Risk Assessment Models for Predicting Venous Thromboembolism in Patients with Pancreatic Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
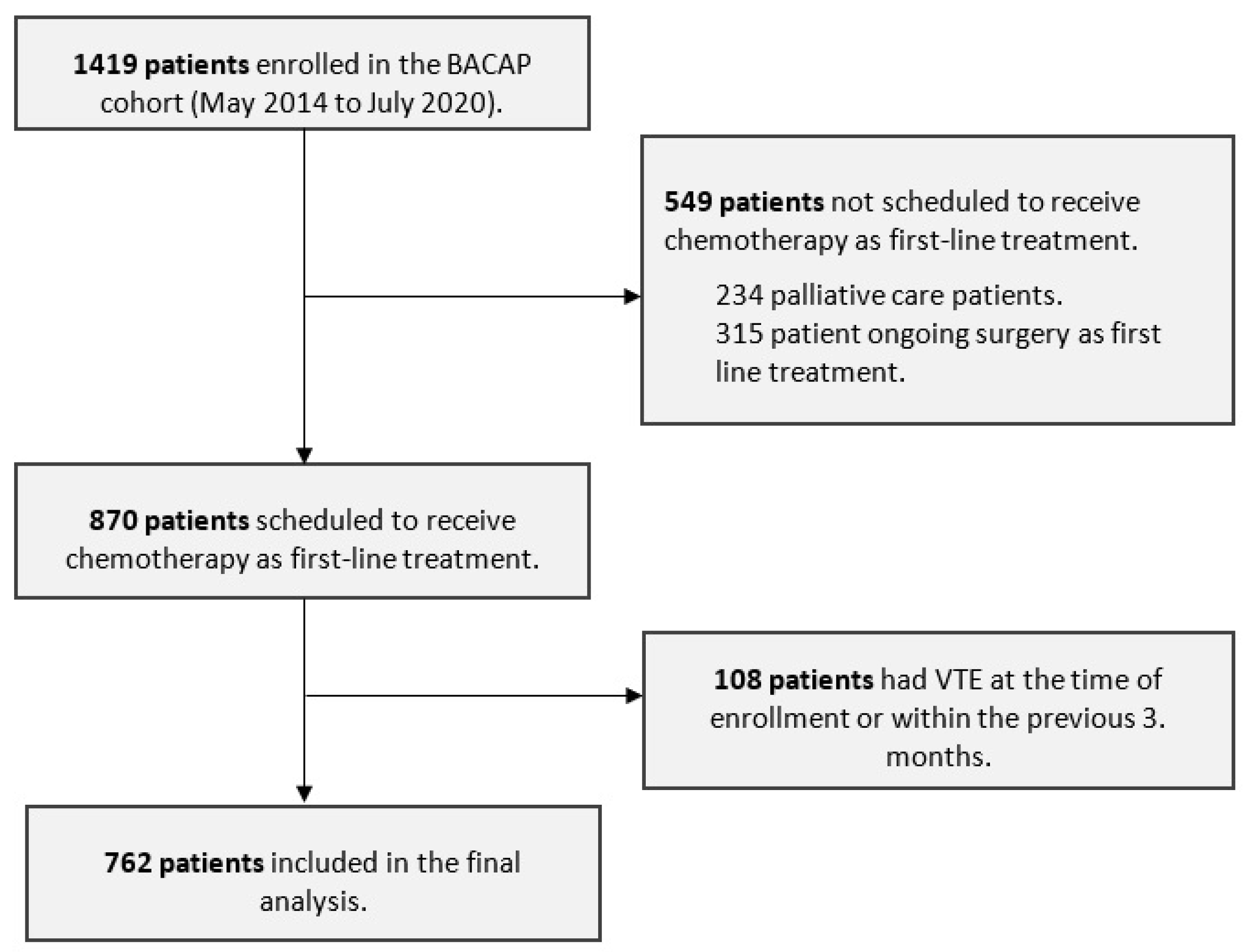
2.1. Study Design and Participants
2.2. Risk Assessment Models
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Outcomes
3.3. Accuracy and Discriminatory Performance of the Scores to Predict the 6-Month Risk of VTE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Members of the BACAP Consortium
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic Cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Horváth-Puhó, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous Thromboembolism in Cancer Patients: A Population-Based Cohort Study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Reni, M.; Cascinu, S.; Barni, S.; Floriani, I.; Cereda, S.; Berardi, R.; Mosconi, S.; Torri, V.; Labianca, R. Venous Thromboembolism Predicts Poor Prognosis in Irresectable Pancreatic Cancer Patients. Ann. Oncol. 2007, 18, 1660–1665. [Google Scholar] [CrossRef]
- Menapace, L.A.; Peterson, D.R.; Berry, A.; Sousou, T.; Khorana, A.A. Symptomatic and Incidental Thromboembolism Are Both Associated with Mortality in Pancreatic Cancer. Thromb. Haemost. 2011, 106, 371–378. [Google Scholar] [CrossRef]
- Berger, A.K.; Singh, H.M.; Werft, W.; Muckenhuber, A.; Sprick, M.R.; Trumpp, A.; Weichert, W.; Jäger, D.; Springfeld, C. High Prevalence of Incidental and Symptomatic Venous Thromboembolic Events in Patients with Advanced Pancreatic Cancer under Palliative Chemotherapy: A Retrospective Cohort Study. Pancreatology 2017, 17, 629–634. [Google Scholar] [CrossRef]
- Ouaissi, M.; Frasconi, C.; Mege, D.; Panicot-Dubois, L.; Boiron, L.; Dahan, L.; Debourdeau, P.; Dubois, C.; Farge, D.; Sielezneff, I. Impact of Venous Thromboembolism on the Natural History of Pancreatic Adenocarcinoma. HBPD INT 2015, 14, 436–442. [Google Scholar] [CrossRef]
- Frere, C.; Bournet, B.; Gourgou, S.; Fraisse, J.; Canivet, C.; Connors, J.M.; Buscail, L.; Farge, D. BACAP Consortium Incidence of Venous Thromboembolism in Patients with Newly Diagnosed Pancreatic Cancer and Factors Associated With Outcomes. Gastroenterology 2020, 158, 1346–1358.e4. [Google Scholar] [CrossRef]
- García Adrián, S.; González, A.R.; de Castro, E.M.; Olmos, V.P.; Morán, L.O.; Del Prado, P.M.; Fernández, M.S.; Burón, J.D.C.; Escobar, I.G.; Galán, J.M.; et al. Incidence, Risk Factors, and Evolution of Venous Thromboembolic Events in Patients Diagnosed with Pancreatic Carcinoma and Treated with Chemotherapy on an Outpatient Basis. Eur. J. Intern. Med. 2022, 105, 30–37. [Google Scholar] [CrossRef]
- Laderman, L.; Sreekrishnanilayam, K.; Pandey, R.K.; Handorf, E.; Blumenreich, A.; Sorice, K.A.; Lynch, S.M.; Cheema, K.; Nagappan, L.; Sosa, I.R.; et al. Venous Thromboembolism in Metastatic Pancreatic Cancer. Eur. J. Haematol. 2023, 110, 706–714. [Google Scholar] [CrossRef]
