Efficacy of Conversion Surgery for Initially Unresectable Biliary Tract Cancer That Has Responded to Down-Staging Chemotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
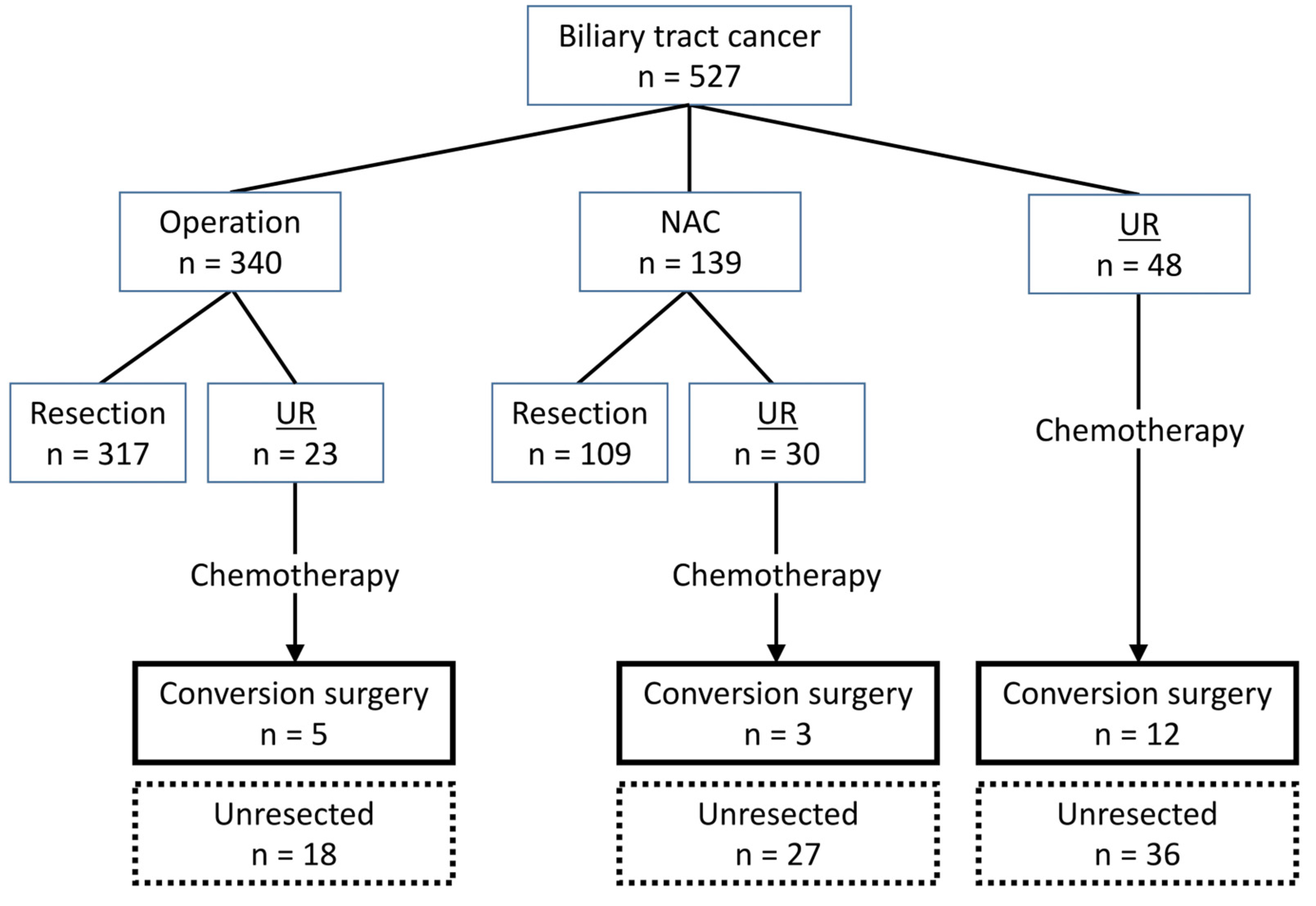
3. Results
3.1. Patients
3.2. Characteristics of Patients with Unresectable Biliary Tract Cancer (n = 101)
3.3. Details of Patients with Initially Unresectable Biliary Tract Cancer Who Underwent Conversion Surgery (n = 20)
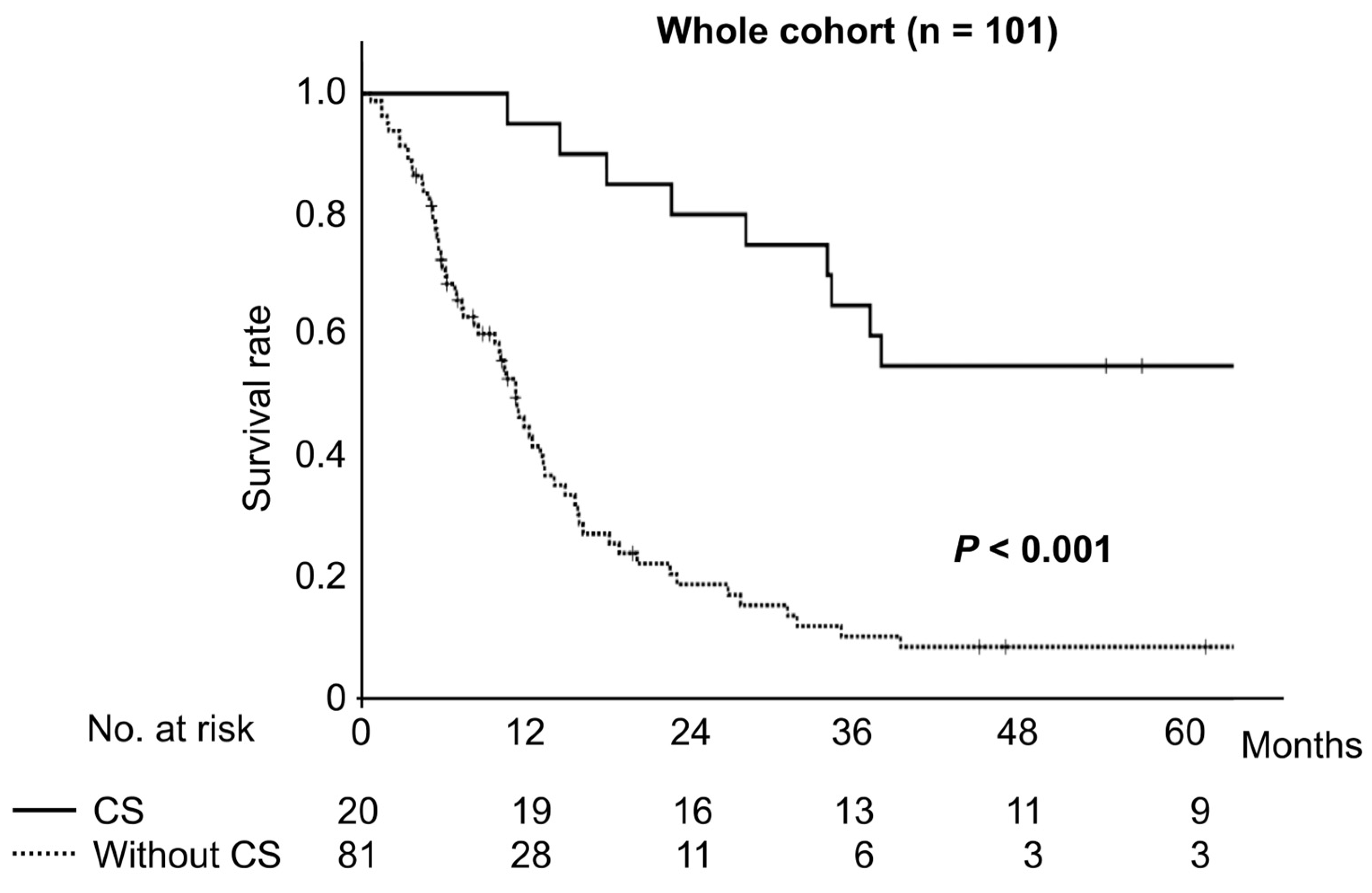
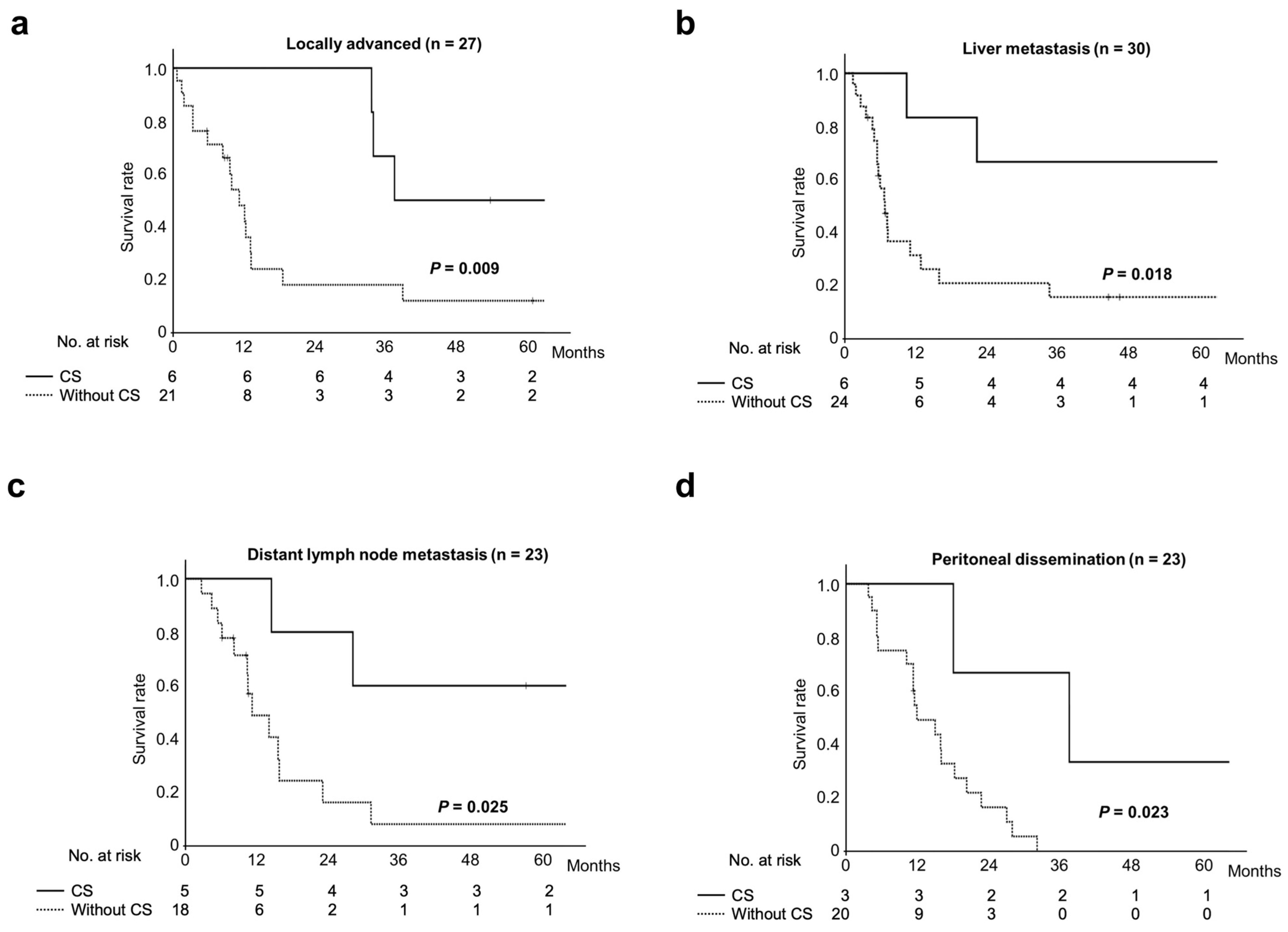
3.4. Survival Comparison Between Patients with Unresectable Biliary Tract Cancer Who Underwent Conversion Surgery and Those Treated by Chemotherapy Alone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomography |
| NAC | Neoadjuvant chemotherapy |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| PD | Progressive disease |
| SD | Stable disease |
| PR | Partial response |
| CDDP | Cisplatin |
References
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. In American Society of Clinical Oncology Educational Book, Proceedings of the 52nd Annual Meeting, Chicago, IL, USA, 3–7 June 2016; American Society of Clinical Oncology: Alexandria, VA, USA, 2016; Volume 35, pp. e194–e203. [Google Scholar]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Horiguchi, A.; Miyakawa, S.; Endo, I.; Miyazaki, M.; Takada, T. Biliary tract cancer registry in Japan from 2008 to 2013. J. Hepatobiliary Pancreat. Sci. 2016, 23, 149–157. [Google Scholar] [CrossRef]
- Koerkamp, B.G.; Fong, Y. Outcomes in biliary malignancy. J. Surg. Oncol. 2014, 110, 585–591. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Kim, S.J.; An, S.; Kang, H.J.; Kim, J.Y.; Jang, M.A.; Lee, J.H.; Song, K.-B.; Hwang, D.W.; Cho, H.; Kim, S.C.; et al. Validation of the eighth edition of the American Joint Committee on Cancer staging system for ampulla of Vater cancer. Surgery. 2018, 163, 1071–1079. [Google Scholar] [CrossRef]
