Enhancing Progestin Therapy with a Glucagon-Like Peptide 1 Agonist for the Conservative Management of Endometrial Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
| Gene | 5′-SEQUENCE-3′ | Tm | |
| GLP1R | For | CAGGCTCGTTCGTGAATGT | 55.4 |
| Rev | GCGATAACCAGAGCAGAGAAG | 55.3 | |
| PGRMC1 | For | AGCAGGAGACTCTGAGTGACTG | 58.0 |
| Rev | CCTCATCTGCGTACACAGTGGG | 56.7 | |
| PGRMC2 | For | CGTGACCAAAGGCAGCAAGTTC | 59.1 |
| Rev | CTCTAAGTGCATCTTTATCTAGGC | 52.8 | |
| FOXO1 | For | CTACGAGTGGATGGTCAAGAGC | 57.1 |
| Rev | CCAGTTCCTTCATTCTGCACACG | 58.4 | |
| ERα | For | TGGGCTTACTGACCAACCTG | 57.1 |
| Rev | CCTGATCATGGAGGGTCAAA | 54.4 | |
| AR | For | ATGGTCCCTGGCAGTCTCCAAA | 60.7 |
| Rev | ATGGTGAGCAGAGTGCCCTATC | 58.8 | |
| GR | For | GGAATAGGTGCCAAGGATCTGG | 57.4 |
| Rev | GCTTACATCTGGTCTCATGCTGG | 57.7 | |
| MR | For | AAATCACACGGCGACCTGTCGT | 61.4 |
| Rev | ATGGCATCCTGAAGCCTCATCC | 59.4 | |
| PGR | For | ATCCTACAAACACGTCAGTGGGCA | 60.5 |
| Rev | ACTGGGTTTGACTTCGTAGCCCTT | 60.3 | |
| 18S rRNA | For | AACCTTTCGATGGTAGTCGCCG | 59.4 |
| Rev | CCTTGGATGTGGTAGCCGTTT | 57.6 |
3. Results
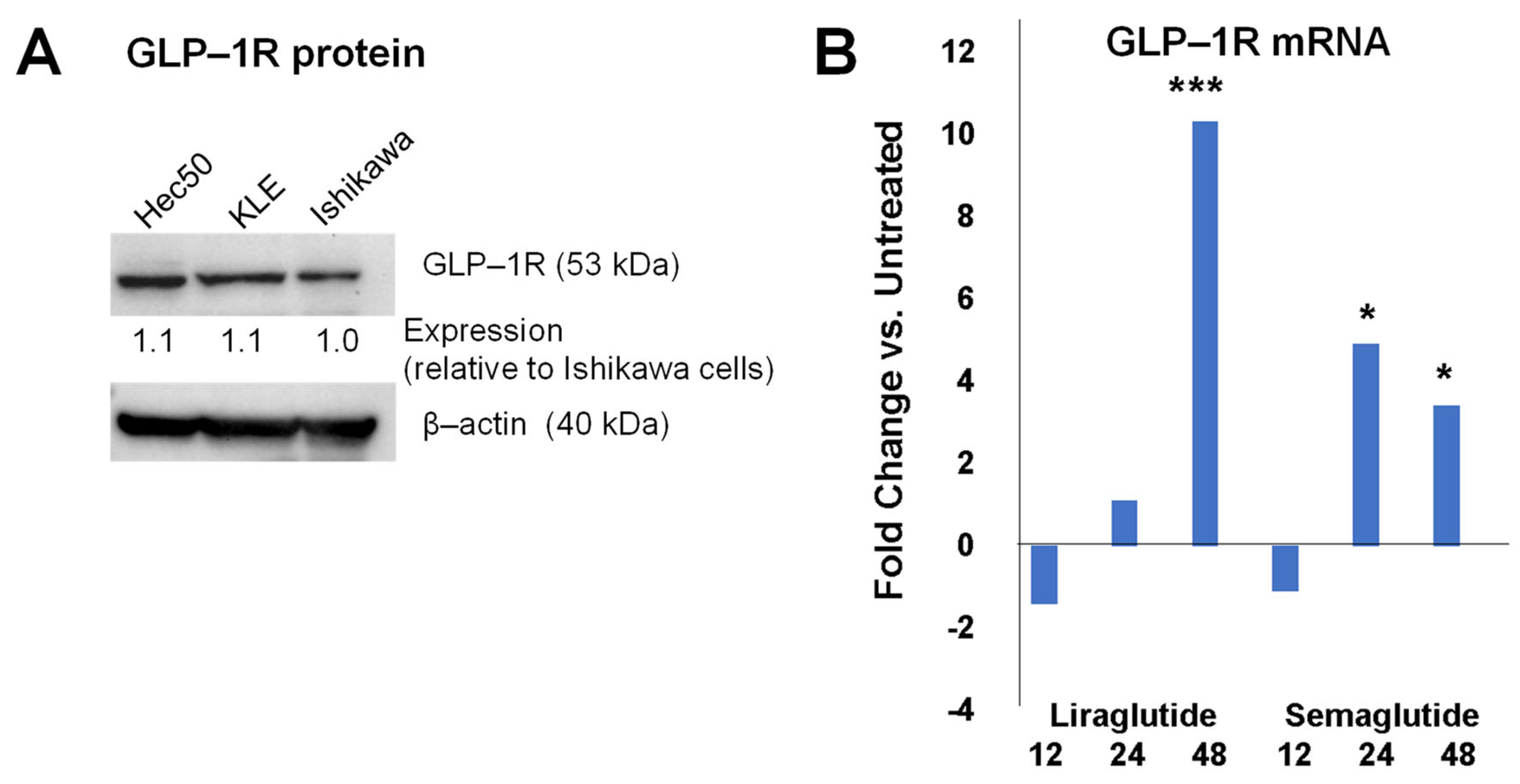
3.1. GLP-1R Expression in Endometrial Cancer Cell Models
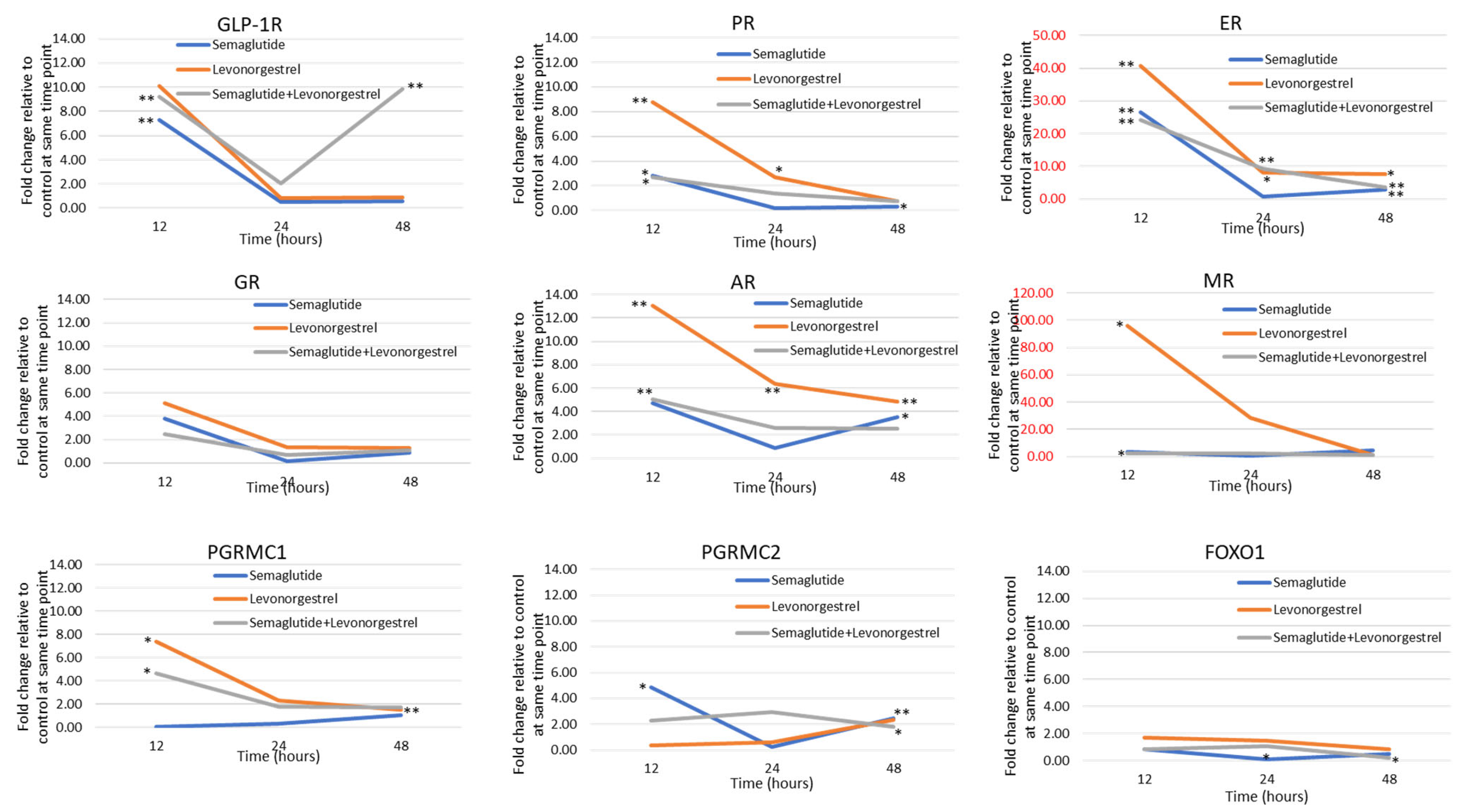
3.2. GLP-1R Agonists and Levonorgestrel Enhance Hormone Signaling Through the Upregulation of Steroid Hormone Receptors and Other Pathway Genes
3.3. Effects of GLP-1 Agonist in Combination with Levonorgestrel on Growth of Patient-Derived Organoid Models of Endometrial Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—Viewpoint of the IARC working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.R.; Gill, P.; Lensen, S.; Thompson, J.M.; Farquhar, C.M. Body mass index trumps age in decision for endometrial biopsy: Cohort study of symptomatic premenopausal women. Am. J. Obstet. Gynecol. 2016, 215, 598.e1–598.e8. [Google Scholar] [CrossRef]
