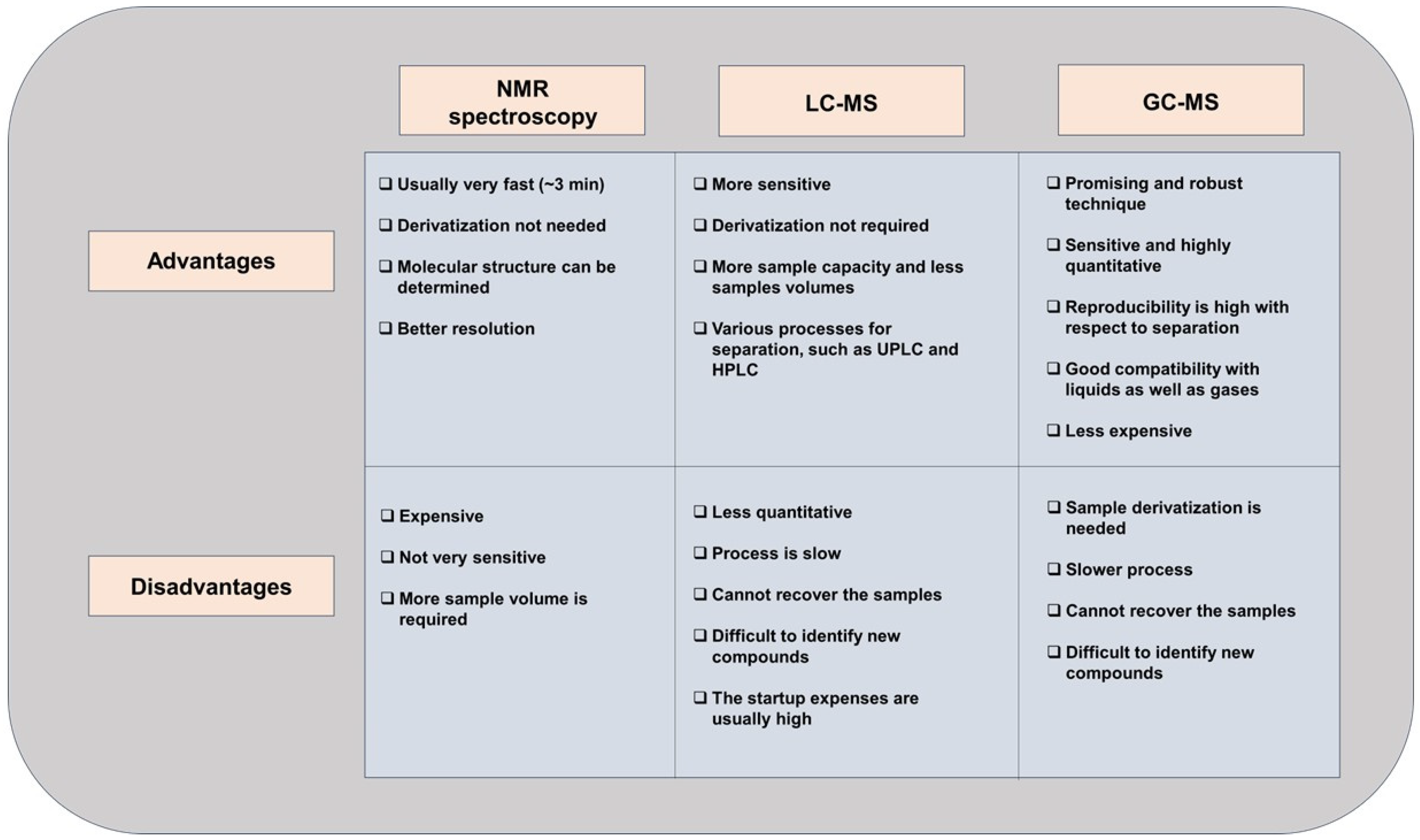
3. Untargeted and Targeted Metabolomics
4. Metabolomics for Biomarker Discovery
| Reference | Review Design | Biospecimen Used | Significantly Altered Metabolites |
|---|---|---|---|
| Khatami et al., 2019 [71] | Systematic review of 31 metabolomic studies (15 targeted and 16 untargeted) investigating metabolite biomarkers of TC. All metabolomic techniques included in search criteria. | Plasma, serum, urine, or FNA specimens. Malignant TC vs. control (healthy, benign nodules, goiter) | Citrate ↓ Lactate ↑ |
| Abooshahab et al., 2022 [53] | Systematic review of metabolomics in endocrine cancers. 35 articles published from 2010–2022 on thyroid cancer metabolomics. Techniques included NMR (15 papers), GC/MS (8 papers), and LC/MS (12 papers). | Tissue, serum/plasma, urine, FNA samples Malignant vs. benign tumors | Lactate ↑ Choline ↓ Mono- and disaccharides, and TCA intermediates altered |
| Coelho et al., 2020 [54] | Review includes 45 original studies on TC metabolomic biomarkers. NMR (21 papers), MS (19 papers), other techniques (5 papers). Spatial metabolomics applied in several listed studies. | Tissue, plasma, serum, urine, feces, breath TC vs. healthy/benign controls | Choline ↑ Lactate ↑ Tyrosine ↑ |
| Abooshahab et al., 2024 [72] | Review of metabolomic studies on TC cell lines. 7 papers identified. MS (6 papers) and NMR (1 paper). | TC cell lines | Various alterations in glycolysis and TCA cycle metabolites |
| Neto et al., 2022 [55] | Review of studies using FTIR spectroscopy to characterize normal vs. tumor samples. 13 papers met the criteria. | Thyroid tissue and cytology samples | Lipids ↓ Carbohydrates ↓ Lipid metabolism ↑ |
| Razavi et al., 2024 [56] | Systematic review and meta-analysis of NMR-based metabolomic studies. 12 studies met the search criteria. | Tissue and FNAB specimens. Malignant vs. benign. | Lactate ↑ Alanine ↑ Citrate ↓ |
| Nagayama et al., 2022 [51] | Summarize the recent findings of metabolic reprogramming in TC as well as recent reports of metabolism-targeted therapies. | Thyroid tissue and cytology samples | Glucose metabolism ↑ Amino acid metabolism ↑ Lipid metabolism ↑ |