- Nagakawa, T.; Kayahara, M.; Ikeda, S.; Futakawa, S.; Kakita, A.; Kawarada, H.; Matsuno, M.; Takada, T.; Takasaki, K.; Tanimura, H.; et al. Biliary tract cancer treatment: Results from the Biliary Tract Cancer Statistics Registry in Japan. J. Hepatobiliary Pancreat. Surg. 2002, 9, 569–575. [Google Scholar] [CrossRef]
- Miyakawa, S.; Ishihara, S.; Horiguchi, A.; Takada, T.; Miyazaki, M.; Nagakawa, T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009, 16, 1–7. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology; Biliary Tract Cancers, Version 6.2024; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Sasaki, T.; Takeda, T.; Okamoto, T.; Ozaka, M.; Sasahira, N. Chemotherapy for Biliary Tract Cancer in 2021. J. Clin. Med. 2021, 10, 3108. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Kondo, S.; Hirano, S.; Tanaka, E.; Shichinohe, T.; Tsuchikawa, T.; Matsumoto, J. Adjuvant surgical therapy for patients with initially-unresectable pancreatic cancer with long-term favorable responses to chemotherapy. J. Hepatobiliary Pancreat. Sci. 2011, 18, 712–716. [Google Scholar] [CrossRef]
- Satoi, S.; Yamaue, H.; Kato, K.; Takahashi, S.; Hirono, S.; Takeda, S.; Eguchi, H.; Sho, M.; Wada, K.; Shinchi, H.; et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: Results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2013, 20, 590–600. [Google Scholar]
- Kato, H.; Horiguchi, A.; Ito, M.; Asano, Y.; Arakawa, S. Essential updates 2019/2020: Multimodal treatment of localized pancreatic adenocarcinoma: Current topics and updates in survival outcomes and prognostic factors. Ann. Gastroenterol. Surg. 2021, 5, 132–151. [Google Scholar] [CrossRef]
- Union for International Cancer Control. TNM Classification of Malignant Tumours, 8th ed.; Union for International Cancer Control: Geneva, Switzerland, 2016. [Google Scholar]