- Frere, C.; Crichi, B.; Bournet, B.; Canivet, C.; Abdallah, N.A.; Buscail, L.; Farge, D. Primary Thromboprophylaxis in Ambulatory Pancreatic Cancer Patients Receiving Chemotherapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and Validation of a Predictive Model for Chemotherapy-Associated Thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Barni, S.; Gasparini, G.; LaBianca, R. A Modified Khorana Risk Assessment Score for Venous Thromboembolism in Cancer Patients Receiving Chemotherapy: The Protecht Score. Intern. Emerg. Med. 2012, 7, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Cella, C.A.; Di Minno, G.; Carlomagno, C.; Arcopinto, M.; Cerbone, A.M.; Matano, E.; Tufano, A.; Lordick, F.; De Simone, B.; Muehlberg, K.S.; et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. Oncologist 2017, 22, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Godinho, J.; Casa-Nova, M.; Moreira-Pinto, J.; Simões, P.; Paralta Branco, F.; Leal-Costa, L.; Faria, A.; Lopes, F.; Teixeira, J.A.; Passos-Coelho, J.L. ONKOTEV Score as a Predictive Tool for Thromboembolic Events in Pancreatic Cancer-A Retrospective Analysis. Oncologist 2020, 25, e284–e290. [Google Scholar] [CrossRef]
- Canivet, C.; Gourgou-Bourgade, S.; Napoléon, B.; Palazzo, L.; Flori, N.; Guibert, P.; Piessen, G.; Farges-Bancel, D.; Seitz, J.-F.; Assenat, E.; et al. A Prospective Clinical and Biological Database for Pancreatic Adenocarcinoma: The BACAP Cohort. BMC Cancer 2018, 18, 986. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the Pancreas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 131, 211–219. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- White, I.R.; Royston, P.; Wood, A.M. Multiple Imputation Using Chained Equations: Issues and Guidance for Practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Gerds, T.A.; Schumacher, M. Consistent Estimation of the Expected Brier Score in General Survival Models with Right-Censored Event Times. Biom. J. 2006, 48, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Brier, G.W. Verification of Forecasts Expressed in Terms of Probability. Mon. Weather. Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Sweeney, C.J.; Connors, J.M. Re: Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. Eur. Urol. 2020, 77, 388–390. [Google Scholar] [CrossRef]
- Li, A.; Kuderer, N.M.; Garcia, D.A.; Khorana, A.A.; Wells, P.S.; Carrier, M.; Lyman, G.H. Direct Oral Anticoagulant for the Prevention of Thrombosis in Ambulatory Patients with Cancer: A Systematic Review and Meta-analysis. J. Thromb. Haemost. 2019, 17, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Martin, K.A.; Molsberry, R.; Khan, S.S.; Linder, J.A.; Cameron, K.A.; Benson, A. Preventing Venous Thromboembolism in Oncology Practice: Use of Risk Assessment and Anticoagulation Prophylaxis. Res. Pract. Thromb. Haemost. 2020, 4, 1211–1215. [Google Scholar] [CrossRef]