5. Metabolomics in Disease Subtyping
6. Metabolomics for Overcoming Therapy Resistance
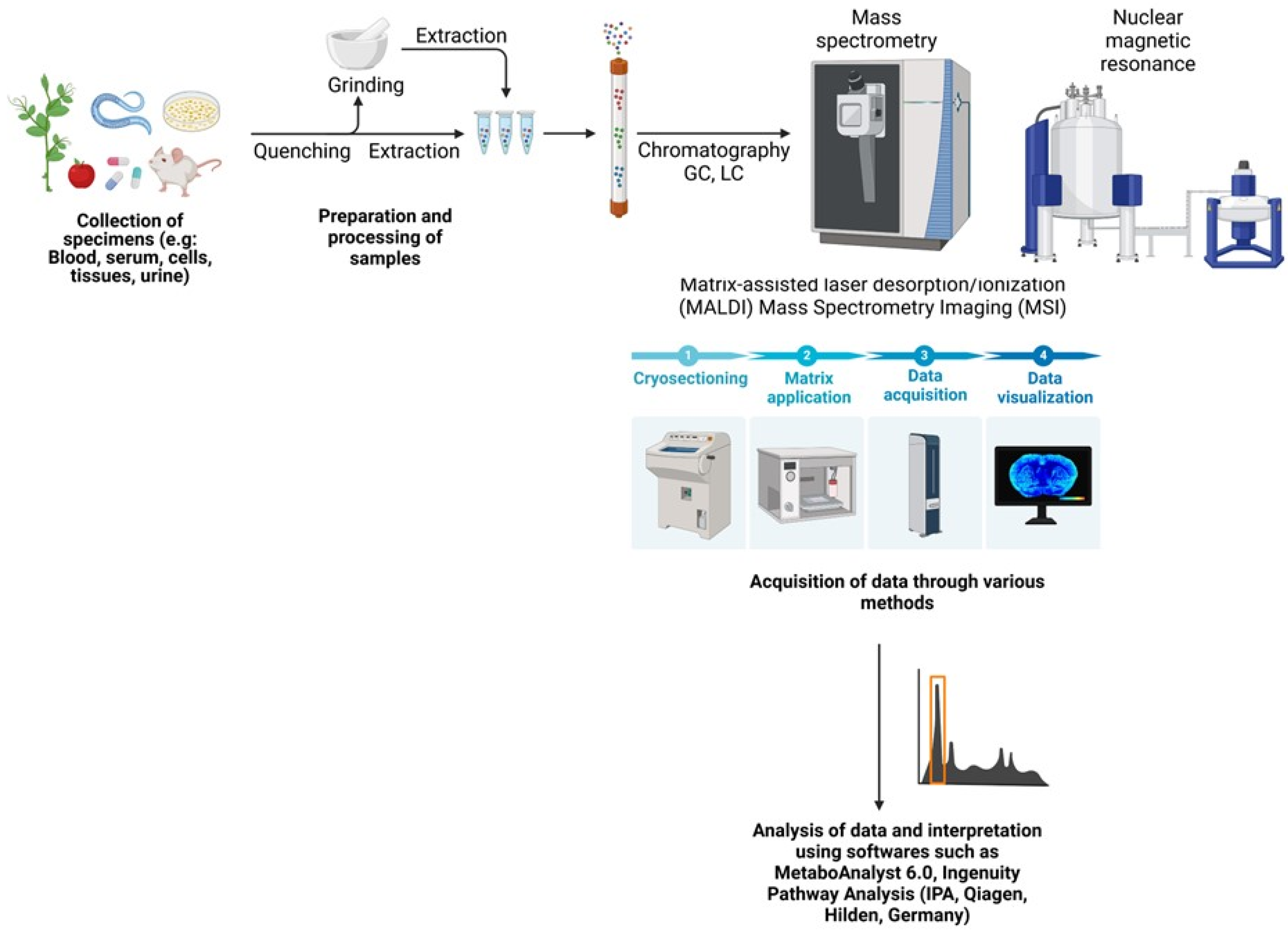
7. Models for Metabolomics Studies
8. Metabolomics: Bridging Other Omics
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TC | Thyroid cancer |
| TCA | Tricarboxylic acid |
| FDG-PET | 18F-deoxyglucose positron emission tomography |
| OXPHOS | Oxidative phosphorylation |
| MS | Mass spectrometry |
| FNA | Fine-needle aspiration |
| NMR | Nuclear magnetic resonance |
| FNAB | Fine-needle aspiration biopsy |
| LC-MS | Liquid chromatography- mass spectrometry |
| GC-MS | Gas chromatography- mass spectrometry |
| LCM | Laser capture microdissection |
| AUS | Atypia of undetermined significance |
| FLUS | Follicular lesion of undetermined significance |
| FN | Follicular neoplasm |
| SMC | Suspicious for malignant cells |
| MALDI-MSI | Matrix-assisted laser desorption/ionization– mass spectrometry imaging |
| HR-MAS NMR | High-resolution magic angle spinning nuclear magnetic resonance |
| LASS2 | Longevity assurance homologue 2 |
| BUME | Butanol/methanol |
| MTBE | Methyl tert-butyl ether |
| NIS | Sodium/iodide symporter |
| TSHR | Thyroid-stimulating hormone receptor |
| DTC | Differentiated thyroid cancer |
| PTC | Papillary thyroid cancer |
| FTC | Follicular thyroid cancer |
| MTC | Medullary thyroid cancer |
| ATC | Anaplastic thyroid cancer |
| TSP | Trimethylsilylpropanoic acid |
| DSS | 4, 4-dimethyl-4-silapentane-1-sulfonic acid |
| TKI | Tyrosine kinase inhibitor |
References
- Sipos, J.A.; Mazzaferri, E.L. Thyroid cancer epidemiology and prognostic variables. Clin. Oncol. 2010, 22, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Thyroid carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Perrild, H.; Bülow, I.; Ovesen, L.; Jørgensen, T. Risk factors for goiter and thyroid nodules. Thyroid. 2002, 12, 879–888. [Google Scholar] [CrossRef]
- Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 911. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Cancer Facts & Figures. 2025. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf (accessed on 16 March 2025).