- Endo, I.; Shimada, H.; Sugita, M.; Fujii, Y.; Morioka, D.; Takeda, K.; Sugae, S.; Tanaka, K.; Togo, S.; Bourquain, H.; et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery 2007, 142, 666–675. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Matuyama, R.; Hiroshima, Y.; Murakami, T.; Bouvet, M.; Morioka, D.; Hoffman, R.M.; Endo, I. Surgical and histological boundary of the hepatic hilar plate system: Basic study relevant to surgery for hilar cholangiocarcinoma regarding the “true” proximal ductal margin. J. Hepatobiliary Pancreat. Sci. 2019, 26, 159–168. [Google Scholar] [CrossRef]
- Noji, T.; Tsuchikawa, T.; Okamura, K.; Shichinohe, T.; Tanaka, E.; Hirano, S. Surgical outcome of hilar plate resection: Extended hilar bile duct resection without hepatectomy. J. Gastrointest. Surg. 2014, 18, 1131–1137. [Google Scholar] [CrossRef]
- Shimada, H.; Endo, I.; Fujii, Y.; Kunihiro, O.; Tanaka, K.; Misuta, K.; Togo, S. Procedure of extended hilar bile duct resection and its application for hilar cholangiocarcinoma. Hepatogastroenterology 2002, 49, 300–305. [Google Scholar]
- Noji, T.; Tanaka, K.; Matsui, A.; Nakanishi, Y.; Asano, T.; Nakamura, T.; Tsuchikawa, T.; Okamura, K.; Hirano, S. Transhepatic Direct Approach to the “Limit of the Division of the Hepatic Ducts” Leads to a High R0 Resection Rate in Perihilar Cholangiocarcinoma. J. Gastrointest. Surg. 2021, 25, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Nimura, Y.; Nagino, M. Changes in hepatic lobar function after right portal vein embolization. An appraisal by biliary indocyanine green excretion. Ann. Surg. 1996, 223, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Kamiya, J.; Nishio, H.; Ebata, T.; Arai, T.; Nimura, Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: Surgical outcome and long-term follow-up. Ann. Surg. 2006, 243, 364–372. [Google Scholar] [CrossRef]
- Matsuyama, R.; Mori, R.; Ota, Y.; Homma, Y.; Yabusita, Y.; Hiratani, S.; Murakami, T.; Sawada, Y.; Miyake, K.; Shimizu, Y.; et al. Impact of gemcitabine plus S1 Neoadjuvant chemotherapy on borderline resectable perihilar cholangiocarcinoma. Ann. Surg. Oncol. 2022, 29, 2393–2405. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer. 