Integrating CT Radiomics and Clinical Features to Optimize TACE Technique Decision-Making in Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
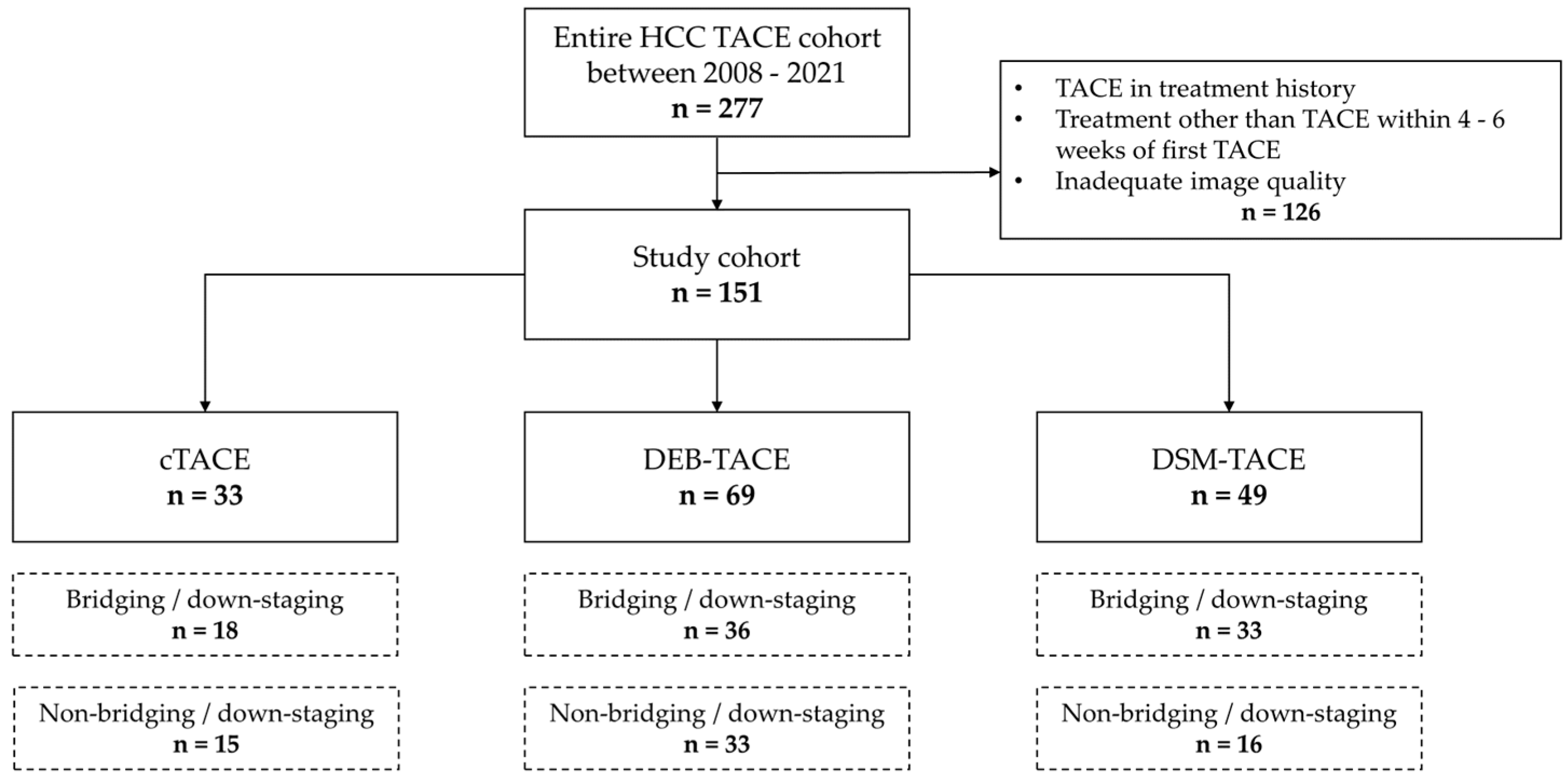
2.1. Study Design and Patient Selection
2.2. TACE Procedures
2.3. Data Collection and Follow-Up
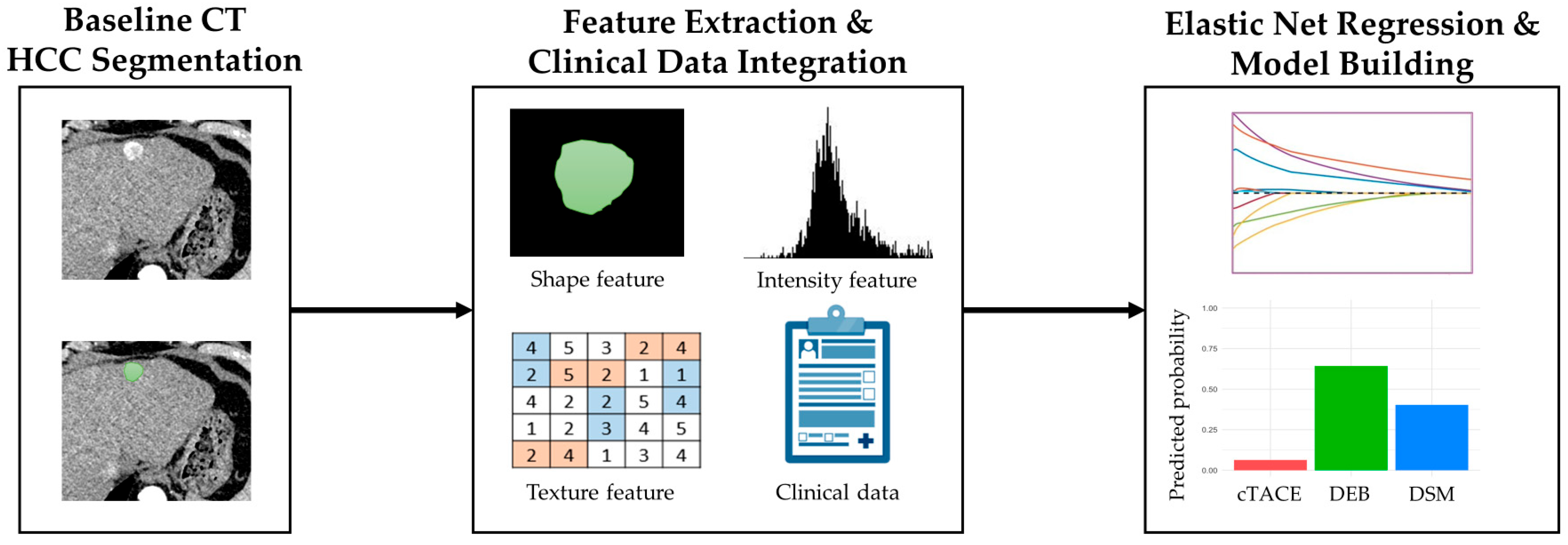
2.4. Imaging and Radiomics Analysis
2.5. Development of Best TACE Technique Decision Framework
2.6. Statistical Analysis
3. Results
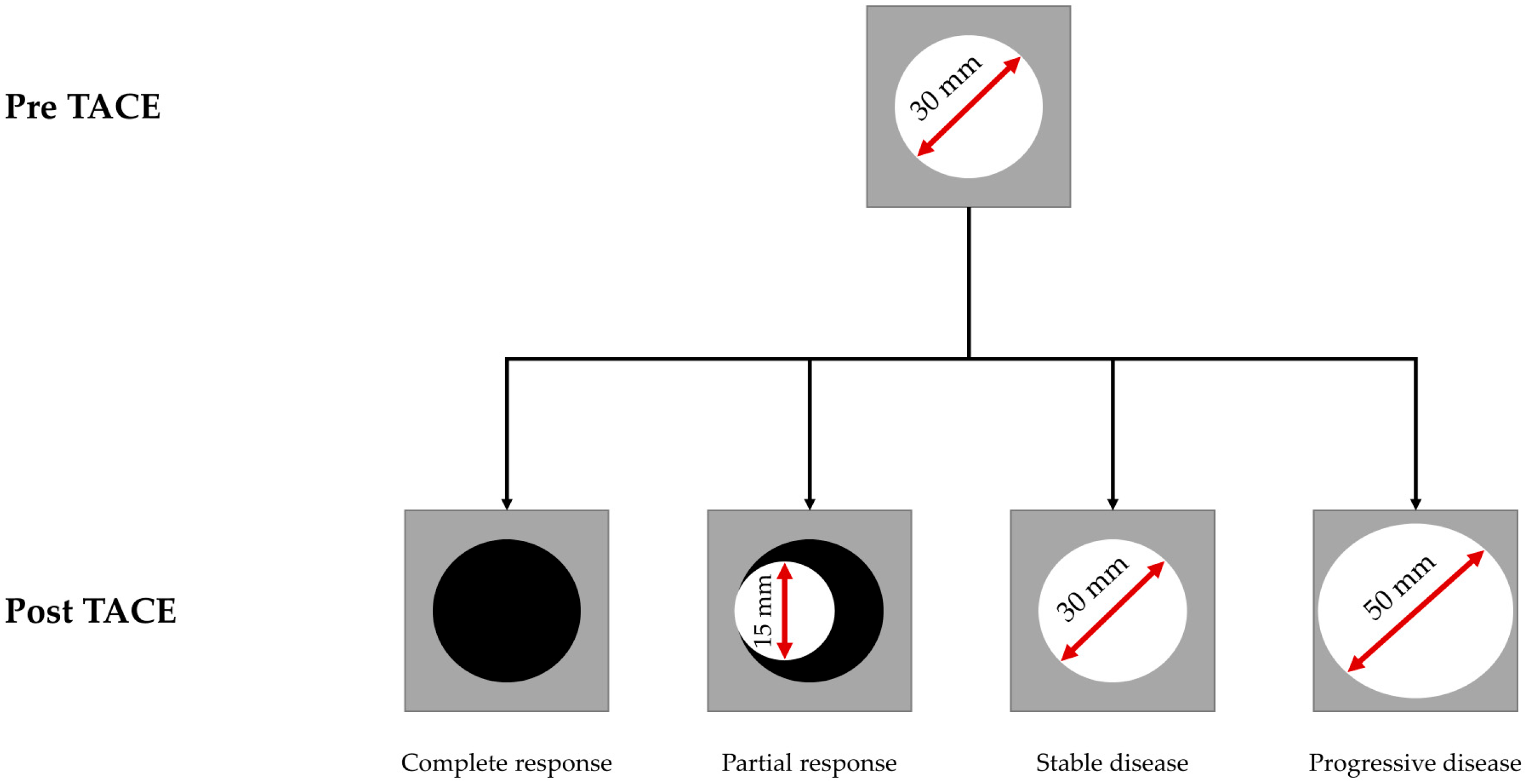
3.1. Patient Characteristics and Response Assessment
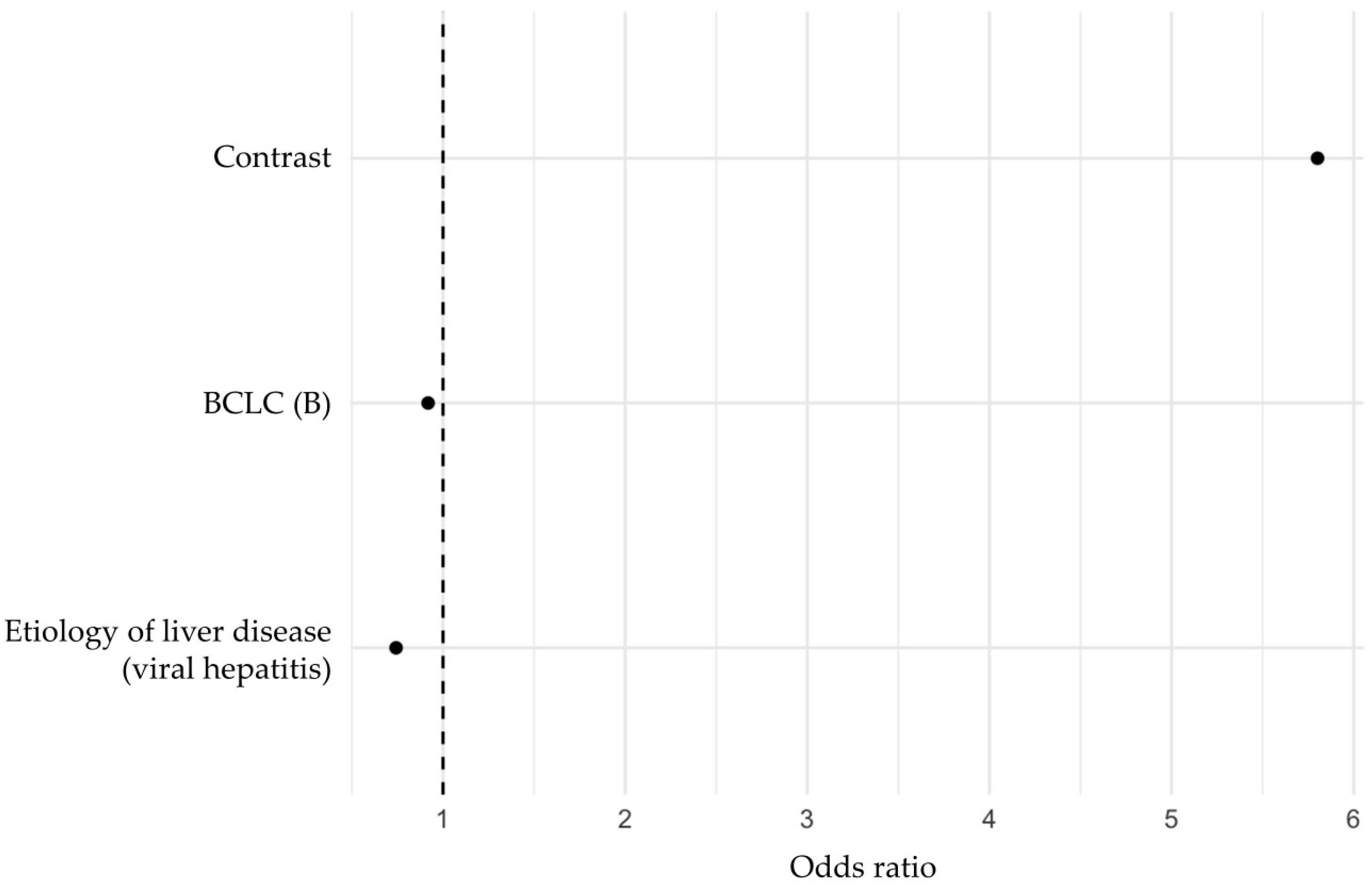
3.2. Multivariable Regression Analysis of Predictors of TACE Response
3.3. Development and Evaluation of a Prediction Model Based on Independent Predictors Identified by Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Corrigendum to “EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma” [J Hepatol 69 (2018) 182–236]. J. Hepatol. 2019, 70, 817. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Stoehr, F.; Mahringer-Kunz, A.; Hahn, F.; Weinmann, A.; Kloeckner, R. Current Strategies to Identify Patients That Will Benefit from TACE Treatment and Future Directions a Practical Step-by-Step Guide. J. Hepatocell. Carcinoma 2021, 8, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Claasen, M.; Sneiders, D.; Rakke, Y.S.; Adam, R.; Bhoori, S.; Cillo, U.; Fondevila, C.; Reig, M.; Sapisochin, G.; Tabrizian, P.; et al. European Society of Organ Transplantation (ESOT) Consensus Report on Downstaging, Bridging and Immunotherapy in Liver Transplantation for Hepatocellular Carcinoma. Transpl. Int. 2023, 36, 11648. [Google Scholar] [CrossRef]
- Saghafian Larijani, R.; Shabani Ravari, N.; Goodarzi, N.; Akhlaghpour, S.; Saghafian Larijani, S.; Rouini, M.R.; Dinarvand, R. Current Status of Transarterial Chemoembolization (TACE) Agents in Hepatocellular Carcinoma Treatment. J. Drug Deliv. Sci. Technol. 2022, 77, 103905. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Systematic Review of Randomized Trials for Unresectable Hepatocellular Carcinoma: Chemoembolization Improves Survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef]