- Zhou, Q.; Zhang, L.Y.; Xie, C.; Zhang, M.L.; Wang, Y.J.; Liu, G.H. Metabolomics as a potential method for predicting thyroid malignancy in children and adolescents. Pediatr. Surg. Int. 2020, 36, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zaballos, M.A.; Santisteban, P. Key signaling pathways in thyroid cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009, 19, 1159–1165. [Google Scholar] [CrossRef]
- Yang, J.; Schnadig, V.; Logrono, R.; Wasserman, P.G. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer 2007, 111, 306–315. [Google Scholar] [CrossRef]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for reporting thyroid cytopathology. J. Am. Soc. Cytopathol. 2023, 12, 319–325. [Google Scholar] [CrossRef]
- Yassa, L.; Cibas, E.S.; Benson, C.B.; Frates, M.C.; Doubilet, P.M.; Gawande, A.A.; Moore, F.D., Jr.; Kim, B.W.; Nosé, V.; Marqusee, E.; et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 2007, 111, 508–516. [Google Scholar] [CrossRef]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W.; European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda System for Reporting Thyroid Cytopathology: A meta-analysis. Acta Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef]
- Ho, A.S.; Sarti, E.E.; Jain, K.S.; Wang, H.; Nixon, I.J.; Shaha, A.R.; Shah, J.P.; Kraus, D.H.; Ghossein, R.; Fish, S.A.; et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid 2014, 24, 832–839. [Google Scholar] [CrossRef]
- Mavromati, M.; Saiji, E.; Demarchi, M.S.; Lenoir, V.; Seipel, A.; Kuczma, P.; Jornayvaz, F.R.; Becker, M.; Fernandez, E.; De Vito, C.; et al. Unnecessary thyroid surgery rate for suspicious nodule in the absence of molecular testing. Eur. Thyroid. J. 2023, 12, e230114. [Google Scholar] [CrossRef]
- Hannoush, Z.C.; Ruiz-Cordero, R.; Jara, M.; Kargi, A.Y. Current State of Molecular Cytology in Thyroid Nodules: Platforms and Their Diagnostic and Theranostic Utility. J. Clin. Med. 2024, 13, 1759. [Google Scholar] [CrossRef]
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur. Thyroid J. 2023, 12, e230067. [Google Scholar] [CrossRef]
- MacKay, C.; Turner, B.; Clarke, S.; Wallace, T.; Rigby, M.H. Cost-Effectiveness Analysis of Molecular Testing for Indeterminate Thyroid Nodules in Nova Scotia. J. Otolaryngol. Head Neck Surg. 2024, 53, 19160216241291806. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ’Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Wojakowska, A.; Chekan, M.; Widlak, P.; Pietrowska, M. Application of metabolomics in thyroid cancer research. Int. J. Endocrinol. 2015, 2015, 258763. [Google Scholar] [CrossRef]
- Spratlin, J.L.; Serkova, N.J.; Eckhardt, S.G. Clinical applications of metabolomics in oncology: A review. Clin. Cancer Res. 2009, 15, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Bucher, E.; Hilvo, M.; Salek, R.; Orešič, M.; Griffin, J.; Brockmöller, S.; Klauschen, F.; Loibl, S.; Barupal, D.K.; et al. Metabolomics of human breast cancer: New approaches for tumor typing and biomarker discovery. Genome Med. 2012, 4, 37. [Google Scholar] [CrossRef]
- Thysell, E.; Surowiec, I.; Hörnberg, E.; Crnalic, S.; Widmark, A.; Johansson, A.I.; Stattin, P.; Bergh, A.; Moritz, T.; Antti, H.; et al. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS ONE 2010, 5, e14175. [Google Scholar] [CrossRef]
- Kind, T.; Tolstikov, V.; Fiehn, O.; Weiss, R.H. A comprehensive urinary metabolomic approach for identifying kidney cancerr. Anal. Biochem. 2007, 363, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Howe, F.A.; Barton, S.J.; Cudlip, S.A.; Stubbs, M.; Saunders, D.E.; Murphy, M.; Wilkins, P.; Opstad, K.S.; Doyle, V.L.; McLean, M.A.; et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 2003, 49, 223–232. [Google Scholar] [CrossRef]
- Claudino, W.M.; Goncalves, P.H.; di Leo, A.; Philip, P.A.; Sarkar, F.H. Metabolomics in cancer: A bench-to-bedside intersection. Crit. Rev. Oncol. Hematol. 2012, 84, 1–7. [Google Scholar] [CrossRef]
- Miccoli, P.; Torregrossa, L.; Shintu, L.; Magalhaes, A.; Chandran, J.; Tintaru, A.; Ugolini, C.; Minuto, M.N.; Miccoli, M.; Basolo, F.; et al. Metabolomics approach to thyroid nodules: A high-resolution magic-angle spinning nuclear magnetic resonance-based study. Surgery 2012, 152, 1118–1124. [Google Scholar] [CrossRef]
- Grogan, R.H.; Mitmaker, E.J.; Clark, O.H. The evolution of biomarkers in thyroid cancer-from mass screening to a personalized biosignature. Cancers 2010, 2, 885–912. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Chi, C.; Wang, X.; Liu, S.; Zhao, W.; Ke, C.; Xu, G.; Li, E. Exhaled breath volatile biomarker analysis for thyroid cancer. Transl. Res. 2015, 166, 188–195. [Google Scholar] [CrossRef]