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Oneda, E.; Abu Hilal, M.; Zaniboni, A. Biliary Tract Cancer: Current Medical Treatment Strategies. Cancers 2020, 12, 1237. [Google Scholar] [CrossRef]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J. Hepatobiliary Pancreat. Sci. 2023, 30, 102–110. [Google Scholar] [CrossRef]
- Cowzer, D.; Harding, J.J. Advanced Bile Duct Cancers: A Focused Review on Current and Emerging Systemic Treatments. Cancers 2022, 14, 1800. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: A retrospective single-center study. Ann. Surg. Oncol. 2013, 20, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshitomi, H.; Furukawa, K.; Takayashiki, T.; Nakadai, E.; Kishimoto, T.; Nakatani, Y.; Yoshidome, H.; et al. Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer Patients Treated with Gemcitabine Plus Cisplatin Combination Therapy Followed by Radical Surgery. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S1093–S1099. [Google Scholar] [CrossRef] [PubMed]
- Noji, T.; Nagayama, M.; Imai, K.; Kawamoto, Y.; Kuwatani, M.; Imamura, M.; Okamura, K.; Kimura, Y.; Hirano, S. Conversion surgery for initially unresectable biliary malignancies: A multicenter retrospective cohort study. Surg. Today. 2020, 50, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Seo, S.; Yoh, T.; Fukumitsu, K.; Ishii, T.; Taura, K.; Morita, S.; Kaido, T.; Uemoto, S. Proposed Definition for Oligometastatic Recurrence in Biliary Tract Cancer Based on Results of Locoregional Treatment: A Propensity-Score-Stratified Analysis. Ann. Surg. Oncol. 2020, 27, 1908–1917. [Google Scholar] [CrossRef]
- Nakachi, K.; Ikeda, M.; Konishi, M.; Nomura, S.; Katayama, H.; Kataoka, T.; Todaka, A.; Yanagimoto, H.; Morinaga, S.; Kobayashi, S.; et al. Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2023, 401, 95–203. [Google Scholar] [CrossRef]
- Choti, M.A. Chemotherapy-associated hepatotoxicity: Do we need to be concerned? Ann. Surg. Oncol. 2009, 16, 2391–2394. [Google Scholar] [CrossRef]