- Lucatelli, P.; Burrel, M.; Guiu, B.; de Rubeis, G.; van Delden, O.; Helmberger, T. CIRSE Standards of Practice on Hepatic Transarterial Chemoembolisation. Cardiovasc. Interv. Radiol. 2021, 44, 1851–1867. [Google Scholar] [CrossRef]
- Zarisfi, M.; Kasaeian, A.; Wen, A.; Liapi, E. Systematic Review and Pharmacokinetic Meta-analysis of Doxorubicin Exposure in Transcatheter Arterial Chemoembolization and Doxorubicin-Eluted Beads Chemoembolization for Treatment of Unresectable Hepatocellular Carcinoma. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 449–466. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Cannella, R.; Santinha, J.; Beaufrere, A.; Ronot, M.; Sartoris, R.; Cauchy, F.; Bouattour, M.; Matos, C.; Papanikolaou, N.; Vilgrain, V.; et al. Performances and Variability of CT Radiomics for the Prediction of Microvascular Invasion and Survival in Patients with HCC: A Matter of Chance or Standardisation? Eur. Radiol. 2023, 33, 7618–7628. [Google Scholar] [CrossRef]
- Lu, M.; Wang, C.; Zhuo, Y.; Gou, J.; Li, Y.; Li, J.; Dong, X. Preoperative Prediction Power of Radiomics and Non-Radiomics Methods Based on Mri for Early Recurrence in Hepatocellular Carcinoma: A Systemic Review and Meta-Analysis. Abdom. Radiol. 2024, 49, 3397–3411. [Google Scholar] [CrossRef]
- Miranda, J.; Horvat, N.; Fonseca, G.M.; Araujo-Filho, J.A.B.; Fernandes, M.C.; Charbel, C.; Chakraborty, J.; Coelho, F.F.; Nomura, C.H.; Herman, P. Current Status and Future Perspectives of Radiomics in Hepatocellular Carcinoma. World J. Gastroenterol. 2023, 29, 43–60. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.L.; Liu, Q.P.; Sun, S.W.; Zhang, J.; Zhu, F.P.; Yang, G.; Yan, X.; Zhang, Y.D.; Liu, X.S. Radiomic Analysis of Contrast-Enhanced CT Predicts Microvascular Invasion and Outcome in Hepatocellular Carcinoma. J. Hepatol. 2019, 70, 1133–1144. [Google Scholar] [CrossRef]
- Schindler, P.; Kaldewey, D.; Rennebaum, F.; Trebicka, J.; Pascher, A.; Wildgruber, M.; Kohler, M.; Masthoff, M. Safety, Efficacy, and Survival of Different Transarterial Chemoembolization Techniques in the Management of Unresectable Hepatocellular Carcinoma: A Comparative Single-Center Analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 235. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J. MModified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Garzelli, L.; Cannella, R.; Ronot, M.; Vilgrain, V. Hepatocellular Carcinoma: Optimal Radiological Evaluation before Liver Transplantation. Life 2023, 13, 2267. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallieres, M.; Abdalah, M.A.; Aerts, H.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Chen, K.; Shuen, T.W.H.; Chow, P.K.H. The Association between Tumour Heterogeneity and Immune Evasion Mechanisms in Hepatocellular Carcinoma and Its Clinical Implications. Br. J. Cancer 2024, 131, 420–429. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Drug-Eluting Beads Versus Conventional Chemoembolization for the Treatment of Unresectable Hepatocellular Carcinoma: A Meta-Analysis. Dig. Liver Dis. 2016, 48, 571–577. [Google Scholar] [CrossRef]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised Controlled Trial of Doxorubicin-Eluting Beads Vs Conventional Chemoembolisation for Hepatocellular Carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef]
- Collettini, F.; Andrasina, T.; Reimer, P.; Schima, W.; Stroszczynski, C.; Lamprecht, Y.; Auer, T.A.; Rohan, T.; Wildgruber, M.; Gebauer, B.; et al. Degradable Starch Microspheres Transarterial Chemoembolization (DSM-TACE) in Patients with Unresectable Hepatocellular Carcinoma: Results from the Prospective Multicenter Observational Hepastar Trial. Eur. Radiol. 2024. [Google Scholar] [CrossRef]
- Vogl, T.J.; Langenbach, M.C.; Hammerstingl, R.; Albrecht, M.H.; Chatterjee, A.R.; Gruber-Rouh, T. Evaluation of Two Different Transarterial Chemoembolization Protocols Using Lipiodol and Degradable Starch Microspheres in Therapy of Hepatocellular Carcinoma: A Prospective Trial. Hepatol. Int. 2021, 15, 685–694. [Google Scholar] [CrossRef]
- Mohr, I.; Vogeler, M.; Pfeiffenberger, J.; Sprengel, S.D.; Klauss, M.; Radeleff, B.; Teufel, A.; Chang, D.H.; Springfeld, C.; Longerich, T.; et al. Clinical Effects and Safety of Different Transarterial Chemoembolization Methods for Bridging and Palliative Treatments in Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2022, 148, 3163–3174. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Forner, A.; Bolondi, L.; Cheung, T.T.; Kloeckner, R.; de Baere, T. Updated Use of TACE for Hepatocellular Carcinoma Treatment: How and When to Use It Based on Clinical Evidence. Cancer Treat. Rev. 2019, 72, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Wei, X.; Xia, X.; Wei, Z.; Li, R.; Jin, J.; Liu, Z.; Hu, X.; Peng, X. Hepatitis B Virus Infection: An Insight into the Clinical Connection and Molecular Interaction between Hepatitis B Virus and Host Extrahepatic Cancer Risk. Front. Immunol. 2023, 14, 1141956. [Google Scholar] [CrossRef]
- Yang, P.; Markowitz, G.J.; Wang, X.F. The Hepatitis B Virus-Associated Tumor Microenvironment in Hepatocellular Carcinoma. Natl. Sci. Rev. 2014, 1, 396–412. [Google Scholar] [CrossRef]
- Elpek, G.O. Molecular Pathways in Viral Hepatitis-Associated Liver Carcinogenesis: An Update. World J. Clin. Cases 2021, 9, 4890–4917. [Google Scholar] [CrossRef]
- Borgia, M.; Dal Bo, M.; Toffoli, G. Role of Virus-Related Chronic Inflammation and Mechanisms of Cancer Immune-Suppression in Pathogenesis and Progression of Hepatocellular Carcinoma. Cancers 2021, 13, 4387. [Google Scholar] [CrossRef]
- Pinter, M.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. Cancer and Liver Cirrhosis: Implications on Prognosis and Management. ESMO Open 2016, 1, e000042. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, C.; Xiong, B.; Xia, X. Tace Versus Tace + Entecavir Versus Tace + Tenofovir in the Treatment of HBV Associated Hepatocellular Carcinoma. BMC Cancer 2023, 23, 235. [Google Scholar] [CrossRef]
- Zhang, S.S.; Liu, J.X.; Zhu, J.; Xiao, M.B.; Lu, C.H.; Ni, R.Z.; Qu, L.S. Effects of Tace and Preventive Antiviral Therapy on Hbv Reactivation and Subsequent Hepatitis in Hepatocellular Carcinoma: A Meta-Analysis. Jpn. J. Clin. Oncol. 2019, 49, 646–655. [Google Scholar] [CrossRef]