- Shang, X.; Zhong, X.; Tian, X. Metabolomics of papillary thyroid carcinoma tissues: Potential biomarkers for diagnosis and promising targets for therapy. Tumour Biol. 2016, 37, 11163–11175. [Google Scholar] [CrossRef]
- Chen, M.; Shen, M.; Li, Y.; Liu, C.; Zhou, K.; Hu, W.; Xu, B.; Xia, Y.; Tang, W. GC-MS-based metabolomic analysis of human papillary thyroid carcinoma tissue. Int. J. Mol. Med. 2015, 36, 1607–1614. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef]
- Bandu, R.; Mok, H.J.; Kim, K.P. Phospholipids as cancer biomarkers: Mass spectrometry-based analysis. Mass. Spectrom. Rev. 2018, 37, 107–138. [Google Scholar] [CrossRef]
- Xing, M.; Haugen, B.R.; Schlumberger, M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013, 381, 1058–1069. [Google Scholar] [CrossRef]
- Krause, K.; Jessnitzer, B.; Fuhrer, D. Proteomics in thyroid tumor research. J. Clin. Endocrinol. Metab. 2009, 94, 2717–2724. [Google Scholar] [CrossRef]
- Carpi, A.; Mechanick, J.I.; Saussez, S.; Nicolini, A. Thyroid tumor marker genomics and proteomics: Diagnostic and clinical implications. J. Cell Physiol. 2010, 224, 612–619. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Guo, S.; Qiu, L.; Wang, Y.; Qin, X.; Liu, H.; He, M.; Zhang, Y.; Li, Z.; Chen, X. Tissue imaging and serum lipidomic profiling for screening potential biomarkers of thyroid tumors by matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 4357–4370. [Google Scholar] [CrossRef]
- Deja, S.; Dawiskiba, T.; Balcerzak, W.; Orczyk-Pawiłowicz, M.; Głód, M.; Pawełka, D.; Młynarz, P. Follicular adenomas exhibit a unique metabolic profile. ¹H NMR studies of thyroid lesions. PLoS ONE 2013, 8, e84637. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Fiehn, O.; Hankemeier, T.; Kristal, B.S.; van Ommen, B.; Pujos-Guillot, E.; Verheij, E.; Wishart, D.; Wopereis, S. Mass-spectrometry-based metabolomics: Limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 2009, 5, 435–458. [Google Scholar] [CrossRef]
- Beger, R.D. A review of applications of metabolomics in cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef]
- Brennan, L. NMR-based metabolomics: From sample preparation to applications in nutrition research. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 83, 42–49. [Google Scholar] [CrossRef]
- Griffin, J.L.; Kauppinen, R.A. Tumour metabolomics in animal models of human cancer. J. Proteome Res. 2007, 6, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Felli, I.C.; Brutscher, B. Recent advances in solution NMR: Fast methods and heteronuclear direct detection. Chemphyschem 2009, 10, 1356–1368. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Suman, S.; Sharma, R.K.; Kumar, V.; Sinha, N.; Shukla, Y. Metabolic fingerprinting in breast cancer stages through (1)H NMR spectroscopy-based metabolomic analysis of plasma. J. Pharm. Biomed. Anal. 2018, 160, 38–45. [Google Scholar] [CrossRef]
- Michálková, L.; Horník, Š.; Sýkora, J.; Habartová, L.; Setnička, V. Diagnosis of pancreatic cancer via(1)H NMR metabolomics of human plasma. Analyst 2018, 143, 5974–5978. [Google Scholar] [CrossRef]
- An, Y.J.; Cho, H.R.; Kim, T.M.; Keam, B.; Kim, J.W.; Wen, H.; Park, C.K.; Lee, S.H.; Im, S.A.; Kim, J.E.; et al. An NMR metabolomics approach for the diagnosis of leptomeningeal carcinomatosis in lung adenocarcinoma cancer patients. Int. J. Cancer 2015, 136, 162–171. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Nagayama, Y.; Hamada, K. Reprogramming of Cellular Metabolism and Its Therapeutic Applications in Thyroid Cancer. Metabolites 2022, 12, 1214. [Google Scholar] [CrossRef]
- Abooshahab, R.; Gholami, M.; Sanoie, M.; Azizi, F.; Hedayati, M. Advances in metabolomics of thyroid cancer diagnosis and metabolic regulation. Endocrine 2019, 65, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abooshahab, R.; Ardalani, H.; Zarkesh, M.; Hooshmand, K.; Bakhshi, A.; Dass, C.R.; Hedayati, M. Metabolomics-A Tool to Find Metabolism of Endocrine Cancer. Metabolites 2022, 12, 1154. [Google Scholar] [CrossRef]
- Coelho, M.; Raposo, L.; Goodfellow, B.J.; Atzori, L.; Jones, J.; Manadas, B. The Potential of Metabolomics in the Diagnosis of Thyroid Cancer. Int. J. Mol. Sci. 2020, 21, 5272. [Google Scholar] [CrossRef]