- Wise, M.R.; Jordan, V.; Lagas, A.; Showell, M.; Wong, N.; Lensen, S.; Farquhar, C.M. Obesity and endometrial hyperplasia and cancer in premenopausal women: A systematic review. Am. J. Obstet. Gynecol. 2016, 214, e681–e689. e617. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer. J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Orbo, A.; Arnes, M.; Vereide, A.B.; Straume, B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel-impregnated intrauterine system or oral progestogens. BJOG: Int. J. Obstet. Gynaecol. 2016, 123, 1512–1519. [Google Scholar] [CrossRef]
- Chen, J.; Cao, D.; Yang, J.; Yu, M.; Zhou, H.; Cheng, N.; Wang, J.; Zhang, Y.; Peng, P.; Shen, K. Fertility-sparing treatment for endometrial cancer or atypical endometrial hyperplasia patients with obesity. Front. Oncol. 2022, 12, 812346. [Google Scholar] [CrossRef]
- Thiebaut, C.; Vlaeminck-Guillem, V.; Tredan, O.; Poulard, C.; Le Romancer, M. Non-genomic signaling of steroid receptors in cancer. Mol. Cell Endocrinol. 2021, 538, 111453. [Google Scholar] [CrossRef]
- Yang, S.; Thiel, K.W.; Leslie, K.K. Progesterone: The ultimate endometrial tumor suppressor. Trends Endocrinol. Metab. 2011, 22, 145–152. [Google Scholar] [CrossRef]
- Carlson, M.J.; Thiel, K.W.; Yang, S.; Leslie, K.K. Catch it before it kills: Progesterone, obesity, and the prevention of endometrial cancer. Discov. Med. 2012, 14, 215–222. [Google Scholar]