- Endo, I.; Hirahara, N.; Miyata, H.; Yamamoto, H.; Matsuyama, R.; Kumamoto, T.; Homma, Y.; Mori, M.; Seto, Y.; Wakabayashi, G.; et al. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: An analysis of patients registered in the National Clinical Database in Japan. J. Hepatobiliary Pancreat. Sci. 2021, 28, 305–316. [Google Scholar] [CrossRef]
- Miura, F.; Yamamoto, M.; Gotoh, M.; Konno, H.; Fujimoto, J.; Yanaga, K.; Kokudo, N.; Yamaue, H.; Wakabayashi, G.; Seto, Y.; et al. Validation of the board certification system for expert surgeons (hepato-biliary-pancreatic field) using the data of the National Clinical Database of Japan: Part 2—Pancreatoduodenectomy. J. Hepatobiliary Pancreat. Sci. 2016, 23, 353–363. [Google Scholar] [CrossRef]
- Miura, F.; Yamamoto, M.; Gotoh, M.; Konno, H.; Fujimoto, J.; Yanaga, K.; Kokudo, N.; Yamaue, H.; Wakabayashi, G.; Seto, Y.; et al. Validation of the board certification system for expert surgeons (hepato-biliary-pancreatic field) using the data of the National Clinical Database of Japan: Part 1—Hepatectomy of more than one segment. J. Hepatobiliary Pancreat. Sci. 2016, 23, 313–323. [Google Scholar] [CrossRef]
| Factors | Initially Unresectable BTC (n = 101) |
|---|---|
| Age (years) | 66.5 (34–84) |
| Sex | |
| Male | 69 |
| Female | 32 |
| Anatomical tumor location | |
| Intrahepatic cholangiocarcinoma | 35 |
| Perihilar cholangiocarcinoma | 40 |
| Distal bile duct cancer | 2 |
| Gallbladder cancer | 20 |
| Ampullary carcinoma | 4 |
| Reason for unresectability | |
| Locally advanced disease | 27 |
| Metastatic disease | 74 |
| Liver metastasis | 30 |
| Lung metastasis | 7 |
| Bone metastasis | 4 |
| Distant lymph node metastasis | 23 |
| Peritoneal dissemination | 23 |
| Treatment regimen | |
| Gem + CDDP | 79 |
| Gem + S-1 | 56 |
| Gem | 16 |
| Radiation | |
| Yes | 6 |
| No | 0 |
| Cycles of chemotherapy | 13.7 (1–89) |
| RECIST | |
| Complete response | 1 |