- Couri, T.; Pillai, A. Goals and Targets for Personalized Therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Braman, N.; Prasanna, P.; Bera, K.; Alilou, M.; Khorrami, M.; Leo, P.; Etesami, M.; Vulchi, M.; Turk, P.; Gupta, A.; et al. Novel Radiomic Measurements of Tumor-Associated Vasculature Morphology on Clinical Imaging as a Biomarker of Treatment Response in Multiple Cancers. Clin. Cancer Res. 2022, 28, 4410–4424. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wahid, K.A.; van Dijk, L.V.; Farahani, K.; Thompson, R.F.; Fuller, C.D. Radiomic Biomarkers of Tumor Immune Biology and Immunotherapy Response. Clin. Transl. Radiat. Oncol. 2021, 28, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Yang, Z.G.; Li, Y.; Li, M.Q. Radiomic Advances in the Transarterial Chemoembolization Related Therapy for Hepatocellular Carcinoma. World J. Radiol. 2023, 15, 89–97. [Google Scholar] [CrossRef]
- Caputo, W.L.; de Souza, M.C.; Basso, C.R.; Pedrosa, V.A.; Seiva, F.R.F. Comprehensive Profiling and Therapeutic Insights into Differentially Expressed Genes in Hepatocellular Carcinoma. Cancers 2023, 15, 5653. [Google Scholar] [CrossRef]
- Nguyen, T.; Vennatt, J.; Downs, L.; Surabhi, V.; Stanietzky, N. Advanced Imaging of Hepatocellular Carcinoma: A Review of Current and Novel Techniques. J. Gastrointest. Cancer 2024, 55, 1469–1484. [Google Scholar] [CrossRef]
- Poot, A.J.; Lapa, C.; Weber, W.A.; Lam, M.; Eiber, M.; Dierks, A.; Bundschuh, R.A.; Braat, A. [(68)Ga]Ga-RAYZ-8009: A Glypican-3-Targeted Diagnostic Radiopharmaceutical for Hepatocellular Carcinoma Molecular Imaging-A First-in-Human Case Series. J. Nucl. Med. 2024, 65, 1597–1603. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Wang, N.; Xu, Q.; Liu, Y.; Liu, J.; Zhang, Q.; Zhang, X.; Chen, A.; Chen, L.; et al. Intratumoral and Peritumoral Radiomics Based on Contrast-Enhanced MRI for Preoperatively Predicting Treatment Response of Transarterial Chemoembolization in Hepatocellular Carcinoma. BMC Cancer 2023, 23, 1026. [Google Scholar] [CrossRef]
- Tay, J.K.; Narasimhan, B.; Hastie, T. Elastic Net Regularization Paths for All Generalized Linear Models. J. Stat. Softw. 2023, 106, 1–31. [Google Scholar] [CrossRef]
- Ende, T.V.D.; Kuijper, S.C.; Widaatalla, Y.; Noortman, W.A.; van Velden, F.H.P.; Woodruff, H.C.; van der Pol, Y.; Moldovan, N.; Pegtel, D.M.; Derks, S.; et al. Integrating Clinical Variables, Radiomics, and Tumor-derived Cell-Free DNA for Enhanced Prediction of Resectable Esophageal Adenocarcinoma Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2024, 121, 963–974. [Google Scholar] [CrossRef]