- Neto, V.; Esteves-Ferreira, S.; Inácio, I.; Alves, M.; Dantas, R.; Almeida, I.; Guimarães, J.; Azevedo, T.; Nunes, A. Metabolic Profile Characterization of Different Thyroid Nodules Using FTIR Spectroscopy: A Review. Metabolites 2022, 12, 53. [Google Scholar] [CrossRef]
- Razavi, S.A.; Khorsand, B.; Salehipour, P.; Hedayati, M. Metabolite signature of human malignant thyroid tissue: A systematic review and meta-analysis. Cancer Med. 2024, 13, e7184. [Google Scholar] [CrossRef]
- Razavi, S.A.; Kalari, M.; Haghzad, T.; Haddadi, F.; Nasiri, S.; Hedayati, M. Exploring the potential of myo-inositol in thyroid disease management: Focus on thyroid cancer diagnosis and therapy. Front. Endocrinol. 2024, 15, 1418956. [Google Scholar] [CrossRef]
- Lan, L.; Luo, Y.; Zhou, M.; Huo, L.; Chen, H.; Zuo, Q.; Deng, W. Comparison of Diagnostic Accuracy of Thyroid Cancer with Ultrasound-Guided Fine-Needle Aspiration and Core-Needle Biopsy: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Razavi, S.A.; Mahmanzar, M.; Gh, B.F.N.M.; Zamani, Z.; Nasiri, S.; Hedayati, M. Plasma metabolites analysis of patients with papillary thyroid cancer: A preliminary untargeted 1H NMR-based metabolomics. J. Pharm. Biomed. Anal. 2024, 241, 115946. [Google Scholar] [CrossRef]
- Yu, S.; Liu, C.; Hou, Y.; Li, J.; Guo, Z.; Chen, X.; Zhang, L.; Peng, S.; Hong, S.; Xu, L.; et al. Integrative metabolomic characterization identifies plasma metabolomic signature in the diagnosis of papillary thyroid cancer. Oncogene 2022, 41, 2422–2430. [Google Scholar] [CrossRef]
- D’Andréa, G.; Jing, L.; Peyrottes, I.; Guigonis, J.M.; Graslin, F.; Lindenthal, S.; Sanglier, J.; Gimenez, I.; Haudebourg, J.; Vandersteen, C.; et al. Pilot Study on the Use of Untargeted Metabolomic Fingerprinting of Liquid-Cytology Fluids as a Diagnostic Tool of Malignancy for Thyroid Nodules. Metabolites 2023, 13, 782. [Google Scholar] [CrossRef]
- Rezig, L.; Servadio, A.; Torregrossa, L.; Miccoli, P.; Basolo, F.; Shintu, L.; Caldarelli, S. Diagnosis of post-surgical fine-needle aspiration biopsies of thyroid lesions with indeterminate cytology using HRMAS NMR-based metabolomics. Metabolomics 2018, 14, 141. [Google Scholar] [CrossRef]
- Ryoo, I.; Kwon, H.; Kim, S.C.; Jung, S.C.; Yeom, J.A.; Shin, H.S.; Cho, H.R.; Yun, T.J.; Choi, S.H.; Sohn, C.H.; et al. Metabolomic analysis of percutaneous fine-needle aspiration specimens of thyroid nodules: Potential application for the preoperative diagnosis of thyroid cancer. Sci. Rep. 2016, 6, 30075. [Google Scholar] [CrossRef]
- Torregrossa, L.; Shintu, L.; Nambiath Chandran, J.; Tintaru, A.; Ugolini, C.; Magalhães, A.; Basolo, F.; Miccoli, P.; Caldarelli, S. Toward the reliable diagnosis of indeterminate thyroid lesions: A HRMAS NMR-based metabolomics case of study. J. Proteome Res. 2012, 11, 3317–3325. [Google Scholar] [CrossRef]
- Planque, M.; Igelmann, S.; Ferreira Campos, A.M.; Fendt, S.-M. Spatial metabolomics principles and application to cancer research. Curr. Opin. Chem. Biol. 2023, 76, 102362. [Google Scholar] [CrossRef]
- Min, X.; Zhao, Y.; Yu, M.; Zhang, W.; Jiang, X.; Guo, K.; Wang, X.; Huang, J.; Li, T.; Sun, L.; et al. Spatially resolved metabolomics: From metabolite mapping to function visualising. Clin. Transl. Med. 2024, 14, e70031. [Google Scholar] [CrossRef]
- Wojakowska, A.; Cole, L.M.; Chekan, M.; Bednarczyk, K.; Maksymiak, M.; Oczko-Wojciechowska, M.; Jarząb, B.; Clench, M.R.; Polańska, J.; Pietrowska, M.; et al. Discrimination of papillary thyroid cancer from non-cancerous thyroid tissue based on lipid profiling by mass spectrometry imaging. Endokrynol. Pol. 2018, 69, 2–8. [Google Scholar] [CrossRef]
- DeHoog, R.J.; Zhang, J.; Alore, E.; Lin, J.Q.; Yu, W.; Woody, S.; Almendariz, C.; Lin, M.; Engelsman, A.F.; Sidhu, S.B.; et al. Preoperative metabolic classification of thyroid nodules using mass spectrometry imaging of fine-needle aspiration biopsies. Proc. Natl. Acad. Sci. USA 2019, 116, 21401–21408. [Google Scholar] [CrossRef]
- Wallace, E.N.; West, C.A.; McDowell, C.T.; Lu, X.; Bruner, E.; Mehta, A.S.; Aoki-Kinoshita, K.F.; Angel, P.M.; Drake, R.R. An N-glycome tissue atlas of 15 human normal and cancer tissue types determined by MALDI-imaging mass spectrometry. Sci. Rep. 2024, 14, 489. [Google Scholar] [CrossRef]
- Mao, X.; Huang, L.; Li, T.; Abliz, Z.; He, J.; Chen, J. Identification of Diagnostic Metabolic Signatures in Thyroid Tumors Using Mass Spectrometry Imaging. Molecules 2023, 28, 5791. [Google Scholar] [CrossRef]
- Khatami, F.; Payab, M.; Sarvari, M.; Gilany, K.; Larijani, B.; Arjmand, B.; Tavangar, S.M. Oncometabolites as biomarkers in thyroid cancer: A systematic review. Cancer Manag. Res. 2019, 11, 1829–1841. [Google Scholar] [CrossRef]