- Dai, D.; Litman, E.S.; Schonteich, E.; Leslie, K.K. Progesterone regulation of Activating pProtein-1 transcriptional activity: A possible mechanism of progesterone inhibition of endometrial cancer cell growth. J. Steroid. Biochem. Mol. Biol. 2003, 87, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Wolf, D.M.; Litman, E.S.; White, M.J.; Leslie, K.K. Progesterone inhibits human endometrial cancer cell growth and invasiveness: Down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res. 2002, 62, 881–886. [Google Scholar]
- Davies, S.; Dai, D.; Feldman, I.; Pickett, G.; Leslie, K.K. Identification of a novel mechanism of NF-kappaB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: Induction of a20 and abin-2. Gynecol. Oncol. 2004, 94, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Richer, J.; Owen, G.; Litman, E.; Horwitz, K.B.; Leslie, K.K. Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: Implications for unopposed estrogen action. Cancer Res. 1998, 58, 1860–1865. [Google Scholar] [PubMed]
- Leslie, K.K.; Thiel, K.W.; Yang, S. Endometrial cancer: Potential treatment and prevention with progestin-containing intrauterine devices. Obstet. Gynecol. 2012, 119, 419–420. [Google Scholar] [CrossRef]
- Singh, M.; Zaino, R.J.; Filiaci, V.J.; Leslie, K.K. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: A Gynecologic Oncology rGoup study. Gynecol. Oncol. 2007, 106, 325–333. [Google Scholar] [CrossRef]
- Thiel, K.W.; Newtson, A.M.; Devor, E.J.; Zhang, Y.; Malmrose, P.K.; Bi, J.; Losh, H.A.; Davies, S.; Smith, L.E.; Padilla, J.; et al. Global expression analysis of endometrial cancer cells in response to progesterone identifies new therapeutic targets. J. Steroid. Biochem. Mol. Biol. 2023, 234, 106399. [Google Scholar] [CrossRef]
- Yang, S.; Thiel, K.W.; De Geest, K.; Leslie, K.K. Endometrial cancer: Reviving progesterone therapy in the molecular age. Discov. Med. 2011, 12, 205–212. [Google Scholar]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, C.E.; Young, P.C.; Stehman, F.B.; Sutton, G.P.; Alford, W.M. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am. J. Obstet. Gynecol. 1988, 158, 796–807. [Google Scholar] [CrossRef]