- Wong, L.M.; Ai, Q.Y.H.; Zhang, R.; Mo, F.; King, A.D. Radiomics for Discrimination between Early-Stage Nasopharyngeal Carcinoma and Benign Hyperplasia with Stable Feature Selection on MRI. Cancers 2022, 14, 3433. [Google Scholar] [CrossRef]
- Schon, F.; Kieslich, A.; Nebelung, H.; Riediger, C.; Hoffmann, R.T.; Zwanenburg, A.; Lock, S.; Kuhn, J.P. Comparative Analysis of Radiomics and Deep-Learning Algorithms for Survival Prediction in Hepatocellular Carcinoma. Sci. Rep. 2024, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, J.; Choi, G.H.; Yun, J.; Kang, J.; Choi, J.; Kim, K.M.; Kim, N. Deep Learning-Based Prediction of Post-Treatment Survival in Hepatocellular Carcinoma Patients Using Pre-Treatment CT Images and Clinical Data. J. Imaging Inform. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, K.; Krzyzinski, M.; Bartczak, T.; Korzeniowski, K.; Lamparski, K.; Wroblewski, T.; Grat, M.; Holowko, W.; Mech, K.; Lisowska, J.; et al. A Novel Radiomics Approach for Predicting TACE Outcomes in Hepatocellular Carcinoma Patients Using Deep Learning for Multi-Organ Segmentation. Sci. Rep. 2024, 14, 14779. [Google Scholar] [CrossRef] [PubMed]

| Total | cTACE | DEB | DSM | p | |
|---|---|---|---|---|---|
| Clinical and laboratory data | |||||
| N | 151 (100) | 33 (21.9) | 69 (45.7) | 49 (32.5) | |
| Sex | |||||
| Male | 120 (79.5) | 27 (81.8) | 52 (75.4) | 41 (83.7) | 0.508 |
| Female | 31 (20.5) | 6 (18.2) | 17 (24.6) | 8 (16.3) | |
| Age (years), median (IQR) | 64.0 (12.0) | 60.0 (10.0) | 63.0 (17.0) | 65.0 (10.0) | 0.325 |
| Etiology of liver disease | |||||
| Alcoholic | 63 (41.7) | 17 (51.5) | 26 (37.7) | 20 (40.8) | 0.882 |
| Viral hepatitis | 40 (26.5) | 9 (27.3) | 17 (24.6) | 14 (28.6) | |
| Biliary disease | 1 (0.7) | 0 (0) | 1 (1.4) | 0 (0) | |
| NASH | 14 (9.3) | 2 (6.1) | 7 (10.1) | 5 (10.2) | |
| Toxic | 1 (0.7) | 0 (0) | 0 (0) | 1 (2.0) | |
| Autoimmune hepatitis | 1 (0.7) | 0 (0) | 1 (1.4) | 0 (0) | |
| Hemochromatosis | 1 (0.7) | 0 (0) | 1 (1.4) | 0 (0) | |
| Cryptogenic | 30 (19.9) | 5 (15.2) | 16 (23.2) | 9 (18.4) | |