- Abooshahab, R.; Razavi, F.; Ghorbani, F.; Hooshmand, K.; Zarkesh, M.; Hedayati, M. Thyroid cancer cell metabolism: A glance into cell culture system-based metabolomics approaches. Exp. Cell Res. 2024, 435, 113936. [Google Scholar] [CrossRef]
- Berinde, G.M.; Socaciu, A.I.; Socaciu, M.A.; Petre, G.E.; Socaciu, C.; Piciu, D. Metabolic Profiles and Blood Biomarkers to Discriminate between Benign Thyroid Nodules and Papillary Carcinoma, Based on UHPLC-QTOF-ESI(+)-MS Analysis. Int. J. Mol. Sci. 2024, 25, 3495. [Google Scholar] [CrossRef]
- Berinde, G.M.; Socaciu, A.I.; Socaciu, M.A.; Petre, G.E.; Rajnoveanu, A.G.; Barsan, M.; Socaciu, C.; Piciu, D. In Search of Relevant Urinary Biomarkers for Thyroid Papillary Carcinoma and Benign Thyroid Nodule Differentiation, Targeting Metabolic Profiles and Pathways via UHPLC-QTOF-ESI(+)-MS Analysis. Diagnostics 2024, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, X.; Li, X.; Yan, Y.; Wang, J.; Zhao, X.; Tian, Y.; Ling, R.; Duan, Y. Identifying potential breath biomarkers for early diagnosis of papillary thyroid cancer based on solid-phase microextraction gas chromatography-high resolution mass spectrometry with metabolomics. Metabolomics 2024, 20, 59. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, X.; Li, Y.; Zhang, J.; Li, X.; Qian, C.; Tian, Y.; Ling, R.; Duan, Y. Diagnostic approach to thyroid cancer based on amino acid metabolomics in saliva by ultra-performance liquid chromatography with high resolution mass spectrometry. Talanta 2021, 235, 122729. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Q.; Hou, H.; Wang, S.; Zhang, Y.; Luo, Y.; Chen, H.; Deng, H.; Zhu, H.; Zhang, L.; et al. Metabolite analysis-aided diagnosis of papillary thyroid cancer. Endocr. Relat. Cancer 2019, 26, 829–841. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Wang, C.; Wang, X.; Luo, J.; He, Y.; Zhang, Y.; Chen, S.; Zhou, Q.; Sun, D.; et al. Study on identification of diagnostic biomarkers in serum for papillary thyroid cancer in different iodine nutrition regions. Biomarkers 2024, 30, 37–46. [Google Scholar] [CrossRef]
- Wang, F.; Lin, Y.; Qin, L.; Zeng, X.; Jiang, H.; Liang, Y.; Wen, S.; Li, X.; Huang, S.; Li, C.; et al. Serum metabolome associated with novel and legacy per- and polyfluoroalkyl substances exposure and thyroid cancer risk: A multi-module integrated analysis based on machine learning. Environ. Int. 2024, 195, 109203. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.; Peng, J.; Jiang, Y.; Lin, X.; Zhang, J. Identification of serum metabolites associated with polybrominated diphenyl ethers (PBDEs) exposure in papillary thyroid carcinoma: A case-control study. Environ. Geochem. Health 2024, 46, 377. [Google Scholar] [CrossRef]
- Zhao, M.; Li, R.; Miao, C.; Miccoli, P.; Lu, J. Non-invasive diagnosis of papillary thyroid microcarcinoma using a novel metabolomics analysis of urine. Endocrine 2024, 87, 1100–1111. [Google Scholar] [CrossRef]
- Wojakowska, A.; Chekan, M.; Marczak, Ł.; Polanski, K.; Lange, D.; Pietrowska, M.; Widlak, P. Detection of metabolites discriminating subtypes of thyroid cancer: Molecular profiling of FFPE samples using the GC/MS approach. Mol. Cell Endocrinol. 2015, 417, 149–157. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yoon, S.J.; Kim, M.; Kim, H.H.; Song, Y.S.; Jung, J.W.; Han, D.; Cho, S.W.; Kwon, S.W.; Park, Y.J. Integrative Multi-omics Analysis Reveals Different Metabolic Phenotypes Based on Molecular Characteristics in Thyroid Cancer. Clin. Cancer Res. 2024, 30, 883–894. [Google Scholar] [CrossRef]
- Qu, N.; Chen, D.; Ma, B.; Zhang, L.; Wang, Q.; Wang, Y.; Wang, H.; Ni, Z.; Wang, W.; Liao, T.; et al. Integrated proteogenomic and metabolomic characterization of papillary thyroid cancer with different recurrence risks. Nat. Commun. 2024, 15, 3175. [Google Scholar] [CrossRef]
- Chai, Y.J.; Yi, J.W.; Oh, S.W.; Kim, Y.A.; Yi, K.H.; Kim, J.H.; Lee, K.E. Upregulation of SLC2 (GLUT) family genes is related to poor survival outcomes in papillary thyroid carcinoma: Analysis of data from The Cancer Genome Atlas. Surgery 2017, 161, 188–194. [Google Scholar] [CrossRef]
- Wen, S.; Luo, Y.; Wu, W.; Zhang, T.; Yang, Y.; Ji, Q.; Wu, Y.; Shi, R.; Ma, B.; Xu, M.; et al. Identification of lipid metabolism-related genes as prognostic indicators in papillary thyroid cancer. Acta Biochim. Biophys. Sin. 2021, 53, 1579–1589. [Google Scholar] [CrossRef]