- Garcia-Saenz, M.; Ibarra-Salce, R.; Pozos-Varela, F.J.; Mena-Ureta, T.S.; Flores-Villagomez, S.; Santana-Mata, M.; De Los Santos-Aguilar, R.G.; Uribe-Cortes, D.; Ferreira-Hermosillo, A. Understanding progestins: From basics to clinical applicability. J. Clin. Med. 2023, 12, 3388. [Google Scholar] [CrossRef]
- Albitar, L.; Pickett, G.; Morgan, M.; Davies, S.; Leslie, K.K. Models representing Type I and Type II human endometrial cancers: Ishikawa H and Hec50co cells. Gynecol. Oncol. 2007, 106, 52–64. [Google Scholar] [CrossRef]
- Devor, E.J.; Gonzalez-Bosquet, J.; Thiel, K.W.; Leslie, K.K. Genomic characterization of five commonly used endometrial cancer cell lines. Int. J. Oncol. 2020, 57, 1348–1357. [Google Scholar] [CrossRef]
- Bi, J.; Newtson, A.M.; Zhang, Y.; Devor, E.J.; Samuelson, M.I.; Thiel, K.W.; Leslie, K.K. Successful patient-derived organoid culture of gynecologic cancers for disease modeling and drug sensitivity testing. Cancers 2021, 13, 2901. [Google Scholar] [CrossRef]
- Bi, J.; Thiel, K.W.; Litman, J.M.; Zhang, Y.; Devor, E.J.; Newtson, A.M.; Schnieders, M.J.; Gonzalez Bosquet, J.; Leslie, K.K. Characterization of a TP53 somatic variant of unknown function from an ovarian cancer patient using organoid culture and computational modeling. Clin. Obstet. Gynecol. 2020, 63, 109–119. [Google Scholar] [CrossRef]
- Kanda, R.; Hiraike, H.; Wada-Hiraike, O.; Ichinose, T.; Nagasaka, K.; Sasajima, Y.; Ryo, E.; Fujii, T.; Osuga, Y.; Ayabe, T. Expression of the glucagon-like peptide-1 receptor and its role in regulating autophagy in endometrial cancer. BMC Cancer 2018, 18, 657. [Google Scholar] [CrossRef]