| ECOG | |||||
| 0 | 76 (50.3) | 14 (42.4) | 34 (49.3) | 28 (57.1) | 0.406 |
| 1 | 61 (40.4) | 13 (39.4) | 30 (43.5) | 18 (36.7) | |
| 2 | 12 (7.9) | 5 (15.2) | 5 (7.2) | 2 (4.1) | |
| 3 | 2 (1.3) | 1 (3.0) | 0 (0) | 1 (2.0) | |
| BCLC | |||||
| A | 79 (52.3) | 14 (42.4) | 33 (47.8) | 32 (65.3) | 0.322 |
| B | 51 (33.8) | 13 (39.4) | 24 (34.8) | 14 (28.6) | |
| C | 13 (8.6) | 3 (9.1) | 8 (11.6) | 2 (4.1) | |
| D | 8 (5.3) | 3 (9.1) | 4 (5.8) | 1 (2.0) | |
| Diagnosis of HCC by liver biopsy Previous therapy | 15 (9.9) | 4 (12.1) | 7 (10.1) | 4 (8.2) | 0.634 |
| Resection | 8 (5.3) | 2 (6.1) | 4 (5.8) | 2 (4.1) | 0.111 |
| Systemic | 7 (4.6) | 2 (6.1) | 4 (5.8) | 1 (2.0) | 0.575 |
| Local | |||||
| RFA | 2 (1.3) | 1 (3.0) | 1 (1.4) | 0 (0) | 0.350 |
| MWA | 1 (0.7) | 0 (0) | 0 (0) | 1 (2.0) | |
| TARE | 11 7.3) | 2 (6.1) | 6 (8.7) | 3 (6.1) | |
| PEI | 4 (2.6) | 0 (0) | 1 (1.4) | 3 (6.1) | |
| INR, median (IQR) | 1.2 (0.2) | 1.2 (0.3) | 1.2 (0.3) | 1.1 (0.2) | 0.168 |
| Bilirubin (mg/dL), median (IQR) | 1.0 (0.9) | 1.2 (1.5) | 0.9 (0.9) | 1.0 (0.8) | 0.449 |
| ALT (U/L), median (IQR) | 41.5 (38.3) | 39.0 (42.5) | 39.0 (33.0) | 38.0 (33.0) | 0.274 |
| AST (U/L), median (IQR) | 57.5 (42.5) | 51.0 (41.0) | 58.0 (48.0) | 50.0 (42.0) | 0.329 |
| ALP (U/L), median (IQR) | 128.5 (97.3) | 130.0 (101.0) | 122.0 (95.0) | 117.0 (85.0) | 0.226 |
| GGT (U/L), median (IQR) | 140.0 (193.5) | 84.0 (168.5) | 173.0 (151.0) | 117.0 (291.0) | 0.490 |
| AFP (ng/mL), median (IQR) | 16.4 (167.6) | 8.4 (133.3) | 17.6 (277.6) | 17.8 (67.5) | 0.486 |
| Imaging features and procedural data | |||||
| Indication for TACE | |||||
| Bridging/down-staging | 87 (57.6) | 18 (54.4) | 36 (52.2) | 33 (67.3) | 0.239 |
| Non-bridging/down-staging | 64 (42.4) | 15 (45.5) | 33 (47.8) | 16 (32.7) | |
| Hepatic tumor burden | |||||
| 0–25% | 133 (88.1) | 27 (81.8) | 60 (87.0) | 46 (93.9) | 0.309 |
| 26–50% | 16 (10.6) | 6 (18.2) | 8 (11.6) | 2 (4.1) | |
| >50% | 2 (1.3) | 0 (0) | 1 (1.4) | 1 (2.0) | |
| Portal vein invasion | 9 (6.0) | 4 (12.1) | 3 (4.3) | 2 (4.1) | 0.239 |
| Within Milan criteria | 49 (32.5) | 6 (18.2) | 22 (31.9) | 21 (42.9) | 0.105 |