| Partial response | 9 |
| Stable disease | 45 |
| Progressive disease | 42 |
| Not evaluated | 4 |
| Conversion surgery | |
| Yes | 20 |
| No | 81 |
| Case | Age, Sex | Tumor Location | Reason for Unresectability | Staging at Diagnosis | Regimen | RECIST | Staging After Chemotherapy | Surgical Procedure | Final Staging | Residual Tumor | Complications ≥ Grade IIIa | Recurrence | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40s, female | IHCC | Locally advanced (A, PV) | T3N1M0, Stage IIIB | Gem + CDDP × 8 | PR | T3N0M0, Stage IIIA | LT + C + BDR | T1aN0M0, Stage IA | R0 | Abdominal abscess | No | 53.9 months, alive |
| 2 | 50s, female | IHCC | Locally advanced (IVC, HV) | T4N0M0, Stage IIIB | Gem + CDDP × 8 | SD | T4N0M0, Stage IIIB | RT + C + BDR + PVR + IVCR + HVR | T3N0M0, Stage IIIA | R0 | - | Yes | 33.7 months, dead |
| 3 | 50s, male | IHCC | Locally advanced (A) | T4N1M0, Stage IIIB | Gem + CDDP × 10 | SD | T3N1M0, Stage IIIB | RH + C + BDR + PVR | T3N1M1, Stage IV | R1 | Abdominal abscess | Yes | 34.0 months, dead |
| 4 | 30s, female | IHCC | Locally advanced (IVC) | T4N1M0, Stage IIIB | Gem × 11 | SD | T4N0M0, Stage IIIB | LH + C + BDR + IVCR | T1aN0M0, Stage IA | R0 | - | Yes | 113.5 months, alive |
| 5 | 50s, female | IHCC | Locally advanced (IVC) | T4N1M0, Stage IIIB | Gem + CDDP × 16 | PR | T3N0M0, Stage IIIA | LH + C + BDR + AR | T2N0M0, Stage II | R0 | Bowel obstruction | Yes | 84.3 months, alive |
| 6 | 50s, female | IHCC | Locally advanced (IVC) | T4N0M0, Stage IIIB | Gem + CDDP × 24 | PR | T4N0M0, Stage IIIB | RH + C + BDR + IVCR + Sp | T2N1M0, Stage IIIB | R1 | Liver failure, Bile leakage | No | 37.6 months, dead |
| 7 | 70s, female | Perihilar CC | Liver metastasis | T3N1M1, Stage IVB | Gem + S-1 × 3 | SD | T2bN1M1, Stage IVB | RH + C + BDR + PVR | T2bN1M1, Stage IVB | R0 | - | No | 94.2 months, alive |
| 8 | 60s, male | Distal BDC | Liver metastasis | T3N1M1, Stage IV | Gem + S-1 × 9 | PR | T3N1M0, Stage IIIC | PD | T2N1M0, Stage IIB | R0 | POPF | Yes | 22.4 months, dead |