- Lindemann, K.; Vatten, L.J.; Ellstrom-Engh, M.; Eskild, A. The impact of BMI on subgroups of uterine cancer. Br. J. Cancer 2009, 101, 534–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Park, J.Y.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Bae, D.S.; Nam, J.H. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol. Oncol. 2017, 146, 39–43. [Google Scholar] [CrossRef]
- Cholakian, D.; Hacker, K.; Fader, A.N.; Gehrig, P.A.; Tanner, E.J., 3rd. Effect of oral versus intrauterine progestins on weight in women undergoing fertility preserving therapy for complex atypical hyperplasia or endometrial cancer. Gynecol. Oncol. 2016, 140, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Cosmes, P.; Vazquez-Martinez, E.R.; Cerbon, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef]
- Zhang, M.; Robitaille, M.; Showalter, A.D.; Huang, X.; Liu, Y.; Bhattacharjee, A.; Willard, F.S.; Han, J.; Froese, S.; Wei, L.; et al. Progesterone receptor membrane component 1 is a functional part of the glucagon-like peptide-1 (GLP-1) receptor complex in pancreatic beta cells. Mol. Cell Proteom. 2014, 13, 3049–3062. [Google Scholar] [CrossRef] [PubMed]

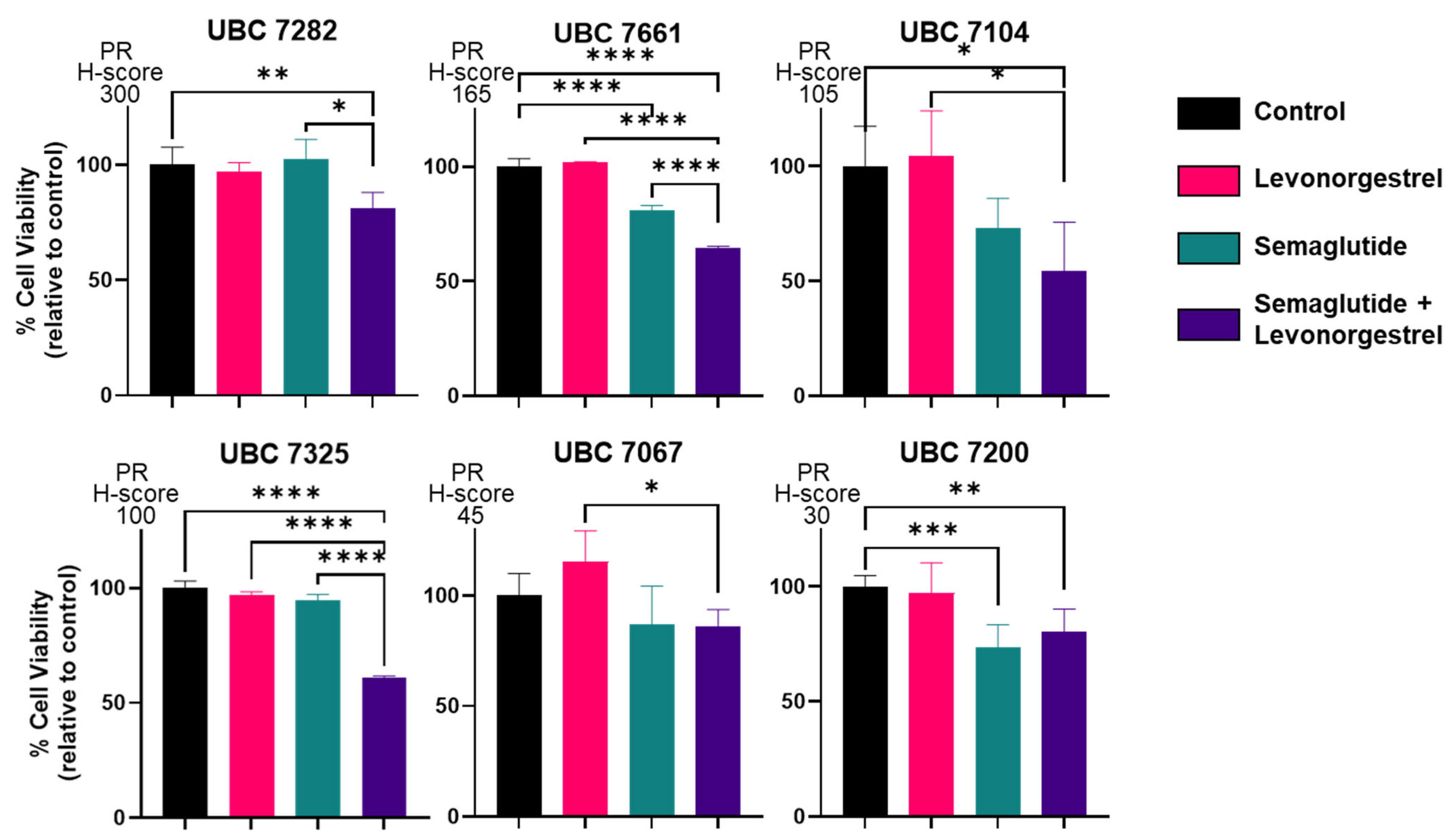

| ID | Age | Race/Ethnicity | Diagnosis (Endometrial Adenocarcinoma) | Primary Adjuvant Treatment | Disease Status (as of 11/2024) | ER IHC | PR IHC |
|---|---|---|---|---|---|---|---|
| UBC 7282 | 68 | White Non-Hispanic | Stage IA/Grade 1 | Clinical surveillance | Dead (metastatic cancer; cannot rule out endometrial cancer as primary site of origin) | 170 | 300 |
| UBC 7661 | 69 | White Non-Hispanic | Stage IA/Grade 1 | Clinical surveillance | NED | 185 | 165 |