- Muñoz Martín, A.J.; García Alfonso, P.; Rupérez Blanco, A.B.; Pérez Ramírez, S.; Blanco Codesido, M.; Martín Jiménez, M. Incidence of Venous Thromboembolism (VTE) in Ambulatory Pancreatic Cancer Patients Receiving Chemotherapy and Analysis of Khorana’s Predictive Model. Clin. Transl. Oncol. 2014, 16, 927–930. [Google Scholar] [CrossRef]
- Kruger, S.; Haas, M.; Burkl, C.; Goehring, P.; Kleespies, A.; Roeder, F.; Gallmeier, E.; Ormanns, S.; Westphalen, C.B.; Heinemann, V.; et al. Incidence, Outcome and Risk Stratification Tools for Venous Thromboembolism in Advanced Pancreatic Cancer—A Retrospective Cohort Study. Thromb. Res. 2017, 157, 9–15. [Google Scholar] [CrossRef]
- van Es, N.; Franke, V.F.; Middeldorp, S.; Wilmink, J.W.; Büller, H.R. The Khorana Score for the Prediction of Venous Thromboembolism in Patients with Pancreatic Cancer. Thromb. Res. 2017, 150, 30–32. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, E.J.; Kim, D.S.; Choi, Y.J.; Lee, S.Y.; Kim, H.J.; Seo, H.Y.; Kim, J.S. Early Venous Thromboembolism at the Beginning of Palliative Chemotherapy Is a Poor Prognostic Factor in Patients with Metastatic Pancreatic Cancer: A Retrospective Study. BMC Cancer 2018, 18, 1260. [Google Scholar] [CrossRef] [PubMed]
- Overvad, T.F.; Ording, A.G.; Nielsen, P.B.; Skjøth, F.; Albertsen, I.E.; Noble, S.; Vistisen, A.K.; Gade, I.L.; Severinsen, M.T.; Piazza, G.; et al. Validation of the Khorana Score for Predicting Venous Thromboembolism in 40 218 Patients with Cancer Initiating Chemotherapy. Blood Adv. 2022, 6, 2967–2976. [Google Scholar] [CrossRef]
- Di Nisio, M.; van Es, N.; Rotunno, L.; Anzoletti, N.; Falcone, L.; De Tursi, M.; Natoli, C.; Tinari, N.; Cavallo, I.; Valeriani, E.; et al. Long-Term Performance of Risk Scores for Venous Thromboembolism in Ambulatory Cancer Patients. J. Thromb. Thrombolysis 2019, 48, 125–133. [Google Scholar] [CrossRef]
- Guman, N.A.M.; van Geffen, R.J.; Mulder, F.I.; van Haaps, T.F.; Hovsepjan, V.; Labots, M.; Cirkel, G.A.; Y F L de Vos, F.; Ten Tije, A.J.; Beerepoot, L.V.; et al. Evaluation of the Khorana, PROTECHT, and 5-SNP Scores for Prediction of Venous Thromboembolism in Patients with Cancer. J. Thromb. Haemost. 2021, 19, 2974–2983. [Google Scholar] [CrossRef]
- Sproul, E.E. Carcinoma and Venous Thrombosis: The Frequency of Association of Carcinoma in the Body or Tail of the Pancreas with Multiple Venous Thrombosis. Am. J. Cancer 1938, 34, 566–585. [Google Scholar] [CrossRef]
- Blom, J.W.; Osanto, S.; Rosendaal, F.R. High Risk of Venous Thrombosis in Patients with Pancreatic Cancer: A Cohort Study of 202 Patients. Eur. J. Cancer 2006, 42, 410–414. [Google Scholar] [CrossRef]
- Mitry, E.; Taleb-Fayad, R.; Deschamps, A.; Mansencal, N.; Lepère, C.; Declety, G.; Lièvre, A.; Vaillant, J.-N.; Lesur, G.; Cramer, E.; et al. Risk of Venous Thrombosis in Patients with Pancreatic Adenocarcinoma. Gastroenterol. Clin. Biol. 2007, 31, 1139–1142. [Google Scholar] [CrossRef]