| 9 | 60s, female | GBC | Liver metastasis | T4N1M1, Stage IVB | Gem + S-1 × 3 Gem + CDDP × 3 | SD | T4N1M1, Stage IVB | Sec + C + BDR + Du + Co | T4N1M1, Stage IVB | R0 | Abdominal abscess | Yes | 10.5 months, dead |
| 10 | 70s, female | GBC | Liver metastasis | T4N1M1, Stage IVB | Gem + S-1 × 6 | PR | T3N1M0, Stage IIIB | Seg + BDR | T2bN0M0, Stage IIB | R0 | - | No | 92.5 months, alive |
| 11 | 70s, female | Ampullary Ca | Liver metastasis | T2N0M1, Stage IV | Gem + S-1 × 4 S-1 × 5 | PR | T2N0M0, Stage IB | PD | T1aN0M0, Stage IA | R0 | POPF | No | 87.5 months, alive |
| 12 | 80s, male | Perihilar CC | Liver metastasis | T3N1M1, Stage IVB | Gem + CDDP × 6 | SD | T3N0M0, Stage IIIA | EHBDR + IVCR + Co | T2aN0M0, Stage II | R0 | - | Yes | 98.8 months, dead |
| 13 | 70s, female | Perihilar CC | Peritoneal dissemination | T4N1M1, Stage IVB | Gem + CDDP × 8 | SD | T4N1M0, Stage IIIC | LH + C + BDR + AR + PVR | T2bN0M0, Stage II | R0 | Abdominal abscess | No | 66.2 months, alive |
| 14 | 60s, female | Perihilar CC | Peritoneal dissemination | T3N2M1, Stage IVB | Gem + CDDP × 8 | SD | T3N1M0, Stage IIIC | RH + C + BDR + PD + PVR | T2bN1M0, Stage IIIC | R0 | - | Yes | 17.7 months, dead |
| 15 | 50s, female | IHCC | Peritoneal dissemination | T2N1M1, Stage IV | Gem + CDDP × 8 | PR | T2N1M0, Stage IIIB | LH + C + BDR + AR | T1aN0M1, Stage IV | R0 | - | Yes | 36.8 months, dead |
| 16 | 60s, female | IHCC | Para-aortic lymph node metastasis | T4N1M1, Stage IV | Gem + S-1 × 6 | SD | T4N1M0, Stage IIIB | RT + C + BDR + PVR + IVCR | T4N1M0, Stage IIIB | R0 | - | Yes | 91.7 months, alive |
| 17 | 50s, male | Perihilar CC | Para-aortic lymph node metastasis | T3N2M1, Stage IVB | Gem + CDDP × 23 | SD | T3N2M1, Stage IVB | RT + C + BDR + AR + PVR | T2bN2M0, Stage IVA | R0 | Enteritis | Yes | 67.4 months, alive |