- Ban, E.J.; Kim, D.; Kim, J.K.; Kang, S.W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Kim, K. Lactate Dehydrogenase A as a Potential New Biomarker for Thyroid Cancer. Endocrinol. Metab. 2021, 36, 96–105. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F. Construction and evaluation of a prognosis prediction model for thyroid carcinoma based on lipid metabolism-related genes. Neuro Endocrinol. Lett. 2022, 43, 323–332. [Google Scholar]
- Enomoto, K.; Hotomi, M. Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer. Endocrinol. Metab. 2020, 35, 227–236. [Google Scholar] [CrossRef]
- Huang, J.; Sun, W.; Wang, Z.; Lv, C.; Zhang, T.; Zhang, D.; Dong, W.; Shao, L.; He, L.; Ji, X.; et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J. Exp. Clin. Cancer Res. 2022, 41, 42. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.; Yi, S.; Choi, N.R.; Lim, M.A.; Chang, J.W.; Won, H.R.; Kim, J.R.; Ko, H.M.; Chung, E.J.; et al. Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses. Nat. Commun. 2024, 15, 1163. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Li, D.; He, W.; Liu, Z.; Liu, L.; Yang, X.; Cheng, X.; Chen, R.; Yang, Y. LASS2 suppresses metastasis in multiple cancers by regulating the ferroptosis signalling pathway through interaction with TFRC. Cancer Cell Int. 2024, 24, 87. [Google Scholar] [CrossRef]
- Xu, B.; Gao, W.; Xu, T.; Liu, C.; Wu, D.; Tang, W. A UPLC Q-Exactive Orbitrap Mass Spectrometry-Based Metabolomic Study of Serum and Tumor Tissue in Patients with Papillary Thyroid Cancer. Toxics 2022, 11, 44. [Google Scholar] [CrossRef]
- Seo, J.W.; Han, K.; Lee, J.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Park, V.Y.; Baek, H.M.; Kwak, J.Y. Application of metabolomics in prediction of lymph node metastasis in papillary thyroid carcinoma. PLoS ONE 2018, 13, e0193883. [Google Scholar] [CrossRef]
- Shen, C.T.; Zhang, Y.; Liu, Y.M.; Yin, S.; Zhang, X.Y.; Wei, W.J.; Sun, Z.K.; Song, H.J.; Qiu, Z.L.; Wang, C.R.; et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin. Endocrinol. 2017, 87, 844–852. [Google Scholar] [CrossRef]
- Cararo Lopes, E.; Sawant, A.; Moore, D.; Ke, H.; Shi, F.; Laddha, S.; Chen, Y.; Sharma, A.; Naumann, J.; Guo, J.Y.; et al. Integrated metabolic and genetic analysis reveals distinct features of human differentiated thyroid cancer. Clin. Transl. Med. 2023, 13, e1298. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, Y.; Zhang, Y.; Liu, Y.; Li, S.; Wang, J.; Tan, L.; Zou, L. Integration of metabolomics and transcriptomics reveals metformin suppresses thyroid cancer progression via inhibiting glycolysis and restraining DNA replication. Biomed. Pharmacother. 2023, 168, 115659. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.; Cheng, S.Y.; Kebebew, E.; et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin. Cancer Res. 2018, 24, 4030–4043. [Google Scholar] [CrossRef]
- Zheng, W.; Tang, X.; Dong, J.; Feng, J.; Chen, M.; Zhu, X. Metabolomic screening of radioiodine refractory thyroid cancer patients and the underlying chemical mechanism of iodine resistance. Sci. Rep. 2024, 14, 10546. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Q.; Xuan, Z.; Mao, Y.; Tang, X.; Yang, K.; Song, F.; Zhu, X. Metabolomics reveals the implication of acetoacetate and ketogenic diet therapy in radioiodine-refractory differentiated thyroid carcinoma. Oncologist 2024, 29, e1120–e1131. [Google Scholar] [CrossRef]
- Yu, Y.; Ning, K.; Liu, X.; Liang, Y.; Jiao, Z.; Zou, B.; Cai, T.; Yang, Z.; Chen, W.; Wu, T.; et al. Per- and polyfluoroalkyl substances (PFAS) exposure is associated with radioiodine therapy resistance and dedifferentiation of differentiated thyroid cancer. Environ. Pollut. 2025, 367, 125629. [Google Scholar] [CrossRef]
- Lu, G.; Gao, D.; Liu, Y.; Yu, X.; Jiang, W.; Lv, Z. Early and long-term responses of intestinal microbiota and metabolites to (131)I treatment in differentiated thyroid cancer patients. BMC Med. 2024, 22, 300. [Google Scholar] [CrossRef]
- Lu, G.; Gao, D.; Jiang, W.; Yu, X.; Tong, J.; Liu, X.; Qiao, T.; Wang, R.; Zhang, M.; Wang, S.; et al. Disrupted gut microecology after high-dose (131)I therapy and radioprotective effects of arachidonic acid supplementation. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Thakur, S.; Cardenas, S.; Makarewicz, A.; Klubo-Gwiezdzinska, J. Metabolic Reprogramming Contributes to Resistance Towards Lenvatinib in Thyroid Cancer. VideoEndocrinology™ 2024, 11, 54–56. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; Mao, Y.; Xuan, Z.; Yang, K.; Tang, X.; Zhu, X. Combined BRAF and PIM1 inhibitory therapy for papillary thyroid carcinoma based on BRAFV600E regulation of PIM1: Synergistic effect and metabolic mechanisms. Neoplasia 2024, 52, 100996. [Google Scholar] [CrossRef]