- Poruk, K.E.; Firpo, M.A.; Huerter, L.M.; Scaife, C.L.; Emerson, L.L.; Boucher, K.M.; Jones, K.A.; Mulvihill, S.J. Serum Platelet Factor 4 Is an Independent Predictor of Survival and Venous Thromboembolism in Patients with Pancreatic Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2605–2610. [Google Scholar] [CrossRef]
- Shaib, W.; Deng, Y.; Zilterman, D.; Lundberg, B.; Saif, M.W. Assessing Risk and Mortality of Venous Thromboembolism in Pancreatic Cancer Patients. Anticancer. Res. 2010, 30, 4261–4264. [Google Scholar]
- Epstein, A.S.; Soff, G.A.; Capanu, M.; Crosbie, C.; Shah, M.A.; Kelsen, D.P.; Denton, B.; Gardos, S.; O’Reilly, E.M. Analysis of Incidence and Clinical Outcomes in Patients with Thromboembolic Events and Invasive Exocrine Pancreatic Cancer. Cancer 2012, 118, 3053–3061. [Google Scholar] [CrossRef]
- Krepline, A.N.; Christians, K.K.; George, B.; Ritch, P.S.; Erickson, B.A.; Tolat, P.; Evans, D.B.; Tsai, S. Venous Thromboembolism Prophylaxis during Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer-Is It Indicated? J. Surg. Oncol. 2016, 114, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Ro, Y.S.; Cho, J.; Park, Y.; Lee, J.H.; Hwang, J.-H.; Choi, H.J.; Lee, S. Characteristics of Venous Thromboembolism in Pancreatic Adenocarcinoma in East Asian Ethnics: A Large Population-Based Observational Study. Medicine 2016, 95, e3472. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.T.M.; Mulder, F.I.; Kamphuisen, P.W.; Middeldorp, S.; Bossuyt, P.M.; Büller, H.R.; van Es, N. Primary Thromboprophylaxis in Ambulatory Cancer Patients with a High Khorana Score: A Systematic Review and Meta-Analysis. Blood Adv. 2020, 4, 5215–5225. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 International Clinical Practice Guidelines for the Treatment and Prophylaxis of Venous Thromboembolism in Patients with Cancer, Including Patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous Thromboembolism in Cancer Patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I.; on behalf of the COMPASS–CAT Working Group. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS–Cancer-Associated Thrombosis Study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef]
- Ay, C.; Dunkler, D.; Marosi, C.; Chiriac, A.-L.; Vormittag, R.; Simanek, R.; Quehenberger, P.; Zielinski, C.; Pabinger, I. Prediction of Venous Thromboembolism in Cancer Patients. Blood 2010, 116, 5377–5382. [Google Scholar] [CrossRef]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.-M.; Nisio, M.D.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A Clinical Prediction Model for Cancer-Associated Venous Thromboembolism: A Development and Validation Study in Two Independent Prospective Cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef]
- Muñoz, A.; Ay, C.; Grilz, E.; López, S.; Font, C.; Pachón, V.; Castellón, V.; Martínez-Marín, V.; Salgado, M.; Martínez, E.; et al. A Clinical-Genetic Risk Score for Predicting Cancer-Associated Venous Thromboembolism: A Development and Validation Study Involving Two Independent Prospective Cohorts. JCO 2023, 41, 2911–2925. [Google Scholar] [CrossRef]