| UBC 7104 | 66 | White Non-Hispanic | Stage IA/Grade 1 | Clinical surveillance | NED | 70 | 105 |
| UBC 7325 | 34 | White Non-Hispanic | Stage IA/Grade 1 | Clinical surveillance | NED | 200 | 100 |
| UBC 7067 | 69 | White Non-Hispanic | Stage 1B/Grade 1 | Vaginal brachytherapy | NED | 255 | 45 |
| UBC 7200 | 45 | White Non-Hispanic | Stage IA/Grade 1 | Clinical surveillance | NED | 240 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagemann, A.R.; Hagemann, I.S.; Mutch, D.G.; Devor, E.J.; Malmrose, P.K.; Zhang, Y.; Morrison, A.M.; Thiel, K.W.; Leslie, K.K. Enhancing Progestin Therapy with a Glucagon-Like Peptide 1 Agonist for the Conservative Management of Endometrial Cancer. Cancers 2025, 17, 598. https://doi.org/10.3390/cancers17040598
Hagemann AR, Hagemann IS, Mutch DG, Devor EJ, Malmrose PK, Zhang Y, Morrison AM, Thiel KW, Leslie KK. Enhancing Progestin Therapy with a Glucagon-Like Peptide 1 Agonist for the Conservative Management of Endometrial Cancer. Cancers. 2025; 17(4):598. https://doi.org/10.3390/cancers17040598
Chicago/Turabian StyleHagemann, Andrea R., Ian S. Hagemann, David G. Mutch, Eric J. Devor, Paige K. Malmrose, Yuping Zhang, Abigail M. Morrison, Kristina W. Thiel, and Kimberly K. Leslie. 2025. "Enhancing Progestin Therapy with a Glucagon-Like Peptide 1 Agonist for the Conservative Management of Endometrial Cancer" Cancers 17, no. 4: 598. https://doi.org/10.3390/cancers17040598
APA StyleHagemann, A. R., Hagemann, I. S., Mutch, D. G., Devor, E. J., Malmrose, P. K., Zhang, Y., Morrison, A. M., Thiel, K. W., & Leslie, K. K. (2025). Enhancing Progestin Therapy with a Glucagon-Like Peptide 1 Agonist for the Conservative Management of Endometrial Cancer. Cancers, 17(4), 598. https://doi.org/10.3390/cancers17040598