- Liu, B.; Peng, Y.; Su, Y.; Diao, C.; Qian, J.; Zhan, X.; Cheng, R. Transcriptome and metabolome sequencing identifies glutamate and LPAR1 as potential factors of anlotinib resistance in thyroid cancer. Anticancer Drugs 2024, 35, 741–751. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, Y.C.; Chen, C.C. Lysophosphatidic Acid Receptor Antagonists and Cancer: The Current Trends, Clinical Implications, and Trials. Cells 2021, 10, 1629. [Google Scholar] [CrossRef]
- Kuang, A.; Kouznetsova, V.L.; Kesari, S.; Tsigelny, I.F. Diagnostics of Thyroid Cancer Using Machine Learning and Metabolomics. Metabolites 2023, 14, 11. [Google Scholar] [CrossRef]
- Kurashige, T.; Shimamura, M.; Hamada, K.; Matsuse, M.; Mitsutake, N.; Nagayama, Y. Characterization of metabolic reprogramming by metabolomics in the oncocytic thyroid cancer cell line XTC.UC1. Sci. Rep. 2023, 13, 149. [Google Scholar] [CrossRef]
- Kumari, S.; Adewale, R.; Klubo-Gwiezdzinska, J. The Molecular Landscape of Hürthle Cell Thyroid Cancer Is Associated with Altered Mitochondrial Function—A Comprehensive Review. Cells 2020, 9, 1570. [Google Scholar] [CrossRef]
- Tronci, L.; Serreli, G.; Piras, C.; Frau, D.V.; Dettori, T.; Deiana, M.; Murgia, F.; Santoru, M.L.; Spada, M.; Leoni, V.P.; et al. Vitamin C Cytotoxicity and Its Effects in Redox Homeostasis and Energetic Metabolism in Papillary Thyroid Carcinoma Cell Lines. Antioxidants 2021, 10, 809. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Qu, Y.; Wang, X.; Wang, Y.; Jia, K.; Du, Q.; Han, J.; Liu, H.; Zhang, X.; et al. High-Performance Metabolic Profiling of High-Risk Thyroid Nodules by ZrMOF Hybrids. ACS Nano 2024, 18, 21336–21346. [Google Scholar] [CrossRef]
- Cristiani, S.; Bertolini, A.; Carnicelli, V.; Contu, L.; Vitelli, V.; Saba, A.; Saponaro, F.; Chiellini, G.; Sabbatini, A.R.M.; Giambelluca, M.A.; et al. Development and primary characterization of a human thyroid organoid in vitro model for thyroid metabolism investigation. Mol. Cell Endocrinol. 2024, 594, 112377. [Google Scholar] [CrossRef]
- Yang, H.; Liang, Q.; Zhang, J.; Liu, J.; Wei, H.; Chen, H.; Wei, W.; Chen, D.; Zhao, Y. Establishment of papillary thyroid cancer organoid lines from clinical specimens. Front. Endocrinol. 2023, 14, 1140888. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tan, Y.; Li, Z.; Li, W.; Yu, L.; Chen, W.; Liu, Y.; Liu, L.; Guo, L.; Huang, W.; et al. Organoid Cultures Derived from Patients with Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, 1410–1426. [Google Scholar] [CrossRef]
- Dhuli, K.; Medori, M.C.; Donato, K.; Donato, K.; Maltese, P.E.; Tanzi, B.; Tezzele, S.; Mareso, C.; Miertus, J.; Generali, D.; et al. Omics sciences and precision medicine in thyroid cancer. Clin. Ter. 2023, 174, 11–20. [Google Scholar] [CrossRef]
- Al-Jundi, M.; Thakur, S.; Gubbi, S.; Klubo-Gwiezdzinska, J. Novel Targeted Therapies for Metastatic Thyroid Cancer—A Comprehensive Review. Cancers 2020, 12, 2104. [Google Scholar] [CrossRef]
- Boufraqech, M.; Nilubol, N. Multi-omics Signatures and Translational Potential to Improve Thyroid Cancer Patient Outcome. Cancers 2019, 11, 1988. [Google Scholar] [CrossRef]
- Gulfidan, G.; Soylu, M.; Demirel, D.; Erdonmez, H.B.C.; Beklen, H.; Ozbek Sarica, P.; Arga, K.Y.; Turanli, B. Systems biomarkers for papillary thyroid cancer prognosis and treatment through multi-omics networks. Arch. Biochem. Biophys. 2022, 715, 109085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Makarewicz, A.; Klubo-Gwiezdzinska, J. Emerging Potential of Metabolomics in Thyroid Cancer—A Comprehensive Review. Cancers 2025, 17, 1017. https://doi.org/10.3390/cancers17061017
Kumari S, Makarewicz A, Klubo-Gwiezdzinska J. Emerging Potential of Metabolomics in Thyroid Cancer—A Comprehensive Review. Cancers. 2025; 17(6):1017. https://doi.org/10.3390/cancers17061017
Chicago/Turabian StyleKumari, Sonam, Andrew Makarewicz, and Joanna Klubo-Gwiezdzinska. 2025. "Emerging Potential of Metabolomics in Thyroid Cancer—A Comprehensive Review" Cancers 17, no. 6: 1017. https://doi.org/10.3390/cancers17061017
APA StyleKumari, S., Makarewicz, A., & Klubo-Gwiezdzinska, J. (2025). Emerging Potential of Metabolomics in Thyroid Cancer—A Comprehensive Review. Cancers, 17(6), 1017. https://doi.org/10.3390/cancers17061017