- Li, A.; La, J.; May, S.B.; Guffey, D.; da Costa, W.L.; Amos, C.I.; Bandyo, R.; Milner, E.M.; Kurian, K.M.; Chen, D.C.R.; et al. Derivation and Validation of a Clinical Risk Assessment Model for Cancer-Associated Thrombosis in Two Unique US Health Care Systems. J. Clin. Oncol. 2023, 41, 2926–2938. [Google Scholar] [CrossRef]
- van Es, N.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Otten, H.-M.; Mahé, I.; Wilts, I.T.; Twint, D.C.; Porreca, E.; Arrieta, O.; et al. Comparison of Risk Prediction Scores for Venous Thromboembolism in Cancer Patients: A Prospective Cohort Study. Haematologica 2017, 102, 1494–1501. [Google Scholar] [CrossRef]
| Characteristics | n = 762 |
|---|---|
| Median age (IQR) | 69 (60–76) |
| Male, n (%) | 412 (54.1) |
| BMI | - |
| Median (IQR), kg/m2 | 23.5 (21.0–26.2) |
| ≥35 kg/m2, n (%) | 14 (1.9) |
| Missing, n | 22 |
| Performance status, n (%) | - |
| ECOG < 2 | 583 (87.3) |
| ECOG ≥ 2 | 85 (12.7) |
| Missing | 94 |
| Comorbidities, n (%) | - |
| Active smokers | 383 (50.5) |
| Hypertension | 301 (39.5) |
| Hyperlipidemia | 168 (22.0) |
| Diabetes | 193 (25.3) |
| Cardiac failure | 15 (2.0) |
| Respiratory failure | 12 (1.6) |
| History of VTE, n (%) | 41 (5.4) |
| Primary tumor location, n (%) | - |
| Head | 402 (53.5) |
| Isthmus | 45 (6.0) |
| Body | 100 (13.3) |
| Tail | 77 (10.2) |
| Multiple | 128 (17.0) |
| Missing | 10 |
| Stage, n (%) | - |
| Resectable tumor | 46 (6.1) |
| Potentially resectable tumor | 76 (10.0) |
| Locally advanced tumor | 350 (46.2) |
| Metastatic tumor | 286 (37.6) |
| Missing | 5 |
| Macroscopic vascular or lymphatic compression, n (%) | - |
| Yes | 317 (47.3) |
| Missing | 92 |
| Scheduled chemotherapy within 6 months, n (%) | |
| Platinum-based therapy | 465(61.0) |
| Gemcitabine-based therapy | 258 (33.9) |
| Platinum- and gemcitabine-based therapy | 29 (3.8) |
| Other chemotherapy | 10 (1.3) |
| Hemoglobin, g/dL | |
| Median (IQR) | 13.0 (11.9–14.0) |
| <10 g/dL, n (%) | 32 (4.4) |
| Missing, n | 38 |
| Leukocyte count, ×109/L | |
| Median (IQR) | 7.5 (6.1–9.3) |
| >11 × 109/L, n (%) | 85 (11.7) |
| Missing, n | 36 |
| Platelet count, ×109/L | |
| Median (IQR) | 259 (205–318) |
| ≥350 × 109/L, n (%) | 124 (16.3) |
| Missing, n | 36 |
| Median CA 19.9 (IQR), µmol/L | 330.1 (46.7–2366.0) |
| Events | Total Study Cohort (n = 762) |
|---|---|
| Total number of events, n (%) | 73 (9.6) |
| Type of events, n (%) | - |
| Pulmonary embolism | 17 (23.3) |
| Deep vein thrombosis | 17 (23.3) |
| Visceral vein thrombosis | 29 (31.5) |
| Catheter-related thrombosis | 4 (5.5) |
| Combined venous thromboembolism events | 6 (8.2) |
| Clinical presentation, n (%) | - |
| Symptomatic | 32 (43.8) |
| Incidental | 41 (56.2) |
| Khorana Score | PROTECHT Score | ONKOTEV Score | |
|---|---|---|---|