| 18 | 70s, male | Perihilar CC | Para-aortic lymph node metastasis | T3N2M1, Stage IVB | Gem + CDDP × 9 | SD | T3N2M0, Stage IVA | RH + C + BDR + PVR | T3N2M0, Stage IVA | R1 | Postoperative bleeding | Yes | 27.8 months, alive |
| 19 | 70s, male | Perihilar CC | Para-aortic lymph node metastasis | T2aN2M1, Stage IVB | Gem + CDDP × 8 | SD | T2aN2M0, Stage IVA | EHBDR + PD | T2aN2M0, Stage IVA | R0 | Cholangitis, septic shock | Yes | 14.3 months, dead |
| 20 | 60s, male | Perihilar CC | Para-aortic lymph node metastasis | T2bN2M1, Stage IVB | Gem + CDDP × 10 | SD | T2bN2M0, Stage IVA | Sec + C + BDR + PD | T1N0M0, Stage I | R0 | - | Yes | 56.5 months, alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Matsuyama, R.; Yabushita, Y.; Homma, Y.; Sawada, Y.; Miyake, K.; Kumamoto, T.; Takeda, K.; Maeda, S.; Yamanaka, S.; et al. Efficacy of Conversion Surgery for Initially Unresectable Biliary Tract Cancer That Has Responded to Down-Staging Chemotherapy. Cancers 2025, 17, 873. https://doi.org/10.3390/cancers17050873
Murakami T, Matsuyama R, Yabushita Y, Homma Y, Sawada Y, Miyake K, Kumamoto T, Takeda K, Maeda S, Yamanaka S, et al. Efficacy of Conversion Surgery for Initially Unresectable Biliary Tract Cancer That Has Responded to Down-Staging Chemotherapy. Cancers. 2025; 17(5):873. https://doi.org/10.3390/cancers17050873
Chicago/Turabian StyleMurakami, Takashi, Ryusei Matsuyama, Yasuhiro Yabushita, Yuki Homma, Yu Sawada, Kentaro Miyake, Takafumi Kumamoto, Kazuhisa Takeda, Shin Maeda, Shoji Yamanaka, and et al. 2025. "Efficacy of Conversion Surgery for Initially Unresectable Biliary Tract Cancer That Has Responded to Down-Staging Chemotherapy" Cancers 17, no. 5: 873. https://doi.org/10.3390/cancers17050873
APA StyleMurakami, T., Matsuyama, R., Yabushita, Y., Homma, Y., Sawada, Y., Miyake, K., Kumamoto, T., Takeda, K., Maeda, S., Yamanaka, S., & Endo, I. (2025). Efficacy of Conversion Surgery for Initially Unresectable Biliary Tract Cancer That Has Responded to Down-Staging Chemotherapy. Cancers, 17(5), 873. https://doi.org/10.3390/cancers17050873