| Sum of target lesion diameter (mm), median (IQR) | 39.1 (31.5) | 34.4 (19.9) | 44.5 (36.5) | 37.0 (30.3) | 0.137 |
| Catheter application position | |||||
| Unselective | 73 (48.3) | 14 (42.2) | 31 (44.9) | 28 (57.1) | 0.102 |
| Selective | 51 (33.7) | 14 (42.4) | 23 (33.3) | 14 (28.6) | |
| Superselective | 27 (17.9) | 5 (15.1) | 15 (21.7) | 7 (14.3) | |
| Follow-up | |||||
| Response at 4–6-week follow-up | |||||
| CR/PR | 72 (47.7) | 10 (30.3) a | 39 (56.5) b | 23 (46.9) a,b | 0.046 |
| SD/PD | 79 (52.3) | 23 (69.7) a | 30 (43.5) b | 26 (53.1) a,b |
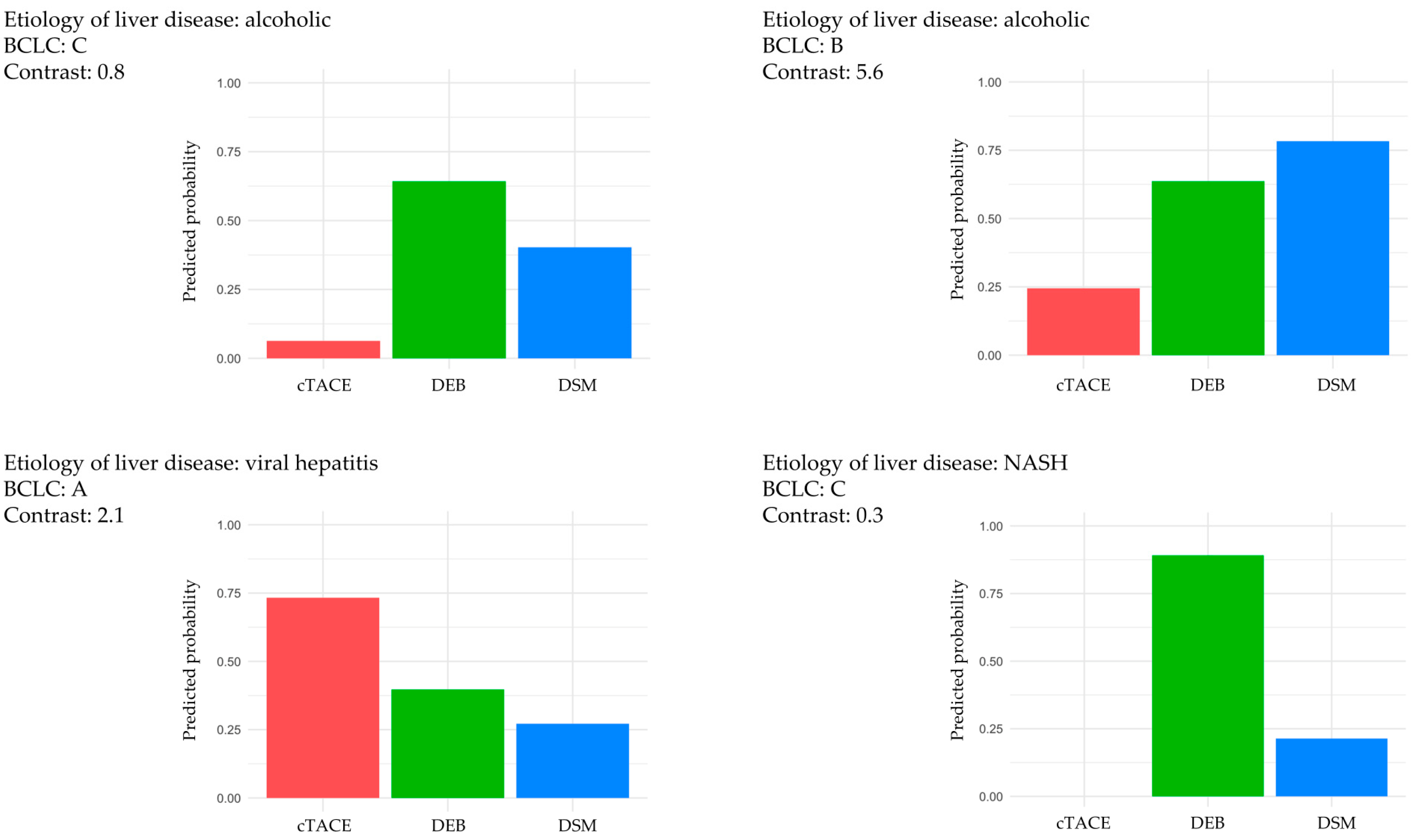
| Parameter | Regression Coefficient | Odds Ratio |
|---|---|---|
| Contrast | 1.75808882 | 5.8013394 |
| BCLC (B) | −0.08530392 | 0.9182332 |
| Etiology of liver disease (viral hepatitis) | −0.29781417 | 0.7424393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masthoff, M.; Irle, M.; Kaldewey, D.; Rennebaum, F.; Morgül, H.; Pöhler, G.H.; Trebicka, J.; Wildgruber, M.; Köhler, M.; Schindler, P. Integrating CT Radiomics and Clinical Features to Optimize TACE Technique Decision-Making in Hepatocellular Carcinoma. Cancers 2025, 17, 893. https://doi.org/10.3390/cancers17050893
Masthoff M, Irle M, Kaldewey D, Rennebaum F, Morgül H, Pöhler GH, Trebicka J, Wildgruber M, Köhler M, Schindler P. Integrating CT Radiomics and Clinical Features to Optimize TACE Technique Decision-Making in Hepatocellular Carcinoma. Cancers. 2025; 17(5):893. https://doi.org/10.3390/cancers17050893
Chicago/Turabian StyleMasthoff, Max, Maximilian Irle, Daniel Kaldewey, Florian Rennebaum, Haluk Morgül, Gesa Helen Pöhler, Jonel Trebicka, Moritz Wildgruber, Michael Köhler, and Philipp Schindler. 2025. "Integrating CT Radiomics and Clinical Features to Optimize TACE Technique Decision-Making in Hepatocellular Carcinoma" Cancers 17, no. 5: 893. https://doi.org/10.3390/cancers17050893
APA StyleMasthoff, M., Irle, M., Kaldewey, D., Rennebaum, F., Morgül, H., Pöhler, G. H., Trebicka, J., Wildgruber, M., Köhler, M., & Schindler, P. (2025). Integrating CT Radiomics and Clinical Features to Optimize TACE Technique Decision-Making in Hepatocellular Carcinoma. Cancers, 17(5), 893. https://doi.org/10.3390/cancers17050893