| Brier score (95% CI) | 0.14 (0.12–0.15) | 0.14 (0.12–0.15) | 0.14 (0.12–0.15) |
| Time-dependent c-index (95% CI) | 0.50 (0.46–0.55) | 0.50 (0.49–0.51) | 0.53 (0.48–0.58) |
| Cumulative incidence of VTE, % (95% CI) | - | - | - |
| High-risk group | 16.1 (11.4–21.5) | 16.5 (13.9–19.3) | 19.0 (14.4–24.2) |
| Intermediate-risk group | 16.5 (13.4–19.8) | Not estimable * | 15.0 (12.0–18.4) |
| SHR high- vs. intermediate-risk group (95% CI) | 1.06 (0.77–1.45) | 1.87 (0.29–12.05) | 1.05 (0.76–1.44) |
| Khorana, SHR (95% CI) | PROTECHT, SHR (95% CI) | ONKOTEV, SHR (95% CI) | |
|---|---|---|---|
| Platelet count | |||
| <350 × 109/L | Ref | Ref | |
| ≥350 × 109/L | 1.07 (0.73–1.55) | 1.08 (0.74–1.58) | |
| Hemoglobin level | - | - | - |
| ≥10 g/dL | Ref | Ref | - |
| <10 g/dL | 1.35 (0.72–2.52) | 1.35 (0.72–2.55) | - |
| Leukocyte count | - | - | - |
| ≤11 × 109/L | Ref | Ref | - |
| >11 × 109/L | 0.99 (0.62–1.57) | 0.98 (0.61–1.56) | |
| Body mass index | - | - | - |
| <35 kg/m2 | Ref | Ref | |
| ≥35 kg/m2 | 1.43 (0.58–3.56) | 1.44 (0.57–3.59) | |
| Gemcitabine therapy | - | - | |
| No | - | Ref | - |
| Yes | - | 1.26 (0.70–2.24) | |
| Platinum-based therapy | - | - | - |
| No | - | Ref | |
| Yes | - | 1.18 (0.65–2.13) | |
| Khorana score | - | - | - |
| ≤2 | - | - | Ref |
| >2 | - | - | 0.98 (0.72–1.35) |
| Previous VTE | - | - | - |
| No | - | - | Ref |
| Yes | - | - | 1.37 (0.79–2.37) |
| Metastatic disease | - | - | - |
| No | - | - | Ref |
| Yes | - | - | 1.56 (1.17–2.07) |
| Macroscopic vascular compression | - | - | - |
| No | - | - | Ref |
| Yes | - | - | 0.79 (0.59–1.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frere, C.; Gourgou, S.; Winter, A.; Gauthier, L.; Canivet, C.; Crichi, B.; Marjanovic, Z.; Yannoutsos, A.; Bensaoula, O.; Buscail, L.; et al. Risk Assessment Models for Predicting Venous Thromboembolism in Patients with Pancreatic Cancer. Cancers 2025, 17, 597. https://doi.org/10.3390/cancers17040597
Frere C, Gourgou S, Winter A, Gauthier L, Canivet C, Crichi B, Marjanovic Z, Yannoutsos A, Bensaoula O, Buscail L, et al. Risk Assessment Models for Predicting Venous Thromboembolism in Patients with Pancreatic Cancer. Cancers. 2025; 17(4):597. https://doi.org/10.3390/cancers17040597
Chicago/Turabian StyleFrere, Corinne, Sophie Gourgou, Audrey Winter, Ludovic Gauthier, Cindy Canivet, Benjamin Crichi, Zora Marjanovic, Alexandra Yannoutsos, Okba Bensaoula, Louis Buscail, and et al. 2025. "Risk Assessment Models for Predicting Venous Thromboembolism in Patients with Pancreatic Cancer" Cancers 17, no. 4: 597. https://doi.org/10.3390/cancers17040597
APA StyleFrere, C., Gourgou, S., Winter, A., Gauthier, L., Canivet, C., Crichi, B., Marjanovic, Z., Yannoutsos, A., Bensaoula, O., Buscail, L., Bournet, B., & Farge, D., on behalf of the BACAP Consortium. (2025). Risk Assessment Models for Predicting Venous Thromboembolism in Patients with Pancreatic Cancer. Cancers, 17(4), 597. https://doi.org/10.3390/cancers17040597







